Abstract
The anthelmintic activity of the ethanol extract of Acacia oxyphylla Graham ex Bentham (Mimosaceae) stem bark was tested against Ascaridia galli Schrank (Nematoda), the intestinal roundworm of domestic fowl. Different concentrations of the extract (0.5, 1, 2, 5, 10 and 20 mg/mL) were prepared in 0.9% phosphate buffered saline (PBS) with 1% dimethylsulfoxide (DMSO). In vitro treatment of the adult roundworms indicated concentration-dependent efficacy of the plant extract. Similar concentrations of a broad-spectrum antiparasitic drug, albendazole, were used as a standard reference. Control experiments consisted of nematodes maintained in 0.9% PBS with 1% DMSO. Albendazole was significantly effective (P < 0.05) at all concentrations tested in causing mortality of A. galli. However, the plant extract showed concentration-dependent efficacy only at the concentrations of 2, 5, 10, and 20 mg/mL. In order to ascertain the anthelmintic effect, scanning electron microscopy was performed, which indicated devastating structural alterations on the fine topography of A. galli treated with 20 mg/mL of the plant extract, when compared with that of the control specimen. Severe shrinkage of the cuticle, loosening and collapse of the lips, and extensive irregular wrinkles all over the body surface were very distinct on the plant extract-treated nematode. Moreover, high magnification of the cuticle revealed formation of a number of small swellings or blebs, which apparently marked the initiation of disintegration of the entire cuticle.
Introduction
Nematodes constitute the most important gastrointestinal parasites of livestock animals and undoubtedly remain the major factor that compromises successful production resulting in heavy economic losses in animal-based industries. Although there have been remarkable achievements in the discovery and improvement of pharmaceutical anthelmintics, diseases due to nematode infections continue to be the greatest limiting factor in sustainable livestock production worldwide, primarily due to rapid evolution of drug resistance in these parasites to all classes of anthelmintics (CitationStear et al., 2007). In addition, global appreciation and general endorsement of organic farming pose serious restriction to the prophylactic use of synthetic drugs (CitationWaller, 2003).
Ascaridia galli Shrank (Nematoda) is a roundworm parasitizing the small intestine of birds (CitationMcDougald, 2003), and is by far the most prevalent of all helminths infecting poultry (CitationPermin et al., 1999). A. galli infections continue to be the most debilitating factor impeding poultry productivity, resulting in retarded growth, weight loss, diarrhea, poor absorption of nutrients, death and even the spread of fatal bacterial infections (CitationChadfield et al., 2001; CitationGauly et al., 2007).
Despite successful evaluations of a large number of traditionally used medicinal plants for anthelmintic activity against different helminth parasites, the global crisis of helminthic infestation is far from being ameliorated (CitationGithiori et al., 2006). This is primarily due to the fact that although medicinal plants exhibit anthelmintic properties, their chemical nature, safety and, above all, mode of action remain poorly understood. Recent evidence has posited that even certain medicinal plants and their products are highly toxic to the host, and without any appreciable value in clinical and veterinary applications (CitationGithiori et al., 2003; Botros et al., 2004; CitationHansson et al., 2005).
Acacia oxyphylla Graham ex Bentham (synonyms: A. caesia Linnaeus, A. intsia Willdenow) is a leguminous perennial climbing shrub belonging to the family Mimosaceae, and is native to south-east Asian countries. The Mizo tribes of north-east India use the juicy extract from the stem bark as a fish stupefying agent and as a remedy for gastrointestinal infections. In view of its traditional usage we had demonstrated that the crude ethanolic extract of the stem bark indeed exerted significant mortality effects associated with structural damage on the tapeworm, Raillietina echinobothrida Megnin (CitationRoy et al., 2007), and inhibition of vital tegumental enzymes (CitationLalchhandama et al., 2007). The present work, therefore, is an attempt to further explore the possible broad-spectrum anthelmintic activity, if any, on the common poultry roundworm, A. galli.
Materials and methods
Plant material and preparation of the extract
The fresh stems of Acacia oxyphylla were collected from the nearby forest of Aizawl, the capital city of Mizoram, India, in July 2005. The specimens were identified by H.S. Thapa, plant taxonomist at the Department of Botany, Pachhunga University College, Mizoram University, India, and a voucher specimen (PUC-BOT-A 012) is maintained. The stem bark was peeled off, thoroughly washed with deionized water, cut into small pieces, and dried in a hot air oven at 50°C. The dried parts were crushed to fine powder and then refluxed with ethanol (100 g/L) for 8 h at 60°C, as described earlier (CitationRoy et al., 2007). The solution obtained was filtered through Whatman filter paper (No. 1) and then desiccated to complete dryness at 50°C. The ethanol extract was obtained as a deep brown powdered material, which was then refrigerated at 4°C until further use. The net yield from such extraction was 4.4%. One hour prior to experimental assay, varying concentrations of the extract, 0.5, 1, 2, 5, 10, and 20 mg/mL, were prepared by dissolving them in 0.9% phosphate buffered saline (PBS, pH 7-7.3), supplemented with 1% dimethylsulfoxide (DMSO).
