Abstract
In this paper, antibacterial and antimycobacterial activity of five Labiatae plant methanol extracts, commonly used for treating cold, stomachache, and sore throat, Salvia fruticosa Mill., Salvia tomentosa Mill., Sideritis albiflora Hub.-Mor. (endemic), Sideritis leptoclada O. Schwarz & P.H. Davis, (endemic), and Origanum onites L., were investigated, and their phenolic compounds were determined by HPLC. Antibacterial activity was analyzed against Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Bacillus cereus, Escherichia coli, Salmonella typhimurium, Enterobacter aerogenes, and Klebsiella pneumoniae. Antimycobacterial activity was assayed against Mycobacterium tuberculosis. The best antibacterial activity (MIC 640 μg/mL) was shown against S. typhimurium and E. aerogenes by S. fruticosa; E. coli, and S. typhimurium, E. aerogenes by S. tomentosa; S. typhimurium, and E. aerogenes by S. leptoclada and S. typhimurium, E. aerogenes and S. epidermidis by O. onites, respectively. The best antimycobacterial activity (MIC 196 μg/mL) was shown by S. tomentosa. S. fruticosa (MIC 392 μg/mL) and O. onites (MIC 784 μg/mL) showed moderate activity against M. tuberculosis. S. albiflora, with low level rosmarinic acid and carvacrol content, showed inhibition against bacteria except K. pneumoniae, B. cereus and M. tuberculosis. The correlation between in vitro activity and ethnobotanical usage was evaluated.
Introduction
The genus Salvia L. (Lamiaceae) is widely distributed with 90 species growing in Turkey, and has economic and medicinal importance (CitationDavis, 1982; CitationBaytop, 1984; CitationBaser, 1995; CitationBaydar et al., 2004; CitationHamzaoglu et al., 2005; CitationTopcu et al., 2007). Salvia species are known to have diterpenoids (CitationUlubelen & Topcu, 1984; CitationUlubelen et al., 1997), triterpenoids (CitationPedreros et al., 1990; CitationSokovic et al., 2002; CitationUlubelen et al., 2001, Citation2002; CitationMehmood et al., 2006) essential oils (CitationTepe et al., 2004; CitationTabanca et al., 2006), and flavonoids (CitationUlubelen & Topcu, 1984). CitationBagci et al. (2004) studied fatty acid composition of some Salvia species. CitationKamatou et al. (2005) researched some Salvia species for in vitro pharmacological activities and chemical constituents.
Studies on methanol extracts of Salvia species were found to be quite interesting and provided valuable results about plants properties. CitationTepe (2007) showed that rosmarinic acid and derivatives were likely responsible for antioxidant activities of some Salvia species. CitationFiore et al. (2006) examined the in vitro antiproliferative activity of the methanol crude extracts of six Salvia species and found potential antitumor agents. CitationEidi et al. (2005) studied the hypoglycemic effect of sage leaves (Salvia officinalis L.) and found that while the essential oil of sage did not change serum glucose, the methanol extract significantly decreased serum glucose in diabetic rats.
Sideritis L. (Lamiaceae) is represented in Turkey by 52 taxa belonging to 45 species of which 34 are endemic. Dried inflorescences of Sideritis species are used as a popular herbal tea in Turkey and Greece (CitationDavis, 1982; CitationDuman, et al., 1995; CitationTabanca et al., 2001; CitationBaser, 2002). It is used for treatment of colds, stomachache, and sore throat (CitationSezik & Ezer, 1983; CitationBaser et al., 1986). Some Sideritis species have been investigated for non-volatile constituents and ent-kaurane diterpenes were isolated (CitationBaser et al., 1996; CitationTopcu et al., 1999, Citation2002). The main constituents of most Sideritis growing in Turkey were essential oils (α- or β-pinene or both) (CitationBaser, 1995; CitationEzer et al., 1996; CitationTabanca et al., 2001).
