Abstract
There are reports that amifostine protects normal tissues against chemotherapy and radiotherapy in cancer treatment. In this study, daunorubicin effects on cell kinetics parameters and apoptosis were examined both singly and in combination on HeLa cell culture. Optimum doses of daunorubicin and amifostine (0.1, 1 μg/mL, respectively) were applied for 24 and 48 h. Cell kinetic parameters such as growth rate (WST-1 colorimetric assay), mitotic and apoptotic index were applied to identify cytotoxicity that was induced by daunorubicin. In addition, to evaluate the molecular mechanism of cytoprotectivity we studied DNA degradation in agarose gel electrophoresis and expression of two genes, bcl-2 and bax, known to be important for the regulation of apoptosis. The cytotoxic effect correlated with a decline of growth rate and mitotic index, and a significant increase of apoptotic index, when HeLa cells were treated with daunorubicin alone or in combination with amifostine treatment (p < 0.05-0.001). The enhancement of the cytotoxicity by daunorubicin was closely associated with DNA degradation. Investigation of bcl-2/bax expression level by RT-PCR showed that amifostine did not protect tumor cell lines against daunorubicin cytotoxicity.
Keywords::
Introduction
Chemotherapy plays a critical role in virtually every phase of cancer treatment (CitationZeng et al., 2000). The role of chemotherapeutic pharmacodynamic and pharmacokinetic properties has increasingly been investigated during the last few years (CitationPlosker & Faulds, 1993; CitationZeng et al., 2000; CitationÖzcan Arıcan et al., 2007). One of the major limitations of cancer chemotherapy is the indiscriminate injury of normal tissue, leading to organ toxicity and consequent dose limitation/treatment failure. Several antioxidants have been tested and shown to protect normal tissues from chemotherapeutic toxicity (CitationDas et al., 2003). Among these agents, amifostine (AMI) has cytoprotective action. AMI, previously known as WR (Walter-Reed) 2721, was originally developed as a radioprotective reagent during the Cold War in the 1950s. Nowadays, it is considered as a broad-spectrum radioprotective and chemoprotective drug, and it has been clinically used to reduce the toxic side-effects induced by anticancer agents (CitationGrochova & Ŝmardova, 2007).
Chemotherapeutic agents including daunorubicin (DAU) are known to induce apoptosis (CitationSkladanowski & Konopa, 1993; CitationHueber et al., 2003). Apoptosis, as a distinct type of cell death, is governed by a number of regulating genes mediated by apoptotic signals. Bcl-2 family proteins were demonstrated to be important apoptosis regulators after drug treatment (CitationPeriman et al., 1999; CitationBruckheimer et al., 2000). Bcl-2 and bax are two discrete members of the gene family involved in the regulation of cellular apoptosis (CitationHanada et al., 1995). Among the variety of proteins that are produced in response to apoptotic signals are those mediating DNA fragmentation, such as bax, bcl-xs and bak, as well as the anti-apoptotic ones, such as bcl-2, bcl-xl (CitationWerner et al., 2002; CitationOliver et al., 2005).
In recent years, the in vitro cytotoxic activity and cytoprotective effect of antineoplastic agents has been investigated. In vitro, AMI’s cytoprotectivity and DAU sensivity was related to different mechanisms (CitationViera et al., 2002; CitationDas et al., 2003. Citation2008; CitationDai et al., 2005; CitationRoˇźalski et al., 2005). Several factors might explain the discrepancies regarding the influence of bcl-2 and bax on apoptosis (CitationJäckel et al., 1999). Therefore, we have investigated the expression and possible role of bcl-2 and bax on cytoprotectivity in vitro.
In this study we examined the cytotoxicity of DAU with growth inhibition and mitotic index (MI) as the cell kinetic parameters. Also, DNA profiles of treatment groups were screened by agarose gel electrophoresis and bcl-2/bax gene expression by RT-PCR. We examined the apoptotic index (AI) parameter to investigate cytotoxicity, induced by DAU and AMI+DAU.