Chemicals and drug
All the chemicals used were of standard analytical grades, obtained either from Merck or SD Fine-Chemicals, Mumbai, India, except where otherwise stated. Ethanol was supplied by Bengal Chemicals, Kolkata, India, and the reference drug albendazole is a product of GlaxoSmithKline Pharmaceutical, India.
Recovery and in vitro treatments of parasites
Live local fowls (Gallus domesticus Linnaeus) were obtained from the local abattoir in Aizawl, Mizoram, India. They were sacrificed and on immediate necropsy, live adult roundworms, A. galli, were recovered from the small intestines. All the experiments were carried out in accordance with the guidelines laid down by the Institutional Animal Care and Use Committee. The worms were collected in 0.9% PBS and then kept at 37° ± 1°C in an automated glass-chambered incubator. The fresh worms were directly introduced to the different concentrations (0.5, 1, 2, 5, 10, and 20 mg/mL) of the plant extract in separate Petri dishes maintained at 37° ± 1°C. Similar treatment was performed for varying doses of albendazole, prepared by serial dilution of the prescribed concentration (20 mg/mL). The control experiment consisted of nematodes maintained in a medium containing only PBS with 1% DMSO. Each incubation medium consisted of 5 replicates. Vigilant observations were made on the physical activity of the nematodes and time taken to attain death was recorded following the method of CitationTandon et al. (1997). Death was substantiated when complete immobility was noted upon dipping the parasites in tepid PBS (~45°C) that induced movement in sentient worms. Data were presented as means plus or minus the standard error (SE) of the mean. Statistical analyses were performed using Biostat 2007, a product of AnalystSoft, Vancouver, Canada. Comparison of the mean values of the experimental treatments against those of the control groups was made using unpaired Student’s t-test, and the level of probability considered significant when P < 0.05.
Scanning electron microscopy
A set of worms from each of the plant extract-treated and control media was thoroughly washed in PBS and then fixed in 4% or 10% neutral phosphate buffered formaldehyde at 4°C at least for 24 h. After post fixation in 1% buffered osmium tetraoxide for 1 h, the worms were washed again with PBS. Subsequent dehydration was carried out through ascending concentration of acetone up to pure acetone. Following the standardized method of CitationRoy and Tandon (1991) specific for helminth parasites, the specimens were treated with tetramethylsilane for 10 min and then allowed to dry at room temperature (25ºC). The dried specimens were placed on metal stubs and sputter-coated with gold in a fine-coat ion sputter, JFC-1100 (JEOL), and finally observed under scanning electron microscope (LEO 435 VP) at an electron accelerating voltage of 20 kV.
Results
A. galli in the control group did not show any sign of loss of physical activity, and survived sturdily for 84.83 h ± 0.89 h in the medium composed of 0.9% PBS with 1% DMSO at 37° ± 1°C. The nematodes were persistently active, but once their movement ceased, death ensued abruptly.
presents the response in physical activity of A. galli upon treatment with albendazole. Albendazole was found to be a highly effective nematocide exhibiting profound dose-dependent activity at all concentrations tested. Significant mortality was observed at 55.17 h ± 1.04 h in the lowest concentration (0. 5 mg/ mL), and at 5.31h ± 0.77 h in the highest concentration (0. 5 mg/mL).
Table 1. Concentration-dependent efficacy of albendazole and the ethanol extract of A. oxyphylla stem bark on the survival of A. galli.
The extract of A. oxyphylla stem bark also indicated concentration-dependent efficacy on the nematode. However, at lower concentrations, i.e., 0.5 and 1 mg/mL, of the plant extract, the nematodes did not show any significant mortality with respect to the control. At concentrations of 2, 5, 10 and 20 mg/mL, the extract indicated significant nematocidal efficacy at 79.84 ± 0.94 h, 62.58 ± 0.81 h, 44.45 ± 0.92 h and 32.78 ± 1.14 h, respectively.