The genus Origanum L. (Lamiaceae) is represented in Turkey by 23 species or 32 taxa, 21 being endemic (CitationDavis, 1982; CitationDuman et al. 1995; CitationBaser, 2002). It is also antispasmodic (CitationBaser, 1995, Citation2002; CitationSatil et al., 2006a) and antibacterial (CitationLambert et al., 2001). CitationTumen et al. (1995) found the major essential oils of Origanum were terpene-phenol, carvacrol, p-cymene, linalool, and γ-terpene.
The aim of this study was to determine major phenolic compounds of Salvia fruticosa Mill, S. tomentosa Mill, Sideritis albiflora Hub.-Mor. (endemic), S. leptoclada O. Schwarz & P.H. Davis, (endemic), and Origanum onites L. methanol extracts by HPLC, and to determine their antibacterial and antimycobacterial activity.
Materials and methods
Plant materials
Aerial parts (Herba in flowering stage) of plants were collected in June 2005 from different parts of Turkey. The plants were identified by F. Satil in Balıkesir University. Voucher specimens were deposited in the herbarium of Balikesir University, Department of Biology. Locality, altitude, collection time and herbarium number of species are given in .
Table 1. Herbarium data of plants.
Preperation of extracts
The air-dried plants at room temperature of S. fruticosa (140 g), S. tomentosa (84 g), Sideritis albiflora (135 g), S. leptoclada (117 g), and O. onites (135 g) were extracted with 1 L of methanol (98%) at room temperature during ten days according to CitationSeshadri (1962). The methanol extracts were filtered with filter paper, concentrated by using a rotary evaporator and dried in vacuo at 40°C. The total yield quantities were 1.30, 1.98, 1.21, 1.45 and 2.17 g, respectively. All stocks were stored at −20°C.
HPLC conditions
HPLC was performed with a Shimadzu HPLC device using phenolic compound preparation techniques (CitationCaponio et al., 1999). The detector was a DAD detector (max = 278 nm) and the auto sampler was an SIL–10ADvp. The system controller was an SCL-10Avp, the pump was an LC-10ADvp and the degasser was a DGU- 14A. The column oven was a CTO-10Avp and the column was Agilent Zorbax EclipseXDB-C18 (250 × 4. 60 mm) 5 μm. Mobile phases were A) 3% acetic acid, and B) methanol, and flow speed was 0.8 mL/minute. Column temperature was 30°C and injection volume was 20 μL.
Microorganisms and inoculum
A total of eight Gram-positive and Gram-negative bacteria were used for antibacterial activity studies. Gram-positive bacteria: Staphylococcus aureus (6538-P), Staphylococcus epidermidis (ATCC 12228), Enterococcus faecalis (ATCC 29212) and Bacillus cereus (CCM 99). Gram-negative bacteria: Escherichia coli (ATCC 11230), Salmonella typhimurium (CCM 583) Enterobacter aerogenes (CIP 6069) and Klebsiella pneumoniae (CCM 2318) were used for antibacterial testing.
The extracts were tested against the reference strain Mycobacterium tuberculosis H37Ra (ATCC 25177) for inhibitory activity in duplicate. Inoculum was prepared both from solid media and from a positive BACTEC Mycobacteria growth indicator tube (MGIT) according to the “inoculation procedure for susceptibility” recommended by the manufacturer CitationBecton Dickinson (2004).
From solid media cultures less than 15 days old, a suspension was prepared in Middlebrook 7H9 broth. The turbity of the suspension did not exceed 1.0 McFarland standard. The suspension was vortexed for 1–2 minutes, allowed to precipitate larger particles, and held for 20 min. The supernatant was transferred to an empty, sterile tube, and held for 15 min. After transferring to a new sterile tube, the suspension was adjusted to a 0.5 McFarland turbidity standard by visual comparison. One mL of the adjusted suspension was diluted in 4 mL of sterile saline.
Positive BACTEC MGIT tubes were used from the first day it became positive (day 1 positive) up to and including the fifth day (day 5 positive). After five days they were subcultured. Tubes from day 1 and day 2 were used for the inoculation procedure and susceptibility test. The tubes between 3 and 5 days were diluted 1 to 4 in saline; this diluted suspension was used for inoculation procedures.