Materials and methods
Cell culture
The tumor cell line used in our experiments was HeLa, initiated from a human cervical carcinoma in 1951 and continuously grown in cell culture since that date. These cells were grown regularly by passage twice a week in our laboratory. Cells were cultured in minimum essential medium (MEM) (Sigma) containing 10% fetal bovine serum (FBS) (Gibco) at 37°C in a humidified atmosphere of 5% CO2 in air. Cells were washed with Hank’s balanced salt solution (HBSS) and harvested using 0.25% trypsin (Gibco) for 3 min. Then cells were centrifuged at 1500 rpm for 3 min. The supernatant was discarded and the pellet was diluted with MEM. Cells were seeded 30.000 cells/well in 96-well plates. After incubation at 37°C for 24 h, experiments were done.
Drug treatments
AMI (Ethyol®, 50 mg) (Bioscience) and DAU (Daun orubicin, 20 mg) (Carlo Erba) were dissolved in MEM as a 1 mg/mL stock solution supplemented with 10% FBS. The pH of the drug solution was adjusted to 7.4 with NaOH. All assays testing AMI and DAU were protected from light. The inhibitory concentration 50% (IC50) dose of DAU was determined in HeLa cell culture (Citationözcan Arıcan et al., 2005). The experiments (using doses of 0.1 and 1 μg/mL, respectively) were carried out in 4 groups: Control, AMI, DAU and AMI+DAU combinations. HeLa cells were tested for 24 and 48 h.
Cytotoxicity assay
The effect of chemotherapeutic agents on the growth rate of HeLa cells was evaluated with the WST-1 assay kit (Premix WST-1, Takara, cat no: MK400). The WST-1 assay was applied to identify cytotoxicity that was formed by drugs for 24 and 48 h. Therefore, WST-1 reagent was added, and 4 h later, the 96 well plates were read on a Mquant Microelisa reader, using a test wavelength of 570 nm, and a reference wavelength of 630 nm.
Mitotic index analysis
MI was studied by the method of Feulgen. Before the cells were treated with Feulgen, they were prepared with 1 N HCl at room temperature for 1 min and then hydrolyzed with 1 N HCl for 10.5 min at 60°C. After the slides were treated with Feulgen, they were rinsed for a few min in distilled water and stained with 10% Giemsa stain solution pH 6.8, for 3 min and washed twice in phosphate buffer. After staining, the slides were rinsed in distilled water and air-dried. Finally, MI was calculated by counting metaphases, anaphases and telophases for each tested drug concentration and control. At least 3,000 cells were examined from each slide for MI.
Apoptosis assay
For the determination of the AI, cells were fixed under methanol and stained with 49, 6-diamidine-2 phenylindole (DAPI). Following extensive washing in phosphate-buffered saline (PBS), slides were scored (double-blind) under the fluorescence microscope. AI represents the percentage of fragmented nuclei and was determined on a microscopic field of at least 100 area/each experimental point by the same scorer.
DNA extraction
Genomic DNA was isolated from HeLa cells as described in the protocol of manufacturer (Apopladder Ex™ Kit, Takara, Cat. No. MK600). The DNA concentration of samples was at A260 and electrophoresed in 2% (w/v) agarose containing 1 μg/mL ethidium bromide.
Total RNA extraction
Total RNA was isolated from HeLa cells via Invitrogen, Purelink Micro-to-Midi Total RNA Purification System (Cat no. 12183-018). Total RNA content was measured with visible spectrophotometer (GBC Cintra 20).
RT-PCR analysis
HeLa cells (2.5 105) were seeded on plastic culture dishes ( 60 mm in diameter) in 5 mL of MEM. Cells were precultured for 24 and 48 h, and then total RNA from the cells was extracted using a total RNA purification system (Invitrogen, Cat no. 12183-018). RT-PCR was carried out using SuperScript III one-step RT-PCR system with Platinum Taq DNA polymerase described by the manufacturer (Invitrogen, Cat no. 12574-026) with the PCR primers from Takara Apoprimer set (Takara Bcl-2 family, Cat no. 6623). Single strand cDNA was synthesized by incubation at 55°C for 30 min. The reaction mixture was then held at 94°C for 2 min to denature the RNA/cDNA hybrid and inactivate the AMV-RT.