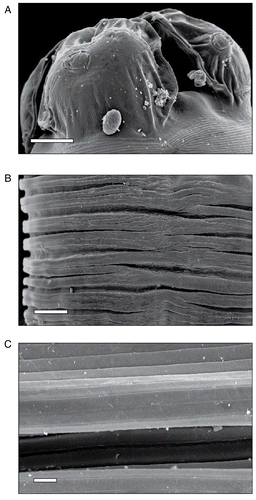
Scanning electron microscopy of the untreated nematode revealed a prominent triangular mouth at the extreme anterior end, which is surrounded by three conspicuous denticulate lips having smooth cuticle and appeared anchored to one another. Prominent cuticular protuberances named labial papillae are situated on the lips, one on each of the latero-ventral lips and two on the dorsal lip; these papillae are sensory in function (). The surface fine topography of the body showed a smooth cuticle characterized by a series of continuous transverse annulations with distinct striations extending from the cephalic region to the posterior end of the body (). The striations were of fine transverse grooves in the form of parallel concentric rings running completely around the cylindrical body. Annulations were deep transverse grooves occurring at regular intervals giving the body a segmented appearance ().
Figure 1. Scanning electron micrographs of untreated control nematode A. galli. A) Anterior end showing three denticulate lips surrounding a central mouth, a latero-ventral lip facing at the center of which is a sensory papilla, × 200 (scale bar, 100 μm). B) The cuticle with distinct ridges and furrows throughout the body, × 270 (scale bar, 200 μm). C) Enlarged portion showing a series of lighter transverse striations and intercalated darker annulations, × 1,000 (scale bar, 50 μm).
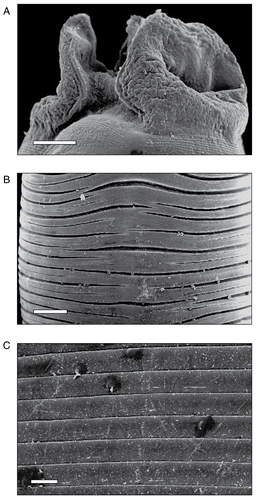
A. galli treated with 20 mg/mL of the ethanol extract of A. oxyphylla stem bark indicated pronounced deformities on the fine topographical structure. At the anterior end, all the three lips showed signs of loosening and collapse, with conspicuous irregular wrinkles all over the surface. The labial papillae were lost from view due to severe shrinkage of the lip. On the general body surface, the cuticle showed considerable disorganization and aberration in the otherwise regular striations (). At higher magnification of the cuticle, the annular rings displayed minute dark spots in the form of blebs all over the surface (). It is obvious that blebbings could result in focal erosion of the cuticle as evidently shown by a ruptured bleb.
Figure 2. Scanning electron micrographs of A. galli treated with 20 mg/mL of the ethanol extract of A. oxyphylla stem bark. A) Anterior end with severe deformity, lips collapsed, papillae destroyed and shrunk, and the cuticle wrinkled, × 200 (scale bar, 100 μm). B) Cuticular surface showing irregular rings bearing a number of unusual dark spots, × 270 (scale bar, 200 μm). C) Transverse rings developing external blebs as conspicuous dark blisters, and a ruptured bleb at the left bottom, × 1,000 (scale bar, 50 μm).
Discussion
A primary procedure often employed for evaluating anthelmintic efficacy is direct observation on the viability of the helminthic worms upon exposure to the anthelmintic agent. Following this basic technique, several medicinal plants such as Albizzia lebbek Bentham (Mimosaceae), Allium sativum Linnaeus (Liliaceae), A. santonica Linnaeus (Liliaceae), Cardiospermum halicacabum Linnaeus (Sapindaceae), Coriandrum sativum Linnaeus, Cucurbita mexicana Damm (Cucurbitaceae), Neurolaena lobata Linnaeus (Asteraceae), Perilla frutescens Linnaeus (Lamiaceae), Spigelia anthelmia Linnaeus (Loganiaceae), Spondias mombin Linnaeus (Anacardiaceae), Polyalthia suaveolens Engler & Diels (Annonaceae), Vernonia amygdalina Linnaeus (Asteraceae), and Zingiber officinale Roscoe (Zingiberaceae) have already been documented to show anthelmintic activity against different nematodes of veterinary importance (CitationAkhtar et al., 2000; CitationEl Garhy & Mahmoud, 2002; CitationIqbal et al., 2003; CitationBoonmars et al., 2005; CitationFujimaki et al., 2005; CitationAdemola et al., 2005, Citation2007; CitationEguale et al., 2007; CitationAdedapo et al., 2007). A closely related species of Acacia used in the present study, A. auriculiformis Cunningham extract, reportedly caused significant activity against the nematode Dirofilaria immitis Leidy of dogs (CitationChakraborty et al., 1995), the bovine nematode Setaria cervi Kunth (CitationGhosh et al., 1993) and a cestode Hymenolepis diminuta Sturdevant (CitationGhosh et al., 1996). Feeding of A. karoo Hayne (Leguminosae) leaves also caused significant decrease in the fecal egg count of the nematode Haemonchus contortus Rudolphi in goats (CitationKahiya et al., 2003).