Antibacterial activity test
Stock solutions of all extracts were prepared in 10% dimethylsulphoxide (DMSO). Determination of minimal inhibitory concentration (MIC) by the microdilution method were performed according to the National Committee of Clinical Laboratory Standard guidelines (CitationNCCLS, 2000) and CitationKoo et al. (2000). Sterile 96-well microplates were used for the assay (0.2 mL volume, Fisher Scientific). Samples were diluted to twice the desired initial test concentration with Trypton soya broth-soybean casein digest medium USP, (TSB), (Oxoid, Code: CM0129); samples that were difficult to dissolve were sonicated. All wells were filled with TSB (80 μL). Test sample (80 μL) was added to the first well and serial two-fold dilutions were made down to the desired minimum concentration. Serial dilutions are performed so extract concentrations in the range of 10240-10245 μg/mL were obtained. Day-old cultures of bacteria grown on Tryptone soy agar (TSA) (Oxoid, Code: CM0131) agar plates were suspended in TSB until turbidity was equal to a 0.5 McFarland Standard (CitationKoneman et al., 1997). Gentamycin (Oxoid) was used as a positive control. Serial dilutions were performed so that gentamycin concentrations in the range of 128–0.06 μg/mL were obtained. The plates were inoculated with the bacterial suspension (10 μL per well) and incubated at 37°C overnight. All tests were done in triplicate in three different experiments.
Antimycobacterial activity test
MGIT mycobacteria growth indicator tubes containing 4 mL of modified Middlebrook 7H9 broth base were used. Each test tube included a fluorescence-quenching-based oxygen sensor embedded in silicone in the bottom of the tubes. The fluorescent compound is sensitive to the presence of dissolved oxygen in the broth. The initial concentration of dissolved oxygen quenches the fluorescent emission from the compound. After actively respiring, the microorganisms consume the oxygen and allow the fluorescence to be observed using a 365 nm UV transilluminator (CitationPalaci et al., 1996; CitationReisner et al., 1995; CitationWalters & Hanna, 1996; CitationBecton Dickinson, 2002).
Assays were performed by instructions of the MGIT manual fluorometric susceptibility test procedure recommended by the manufacturer Becton Dickinson. OADC enrichment (0.5 mL), and a mixture of oleic acid, albumin, dextrose and catalase were added to each tube. Compound was added in a volume of 0.1 mL per MGIT tube.
The final concentration of the extracts adopted to evaluate antimycobacterial activity was included from 1.5 to 0. 012 mg/mL. An uninoculated MGIT tube was used as a negative control. The positive control tube contained only organisms and OADC but not the plant extract. Any suspicious growth of other bacteria was checked using blood agar for each test. The vials were incubated at 37°C and MIC was determined to be the lowest dilution giving negative by MicroMGIT fluorescence reader within 2 days when the controls turned positive. Tubes were read daily starting on the second day of incubation using a MicroMGIT fluorescence reader which has a long wave UV light (CitationBecton Dickinson, 2002).
Results and discussion
Five methanol extracts prepared from the aerial parts of plants were screened against Gram-positive bacteria S. aureus (6538-P), S. epidermidis (ATCC 12228), E. faecalis (ATCC 29212), B. cereus (CCM 99), and Gram-negative bacteria E. coli (ATCC 11230), S. typhimurium (CCM 583) E. aerogenes (CIP 6069) and K. pneumoniae (CCM 2318) ( and ). Gram− bacteria and Gram+ bacteria tested for susceptibility with gentamycin.
On the other hand, methanol extracts obtained from the aerial parts of plants were also tested against M. tuberculosis H37Ra (ATCC 25177) for antimycobacterial activity using the MGIT manual fluorometric susceptibility test. Standard drugs were streptomycin, rifampin, ethambuthol, and isoniasid. M. tuberculosis was found sensitive to standard drugs ().
Table 2. Susceptibility test results against M. tuberculosis H37Ra (ATCC 25177) obtained by the MGIT fluorometric manual method.
S. tomentosa displayed the best activity (MIC 640 μg/ml) against E. coli, S. typhimurium, and E. aerogenes. Other bacteria (K. pneumoni, B. cereus, E. faecalis, S. aureus, and S. epidermidis) were inhibited between MIC 1280-10240 μg/mL ().