The double-stranded cDNA was produced and amplified with the following PCR conditions: 40 cycles of template denaturation at 94°C for 15 s primer annealing at 60°C for 30 s and primer extension at 68°C for 1 min. A final elongation step at 68°C for 5 min was performed. Reaction was stopped at 4°C. PCR products were separated on Tris-acetate-EDTA 1.5% agarose gels containing 100 ng/mL ethidium bromide.
Statistical analysis
The data were analyzed statistically using ANOVA. Dunnett’s test (between control and treatment groups) and Student Newman-Keuls tests (between treatment groups) were used for multiple comparisons. Apoptotic index analysis was evaluated by Student’s t-test. Data analyzed were from a minimum of three independent experiments.
Results
Growth rate effects of AMI and DAU
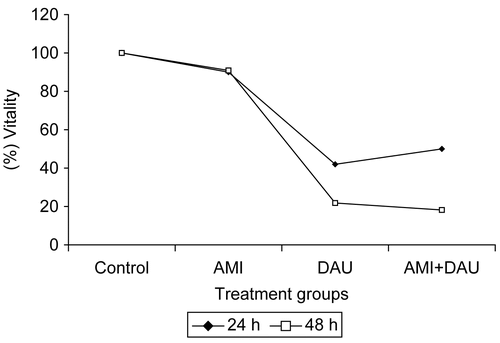
The absorbance value of AMI, DAU and AMI+DAU treatment groups of HeLa cells are shown in . The absorbance value for drugs was higher for 24 h than for 48 h in HeLa cells. When the HeLa cells were treated for 24 and 48 h with DAU, their growth and viability were significantly decreased in time-dependent manners relative to control (p <0.001) ().
Table 1. Effect of AMI and DAU either alone or in combination for 24 and 48 h in HeLa cells, given in ± SD.
Mitotic index of treated cells
The values of MI for 24 and 48 h decreased in HeLa cells when treated with DAU and AMI+DAU, as seen in . These decreases were statistically significant when compared to control and single AMI treatment (p <0.001). Another part of the cytotoxic effect of these drugs is inhibition of MI in HeLa cells.
Table 2. Mitotic index values of treated cells, given in ± SD.
Apoptosis assay of treated cells
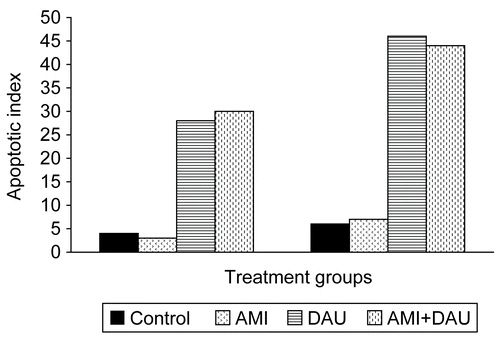
Apoptotic cell number of HeLa cell culture treated with AMI, DAU and AMI+DAU, as shown in , revealed that drugs express part of their cytotoxic effects by induction apoptosis. The values of AI also increased with increasing treatment time (). The apoptotic cell number and AI values in DAU and AMI+DAU treatment was higher than control and single AMI treatment for both treatment times.
Table 3. Apoptotic cell number of drugs in HeLa cell lines.