A number of plants, including Bassia latifolia Roxburgh (Sapotaceae) (CitationAsadullah & Sabir, 1980), Melia azedarach Linnaeus (Meliaceae) (CitationAkhtar & Riffat, 1985), Caesalpinia crista Linnaeus (Leguminosae) (CitationJaved et al., 1994), Piliostigma thonningii Schum (Leguminosae) (CitationAsuzu & Onu, 1994), Embelia ribes Burman (Myrsinaceae) (CitationDama & Kirdak, 2002), Cleome viscosa Linnaeus (Capparaceae) (CitationMali et al., 2007a), Mimusop elengi Linnaeus (Sapotaceae) (CitationMali et al., 2007b), Carica papaya Linnaeus (Caricaceae) (CitationSingh & Nagaich, 1999) and Ocimum sanctum Linnaeus (Lamiaceae) (CitationSingh & Nagaich, 2002), were demonstrated to have efficacy against A. galli. The present investigation also evidently showed that extract of A. oxyphylla stem bark is an effective anthelmintic agent upon A. galli, comparable to the efficacy of M. elengi and C. papaya, but less effective than other reported plants. CitationTandon et al. (1997) had also reported similar observations using the root tuber extract of Flemingia vestita Bentham & Hooker (Fabaceae) on different nematodes such as Ascaris suum Goeze, A. lumbricoides Linnaeus, A. galli and Heterakis gallinarum Schrank.
The cuticle of nematodes is metabolically active and morphologically specialized to perform selective absorption of nutrients, secretion of glycoproteins for immuno-protection, osmoregulation and (insofar as it supports sense organs) sensory reception. Consequently, passive diffusion through the cuticle is the principal mechanism by which anthelmintic compounds enter the nematode body (CitationAlvarez et al., 2007). Apparently, it has been firmly documented that one of the hallmark effects of any anthelmintic is the direct destruction of the worm’s surface (CitationTippawangkosol et al., 2004; CitationSchmahl et al., 2007).
Albendazole reportedly caused structural damage on the cuticle of Trichinella spiralis Owen upon in vivo treatment (CitationHrćkova et al., 1993). Surface distortion and loss of regular cuticular annulations were also observed on Brugia malayi Brug (CitationTippawangkosol et al., 2004). Adult Wuchereria bancrofti Wucherer & Bancroft subjected to albendazole and diethylcarbamazine combination therapy exhibited swollen cuticle, formation of spherical and spike-like projections at the anterior region, and leaf-like expansion on the general cuticle (CitationOliveira-Menezes et al., 2007).
Angiostrongylus cantonensis Chen also developed severe shrinkage and formation of rounded leaf-like expansions on the cuticle throughout the body after in vivo treatment with imidacloprid and moxidectin combination (CitationSchmahl et al., 2007). Cysteine proteinases isolated from different fruits were shown to cause wrinkles and folds of the cuticle, often followed by blistering and gradual digestion of the cuticle on different nematodes (CitationStepek et al., 2005, Citation2006, Citation2007). Thus, structural alterations observed on A. galli cuticle in the present study were definitely due to the anthelmintic activity of the extract of A. oxyphylla stem bark.
Declaration of interest: The authors are grateful to the University Grant Commission, New Delhi, India, for financial assistance.
References
- Adedapo AA, Otesile AT, Soetan KO (2007): Assessment of the anthelmintic efficacy of an aqueous crude extract of Vernonia amygdalina. Pharm Biol 45: 564–568.
- Ademola IO, Akanbi AI, Idowu SO (2005): Comparative nematocidal activity of chromatographic fractions of Leucaena leucocephala seed against gastrointestinal sheep nematodes. Pharm Biol 43: 599–604.
- Ademola IO, Fagbemi BO, Idowu SO (2007): Anthelmintic activity of Spigelia anthelmia extract against gastrointestinal nematodes of sheep. Parasitol Res 101: 63–69.