Table 3. Antibacterial activity of methanol extracts of the plants as MIC (μg/mL).
S. leptoclada and O. onites showed the best activity on Gram− bacteria with MIC 640 μg/mL against S. typhimurium and E. aerogenes. O. onites also inhibited S. aureus (MIC 640 μg/mL). Although S. leptoclada didn’t show any significant activity, O. onites exhibited activity (MIC 784 μg/mL) against M. tuberculosis. S. albiflora, with low level rosmarinic acid and carvacrol content, showed particular inhibition against bacteria, except K. penumoniae, B. cereus, and M. tuberculosis.
Plant methanol extracts showed promising results; S. fruticosa displayed the best activity on Gram− bacteria with MIC 640 μg/mL against S. typhimurium and E. aerogenes. The rest of the bacteria exhibited activity between 1280-10240 μg/mL ().
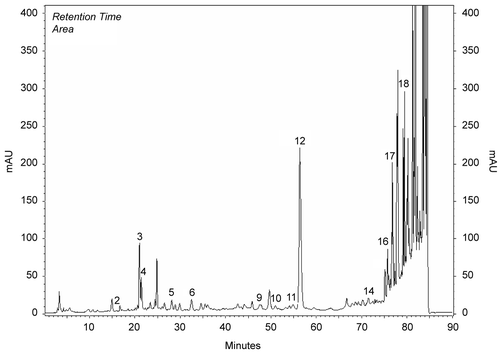
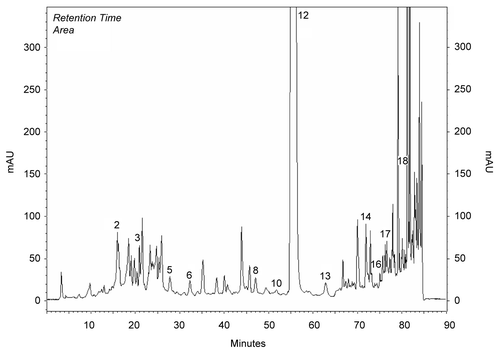
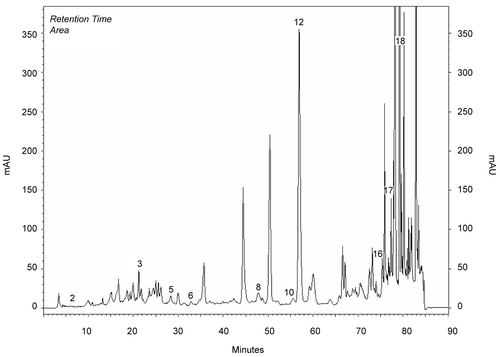
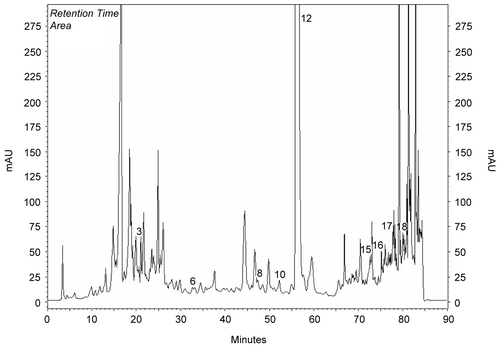
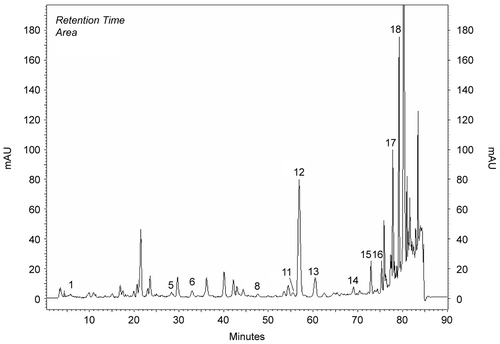
Methanol extracts obtained from the aerial parts of plants were investigated by HPLC analysis. Chemicals in the methanol extracts are given in . Chromatograms of phenolics in methanol extracts compared to chromatograms of standards (). Results were evaluated according to literature. The most prominent phenolic compounds of these plants determined by HPLC analyses were carvacrol and apigenin for O. onites; rosmarinic acid, rutin, and catechin for S. tomentosa; caffeic acid for S. fruticosa. Gallic acid, catechin, epicatechin, rutin, naringin, hesperidin, eridictiol, and quercetin were minor compounds (, ).