DNA profiles of treated cells
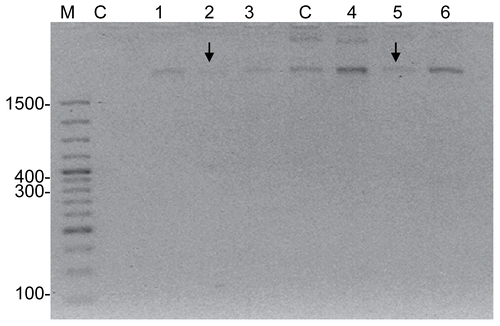
Agarose gel electrophoresis of DNA from HeLa cells treated with AMI, DAU and AMI+DAU is shown in for 24 and 48 h, respectively. As a result, high DNA degradation was determined in single DAU treatment for 24 and 48 h. When the DNA levels of HeLa cells were compared in treatment groups, the most effective result was obtained in single DAU treatment for 48 h.
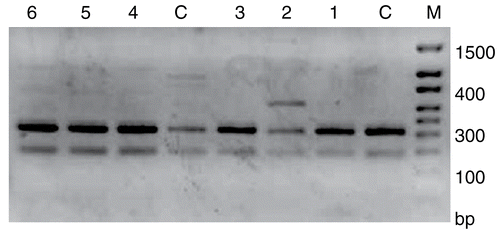
Bcl-2 and bax expression of HeLa cells
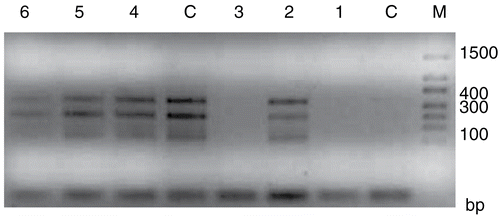
In our study, single DAU treatment modified the expression of bcl-2 and changed the ratio of bcl-2 over bax, thereby inducing more apoptosis than other experimental treatment, especially for 24 h ( and ). Bcl-2/bax gene expression levels showed that both bcl-2 and bax expression levels increase on only DAU experiment groups. There was no expression difference between control and AMI+DAU combined group.
Discussion
In our experiment, assessed parameters showed that DAU has cytotoxic effects both at 24 and 48 h, thus it has been observed that it causes significant (p < 0.001) time-dependent decrease on the proliferation rate of HeLa cells. It also has been shown using proliferation rate, MI, AI, agarose gel electrophoresis and RT-PCR techniques that AMI does not moderate cytoprotective effects over the cytotoxic effects of DAU. As a result, we agree the cytotoxic effect of AMI does not occur on tumor cell lines (CitationÖzcan Arıcan, 2005).
DNA synthesis inhibition, free radical formation and lipid peroxidation, DNA binding and alkylation, DNA cross-linking, interference with DNA strand separation and helicase activity, direct membrane effects, and the initiation of DNA damage via the inhibition of topoisomerase II are mechanisms responsible for the antiproliferative and cytotoxic effects of daunorubicin (CitationMeriwether & Bachur, 1972; CitationKeprtova et al., 1993; CitationGewirtz, 1999). The anthracyclines have been shown to induce apoptotic cell death (CitationLing et al., 1993; CitationSkladanowski & Konopa, 1993; CitationJaffrezou et al., 1996), although this is likely to be the final cellular response to upstream events such as inhibition of topoisomerase II (CitationGewirtz, 1999). CitationGrazide et al. (2004) studied the effect of blocking GSC in their highly drug-sensitive leukemic models. They demonstrated that by using the classic GCS inhibitors, daunorubicin and Ara-C induced apoptosis was blocked. In recent years, several studies have reported the cytoprotectivity and apoptotic activities of anticancer drugs of with cell culture (CitationMirjolet et al., 2000; CitationNitsu et al., 2000; CitationLucianot et al., 2006; CitationTakara et al., 2006).
Criteria such as proliferation rate and DNA fragmentation which were assessed in our experiments are limited to explain the mechanism of cytotoxic and cytoprotective effects of drugs on the cellular level. However, previous studies are consistent with our findings as cytotoxicity mediated by DAU increases depending on time, and the cytoprotective effect of AMI does not occur with tumor cells.
The effect of AMI, DAU, and AMI+DAU combinations on HeLa cell DNA profiles are shown in . The largest DNA sample degradation was caused by DAU; there is no cytoprotective effect of combined AMI application in tumor cells.