Figure 1. Chromatogram of standards: 1) gallic, 2) catechin, 3) caffeic, 4) epicatechin, 5) p-coumaric acid, 6) ferulic acid 7) vitexin, 8) rutin, 9) naringin, 10) hesperidin, 11) apigenin-glucoside, 12) rosmarinic acid, 13) eridictiol, 14) quercetin, 15) naringenin, 16) luteolin, 17) apigenin, 18) carvacrol, 19) acecetin.
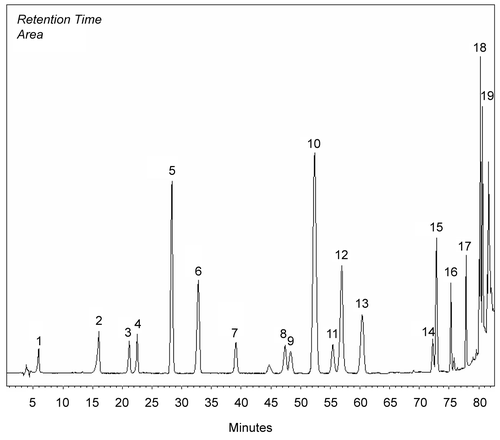
Table 4. Amount of compounds (μg/mL) presented in the methanol extracts of plants.
CitationBotelho et al. (2007) and CitationKunle et al. (2003) reported that carvacrol showed potent antimicrobial activity. CitationSatil et al. (2006b) reported that a solution of S. tomentosa is also used by pouring onto open cuts and is called “Tentürdiyot out” (Iodine herb), “Moşabla” or “Boş yaprak”. Other references about phenolics and their antimicrobial effects are given below. Our results are supported by antibacterial effects, especially on Gram (-) bacteria. S. tomentosa has an inhibitory effect on M. tuberculosis (MIC 196 μg/mL). According to HPLC analyses, we found that the amount of rosmarinic acid was quite high. There is a good correlation between rosmarinic acid and the MIC value. It is our opinion that this activity may come from rosmarinic acid compounds. Therefore, it might be a useful antimicrobial agent, especially on Gram− bacteria and M. tuberculosis for commercial use.
S. fruticosa tea, called “Adaçayı”, or “Elmaçayı”, is commonly used to cure colds and stomachache (CitationKirimer et al., 1999; CitationSezik et al., 2001). It has shown similar effects on Gram− bacteria. Although its content of rosmarinic acid is lower than that of S. tomentosa, we identified a significant amount of carvacrol in the methanol extract. CitationSharififar et al. (2007) and CitationSokmen et al. (2004) showed that carvacrol has antibacterial activity, especially on Gram− bacteria.
While all extracts except O. onites showed efficacy on Gram+ bacteria, O. onites was the only species exhibiting significant activity (MIC 640 μg/mL) against S. epidermidis, but not S. aureus. According to some reports in the literature, luteolin is active against Gram+ bacteria. The luteolin content might be responsible for this activity against S. epidermidis (CitationRamesh et al., 2002; CitationSousa et al., 2006; CitationObied et al., 2007).
As to usage of plants, the genus Salvia L., Sideritis L., and Origanum L., have a wide range of spreading, many species, and economic and medicinal importance in Turkey (CitationDavis, 1982; CitationBaytop, 1984; Baser, 1995; Hamzaoglu, 2005; CitationTopcu et al., 2007). Sideritis L. species, also known by different local names and traditional names, are widely used as herbal tea in Turkey. Sideritis is used as an antispasmodic (CitationEzer et al., 1992), antibacterial (CitationEzer et al., 1994) and as a folk medicine to cure cold (CitationBaser, 1995; CitationKirimer et al., 1999). Origanum, known as “kekik”, is used after distillation to obtain a special medicinal oil (kekik oil) and water (kekik water). It is also sold commercially. It is used for ethnic medicine for colds and respiratory tract infections (CitationBaser, 1995). It is also used as a preservative for dried figs by soaking in boiled kekik water (CitationTumen, 1989).
Therefore, it can be explained why these plants have common usage as a remedy to cure some ailments. These effective phenolics could be responsible for antimicrobial activity. This is the first report describing the antimycobacterial and antibacterial activity of these plants.
Declaration of interest: The authors are grateful to the Scientific and Technological Research Council of Turkey (TUBITAK). This research was supported by a grant from TUBITAK, TBAG (Research grant no.104T336).
References
- Bagci E, Vural M, Dirmenci T, Bruehl L, Aitzetmüller K (2004): Fatty acid and tocochromanol patterns of some Salvia L. species. Zeit Naturforschung 59c: 305–309.
- Baser KHC (1995): Essential oils from aromatic plants which are used as herbal tea in Turkey. in: Baser KHC, ed., Proceedings of the 13th International Congress of Flavours, Fragrances and Essential Oils, 15–19 October 1995, AREP Publications, Istanbul, Vol. 2, pp. 181–199.
- Baser KHC (2002): The Turkish Origanum species, in: Kintzios SE, ed., Oregano, The Genera Origanum and Lippia. London, Taylor and Francis, pp. 109–126.
- Baser KHC, Bondi ML, Bruno M, Krimer N, Piozzi F, Tumen G, Vasallo N (1996): An ent-kaurane from Sideritis huber-morathii. Phytochemistry 43: 1293–1296.
- Baser KHC, Honda, G, Miki, W (1986): Herb Drugs and Herbalists in Turkey. Tokyo, Institute for the Study of Languages and Cultures of Asia and Africa, p. 54.
- Baydar H, Sagdic O, Ozkan G, Karadogan T (2004): Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 15: 169–172.
- Baytop T (1984): Therapy with Medicinal Plants, Past and Present. Istanbul University Publications No. 3255, Istanbul, Istanbul University Press.
- Becton Dickinson (2002): Becton Dickinson and Company Newsletter BD Bactec MGIT 960 SIRE kit now FDA-cleared for susceptibility testing of Mycobacterium tuberculosis. Microbiol News Ideas 13: 4.
- Becton Dickinson (2004): Becton Dickinson and Company BACTEC MIGIT 960 SIRE Kit for the antimycobacterial susceptibility testing of Mycobacterium tuberculosis. Sparks, MD, Becton Dickinson, pp.1–6.
- Botelho M, Nogueira NA, Bastos GM, Fonseca SG, Lemos TL, Matos FJ, Montenegro D, Heukelbach J, Rao VS, Brito GA (2007): Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz J Med Biol Res 40: 349–356.
- Caponio F, Alloggio V, Gomes T (1999): Phenolic compounds of virgin olive oil: Influence of paste preparation techniques. Food Chem 64: 203–204.
- Davis PH (1982): Flora of Turkey and the Aegean Islands, Vol. 7. Edinburgh, Edinburgh University Press, pp. 313–323.
- Duman H, Aytac Z, Ekici M, Karaveliogulları FA, Dönmez A, Duran A (1995): Three new species (Labiatae) from Turkey. Flora Mediterranea 5: 221–228.
- Eidi M, Eidi A, Zamanizadeh H (2005): Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J Ethnopharmacol 100: 310–313.
- Ezer N, Sezik E, Erol K, Özdemir M (1992): The spazmodic activity of some Sideritis species, in: Baser KHC, ed., Proceedings of the 9th Symposium on Plant Drugs, Eskişehir, 16-19 May 1991. Anadolu University Publications No. 641. Eskisehir, Anadolu Universitesi Yayinlari, pp. 88–93.
- Ezer N, Usluer G, Güneş İ, Erol K (1994): Antibacterial activity of some Sideritis species. Fitoterapia 65: 549–551.