In summary, consistent with the literature, our findings indicate a lack of cytoprotective effects of AMI on tumor cells. As shown by AI values, DAU and AMI+DAU treatment groups have high values relative to control and single AMI experimental groups (p <0.05). DNA profiles and RT-PCR results support AI and MI results in single and combined DAU treatments. In addition, we looked at bcl-2/bax gene expression levels, we could see both bcl-2 and bax expression levels increase on only DAU experiment groups. There was no expression difference between control and AMI+DAU groups. For future studies, the cytoprotective effect of AMI mediated by bcl-2 and bax regulation can be investigated. The results of this study show the extent of spontaneous apoptosis seems to be independent of the expression of bcl-2 and bax, but correlates with the mitotic activity and apoptotic index in HeLa cell lines. Our results also suggest the mechanisms underlying this process are complex and largely unknown; it will be difficult to assess the contribution of individual genes to apoptosis in vitro and in vivo.
Acknowledgements
This work was supported by the Research Fund of the University of Istanbul. Project number BYP-749/21072005.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bruckheimer EM, Cho S, Brisbay S, Johnson DJ, Gingrich JR, Greenberg N, McDonnell TJ (2000): The impact of bcl-2 expression and bax deficiency on prostate homeostasis in vivo. Oncogene 19: 2404–2412.
- Dai D, Holmes AM, Nguyen T, Davies S, Theele DP, Verschraegen C, Leslie KK (2005): A potential synergistic anticancer effect of paclitaxel and amifostine on endometrial cancer. Cancer Res 65: 9517–9524.
- Das A, Belagodu A, Relter RJ, Ray SK, Banik NL (2008): Cytoprotective effects melatonin on C6 astroglial cells exposed to glutamate excitotoxicity and oxidative stress. J Pineal Res 45: 117–124.
- Das B, Yeger H, Baruchel H, Freedman MH, Koren G, Baruchel S (2003): In vitro cytoprotective activity of squalene on a bone marrow versus neuroblastoma model of cisplatin-induced toxicity: Implications in cancer chemotherapy. Eur J Cancer 39: 2556–2565.
- Gewirtz DA (1999): A critical evaluation of the mechanism of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57: 727–741.
- Grazide S, Terrisse A-D, Leronge S, Laurent G, Jaffrézou J-P (2004): Cytoprotective effect of glucosylceramide synthase inhibition against daunorubicin-induced apoptosis in human leukemic cell lines. J Biol Chem 30: 18258–18261.
- Grochova D, Ŝmardova J (2007): The antimutagenic and cytoprotective effects of amifostine: the role of p53. J Appl Biomed 5: 171–178.
- Hanada M, Aime-Sempe C, Sato T, Reed JC (1995): Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem 270: 11962–11969.
- Hueber A, Weller M, Welsandt G, Kociok N, Kirchhof B, Eser P (2003): Characterization of dounorubicin-induced apoptosis in retinal pigment epithelial cells: modulation by CD95L. Investigative Ophtalmology & Visual Science 44: 2851–2857.
- Jäckel MC, Dorudian MA, Marx D, Brinck U, Schaurer A, Steiner W (1999): Spontaneous apoptosis in laryngeal squamous cell carcinoma is independent of bcl-2 and bax protein expression. American Cancer Society 85: 591–599.
- Jaffrezou JP, Levade T, Bettaieb A, Andrieu N, Bezombes C, Maestre N, Vermeersch S, Rousse A, Laurent G (1996): Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J 15: 2417–2424.
- Keprtova J, Vyhnakova M, Minarova E, Kleinwachter V (1993): Effects of daunomycin and its macromolecular analog daunophilin on the proliferation of mammalian cells. Neoplasma 40: 161–165.
- Ling YH, Priebe W, Perez-Solar R (1993): Apoptosis induced by anthracycline antibiotics in P388 parent and multidrug resistant cells. Cancer Res 53: 1845–1852.