- Ezer N, Villa R, Canigueral S, Adzet T (1996): Essential oil composition of four Turkish species of Sideritis. Phytochemistry 41: 203–205.
- Fiore G, Nencini C, Cavallo F, Capasso A, Bader A, Giorgi G, Micheli L (2006): In vitro antiproliferative effect of six Salvia species on human tumor cell lines. Phytother Res 20: 701–703.
- Hamzaoğlu E, Duran A, Pınar MN (2005): Salvia anatolica (Lamiaceae), a new species from East Anatolia, Turkey. Ann Bot Fennici 42: 215–220.
- Kamatou G, Viljoen AM, Gono-Bwalya AB, van Zyl RL, van Vuuren SF, Lourens AC, Baser KHC, Demirci B, Lindsey KL, van Staden J, Steenkamp P (2005): The in vitro pharmacological activities and a chemical investigation of three South African Salvia species. J Ethnopharmacol 102: 382–390.
- Kirimer N, Tabanca N, Özek T, Baser KHC, Tumen G (1999): Composition of essential oils from two endemic Sideritis species of Turkey. Chem Nat Comp 35: 76–80.
- Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC (1997): Diagnostic Microbiology, 5th edn, Philadelphia, Lippincott, pp. 803–841.
- Koo H, Gomes BP, Rosalen PL, Ambrosano GM, Park YK, Cury JA. (2000): In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch Oral Biol 45:141–148.
- Kunle O, Okogun J, Egamana E, Emojevwe E, Shok M (2003): Antimicrobial activity of various extracts and carvacrol from Lippia multiflora leaf extract. Phytomedicine 10: 59–61.
- Lambert R, Skandamis PN, Coote PJ, Nychas GJ (2001): A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol, and carvacrol. J Appl Microbiol 91: 453–462.
- Mehmood S, Riaz N, Nawaz S.A (2006): New butyrylcholinesterase inhibitory triterpenes from Salvia santolinifolia. Arch Pharm Res 3: 195–198.
- NCCLS (2000): Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th edn. Approved standard. NCCLS publication no. M7-A5. Wayne, PA, National Committee for Clinical Laboratory Standards.
- Obied HK, Bedgood DR, Prenzler PD, Robards K (2007): Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem Toxicol 45: 1238–1248.
- Palaci M, Ueki SY, Sato DN, Da Silva Telles MA, Curcio M, Silva EA (1996): Evaluation of Mycobacteria growth indicator tube for recovery and drug susceptibility testing of Mycobacterium tuberculosis isolates from respiratory specimens. J Clin Microbiol 34: 762–764.
- Pedreros S, Rodriguez B, de la Torre MC, Bruno M, Savona G, Perales A, Torres MR (1990): Dammarane triterpenes of Salvia hierosolymitana: Structure and absolute stereochemistry of salvilymitone and salvilymitol. Phytochemistry 29: 919–922.
- Ramesh N, Viswanathan MB, Saraswathy A, Balakrishna K, Brindha P, Lakshmanaperumalsamy P (2002): Phytochemical and antimicrobial studies of Begonia malabarica. J Ethnopharmacol 79: 129–132.
- Reisner BS, Gatson AM, Woods GL (1995): Evaluation of Mycobacteria growth indicator tubes for susceptibility testing of Mycobacterium tuberculosis to isoniazid and rifampin. Diagn Microbiol Infect Dis 22: 325–329.
- Satil F, Dirmenci T, Tumen G (2006a): The trade of wild plants that are named as thyme (kekik) collected from Kazdagi, in: Ertug, ZF, ed., Proceedings of the IVth International Congress of Ethnobotany (ICEB 2005), Yeditepe University, Ege Publications, Istanbul, pp. 201–204.
- Satil F, Tümen G, Dirmenci T, Çelik A, Arı Y, Malyer H (2006b): Study on inventory of ethnobotany in Kazdaği National Park and vicinity. TüBA J Cultural Inventory 5: 171–203.
- Seshadri TR (1962): Isolation of flavonoid compounds from plant materials, in: Geissman TA, ed., The Chemistry of Flavonoid Compounds. Oxford, Pergamon, pp. 184–186.
- Sezik E, Ezer N (1983): Morphological and anatomical investigation on the plants used as folk medicine and herbal tea in Turkey I, Sideritis Congesta Davis et Huber-Morath. J Doga Billim 7: 163–168.
- Sezik E, Yeşilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T (2001): Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J Ethnopharmacol 75: 95–115.
- Sharififar F, Moshafi MH, Mansouri SH, Khodashenas M, Khoshnoodi M (2007): In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food Control 18: 800–805.
- Sokmen A, Gulluce M, Akpulat HA, Tepe, B, Sokmen M, Sahin, F (2004): The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 15: 627–634.
- Sokovic M, Tzakou O, Pitarokili D, Couladis M, (2002): Antifungal activities of selected aromatic plants growing wild in Greece. Die Nahrung 5: 317–320.
- Sousa A, Ferreira ICFR, Calhelha R, Andrade PB, Valentao P, Seabra R, Estevinho L, Bento A, Pereira JA (2006): Phenolics and antimicrobial activity of traditional stoned table olives “alcaparra”. Bioorganic Med Chem 14: 8533–8538
- Tabanca N, Demirci B, Baser KHC, Aytac Z, Ekici, M (2006): The chemical composition and antifungal activity of Salvia macrochlamys and Salvia recognita essential oils. J Agric Food Chem 18: 6593–6597.
- Tabanca N, Kirimer N, Baser KHC (2001): The composition of essential oils from two varieties of Sideritis erythrantha var. erythrantha and var. cedretorum. Turk J Chem 25: 201–208.
- Tepe B (2007): Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia virgata (Jacq), Salvia staminea (Montbret & Aucher ex Bentham) and Salvia verbenaca (L.) from Turkey. Bioresour Technol 99: 1584–1588.
- Tepe B, Donmez E, Unlu M, Candan F, Daferera D, Vardar-Unlu G, Polissiou M, Sokmen A (2004): Antimicrobial and antioxidative activity of the essential oils and methanol extracts of Salvia cryptantha (Montbret et Aucher ex Benth.) and Salvia multicaulis (Vahl). Food Chem 84: 519–525.
- Topcu G, Ertas A, Kolak U, öztürk M, Ulubelen A (2007): Antioxidant activity tests on novel triterpenoids from Salvia macrochlamys. Arkivoc 7: 195–208.
- Topcu G, Goren AC, Kiliç T, Yildiz YK, Tumen G (2002): Diterpenes from Sideritis trojana. Nat Prod Lett 16: 33–37.
- Topcu G, Goren AG, Yildiz YK, Tumen G (1999): Ent-Kaurene diterpenes from Sideritis athoa. Nat Prod Lett 14:123–129.
- Tumen G (1989): Labiatae family as medicinal plants from Balikesir district in Turkey. Uludag Univ J Fac Educ 4: 7–12.
- Tumen G, Baser KHC, Kirimer N (1995): The essential oils of Turkish Origanum species: A treatise, in: Baser KHC, ed., Proceedings of the 13th International Congress of Flavours, Fragrances and Essential Oils, 15-19 October 1995, AREP Publications, Istanbul, Vol. 2, p. 200.
- Ulubelen A, Birman H, Oksuz S (2002): Cardioactive diterpenes from the roots of Salvia eriophora. Planta Med 9: 818–821.
- Ulubelen A, Oksuz S, Topcu G, Goren AC, Voelter W (2001): Antibacterial diterpenes from the roots of Salvia blepharochlaena. J Nat Prod 4: 549–551.
- Ulubelen A, Topcu G (1984): Flavonoids and terpenoids from Salvia verticillata and Salvia pinnata. J Nat Prod 6: 1068–1068.
- Ulubelen A, Topcu G, Johansson C.B (1997): Norditerpenoids and diterpenoids from Salvia multicaulis with antituberculous activity. J Nat Prod 60: 1275–1280.
- Walters SB, Hanna BA (1996): Testing of susceptibility of Mycobacterium tuberculosis to isoniazid and rifampin by Mycobacterium growth indicator tube method. J Clin Microbiol 34: 1565–1567.