- Lucianot F, Zhai D, Zhu X, Bailly-Maitre B, Ricci J-E, Satterthwait AC, Reed JC (2006): Cytoprotective peptide humanin binds and inhibits proapoptotic Bcl-2/Bax family protein BimEl. J Biol Chem 280: 15825–15836.
- Meriwether WD, Bachur NR (1972): Inhibition of DNA and RNA metobolism by daunorubicin and adrimycin in L1210 mouse leukemia. Cancer Res 32: 1137–1142.
- Mirjolet J-F, Barberi-Heyob M, Didelot C, Peyrat J-P, Abecassis J, Millon R, Merlin J-L (2000): Bcl-2/Bax protein ratio predicts 5-fluorouracil sensitivity independently of p53 status. Br J Cancer 83: 1380–1388.
- Nitsu N, Kasukabe T, Yokoyama A, Okabe-Kado J, Yamamoto-Yamaguchi Y, Umeda M, Honma Y (2000): Anticancer derivative of butyric acid (pivalyloxymethyl butyrate) specifically potentiates the cytotoxicity of doxorubicin and daunorubicin through the suppression of microsomal glycosidic activity. Mol Pharmacol 58: 27–36.
- Oliver CL, Miranda MB, Shangary S, Land S, Wang S, Johnson DE (2005): (-)-Gossypol acts directly on the mitochondria to overcome Bcl-2 and Bcl-XL-mediated apoptosis resistance. Mol Cancer Therapeut 4: 23–31.
- Özcan Arıcan G (2005): Cytoprotective effects of amifostine and cysteamine on cultured normal and tumor cells treated with paclitaxel in terms of mitotic index and 3H-tymidine labeling index. Cancer Chemo Pharmacol 56: 221–229.
- Özcan Arıcan G, Özalpan A (2007): Evaluation of the effect of PAC, EPI and TAM by cell kinetics parameters in estrogen-receptor-positive Ehrlich Ascites Tumor (EAT) cells growing in vitro. Acta Biologia Hungarica 58: 49–59.
- Özcan Arıcan G, Soy NN (2005): Effects of epirubicin and daunorubicin on cell proliferation and cell death in HeLa cells. J Cell Mol Biol 4: 47–52.
- Periman H, Zhang X, Chen MW, Walsh K, Buttyan R (1999): An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Different 6: 48–54.
- Plosker GL, Faulds D (1993): A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapy. Drugs 45: 788–856.
- Roˇźalski M, Mirowski M, Balcerczak E, Krajewska U, Mlynarski W, Wierzbicki R (2005): Induction of caspase 3 activity, bcl-2 bax and p65 gene expression modulation in human acute promyeloctic leukemia HL-60 cells by doxorubicin with amifostine. Pharmacol Rep 57: 360–366.
- Skladanowski A, Konopa J (1993): Adriamycin and daunomycin induce programmed cell death (apoptosis) in tumor cells. Biochem Pharmacol 46: 375–382.
- Takara K, Obata Y, Yoshikawa E, Kitada N, Sakaeda T, Ohnishi N, Yokoyama T (2006): Molecular changes to HeLa cells on continuous exposure to cisplatin or paclitaxel. Cancer Chemo Pharmacol 58: 785–793.
- Viera HLA, Boya P, Cohen I, El Hamel C, Haouzi D, Druillenec S, Belzacq A-S, Brenner C, Roques B, Kroemer G (2002): Cell permeable BH3-peptides overcome the cytoprotective effect of Bcl-2 and Bcl-XL. Oncogene 21: 1963–1977.
- Werner AB, de Viries E, Stephen WG, Tait SWG, Bontjer I, Borst J (2002): Bcl-2 family member Bfl-1/A1 sequesters truncated bid to inhibit its collaboration with pro-apoptotic Bak or Bax. J Biol Chem 277: 22781–22788.
- Zeng S, Chen YZ, Fu L, Johnson KR, Fan W (2000): In vitro evaluation of schedule-dependent interactions between docataxel and doxorubicin against human breast and ovarian cancer cells. Clin Cancer Res 6: 3766–3773.