- Akhtar MS, Riffat S (1985): Evaluation of Melia azedarach Linn. seeds (Bahain) and piperazine against Ascaridia galli infection in chickens. Pakistan Vet J 5: 34–37.
- Akhtar MS, Iqbal Z, Khan MN, Lateef M (2000): Anthelmintic activity of medicinal plants with particular reference to their use in animals in the Indo-Pakistan subcontinent. Small Rumin Res 38: 99–107.
- Alvarez LI, Mottier ML, Lanusse CE (2007): Drug transfer into target helminth parasites. Trends Parasitol 23: 97–104.
- Asadullah M, Sabir M (1980): In vitro anthelmintic action of Bussia latifolia against Ascaridia galli worms. Vet Res J 3: 131–133.
- Asuzu IU, Onu UO (1994): Anthelmintic activity of the ethanolic extract of Piliostigma thonningii bark in Ascaridia galli infected chickens. Fitoterapia 65: 291–297.
- Boonmars T, Khunkitti W, Sithithaworn P, Fujimaki Y (2005): In vitro antiparasitic activity of extracts of Cardiospermum halicacabum against third-stage larvae of Strongyloides stercoralis. Parasitol Res 97: 417–419.
- Botros S, William S, Ebeid F, Cioli D, Katz N, Day TA, Bennette JL (2004): Lack of evidence for an antischistosomal activity of myrrh in experimental animals. Am J Trop Med Hyg 71: 206–210.
- Chadfield M, Permin A, Nansen P, Bisgaard M (2001): Investigation of the parasitic nematode Ascaridia galli (Shrank 1788) as a potential vector for Salmonella enterica dissemination in poultry. Parasitol Res 87: 317–325.
- Chakraborty T, Sinha Babu SP, Sukul NC (1995): Antifilarial effect of a plant Acacia auriculiformis on canine dirofilariasis. Trop Med 37: 35–37.
- Dama LB, Kirdak RV (2002): Effect of vidhang seed (Embelia ribes L.) extract against Ascaridia galli in naturally infected fowls (Gallus domesticus). J Parasit Dis 26: 48–50.
- Eguale T, Tilahun G, Debella A, Feleke A, Makonnen E (2007): In vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J Ethnopharmacol 110: 428–433.
- El Garhy MF, Mahmoud LH (2002): Anthelminthic efficacy of traditional herbs on Ascaris lumbricoides. J Egyptian Soc Parasitol 32: 893–900.
- Fujimaki Y, Kamachi T, Yanagi T, Cáceres A, Maki J, Aoki Y (2005): Macrofilaricidal and microfilaricidal effects of Neurolaena lobata, a Guatemalan medicinal plant, on Brugia pahangi. J Helminthol 79: 23–28.
- Gauly M, Duss C, Erhardt G (2007): Influence of Ascaridia galli infections and anthelmintic treatments on the behaviour and social ranks of laying hens (Gallus gallus domesticus). Vet Parasitol 146:280.
- Ghosh NK, Sinhababu SP, Sukul NC (1993): Antifilarial effect of two triterpenoid saponins from Acacia auriculiformis. Indian J Exp Biol 31: 604–606.
- Ghosh NK, Babu SP, Sukul NC, Ito A (1996): Cestocidal activity of Acacia auriculiformis. J Helminthol 70: 171–172.
- Githiori JB, Höglund J, Waller PJ, Baker RL (2003): Evaluation of anthelmintic properties of some plants used as livestock dewormers against Haemonchus contortus infections in sheep. Parasitology 129: 245–253.
- Githiori JB, Athanasiadou S, Thamsborg SM (2006): Use of plants in novel approaches for control of gastrointestinal helminths in livestock with emphasis on small ruminants. Vet Parasitol 139: 308–320.
- Hansson A, Zelada JC, Noriega HP (2005): Reevaluation of risks with the use of Ficus insipida latex as a traditional anthelmintic remedy in the Amazon. J Ethnopharmacol 98: 251–257.
- Hrćkova G, Velebný S, Horak J (1993): A morphological study of the effects of liposomized albendazole on the muscle phase of Trichinella spiralis in mice. J Helminthol 67: 24–30.
- Iqbal Z, Akhtar MS, Sindhu ZUD, Khan MN, Jabbar A (2003): Herbal dewormers in livestock – A traditional therapy. Int J Agric Biol 5: 199–206.
- Javed I, Akhtar MS, Rahman ZU, Khaliq T, Ahmad M (1994): Comparative anthelminthic efficacy and safety of Caesalpinia crista seed and piperazine adipate in chickens with artificially induced Ascaridia galli infection. Acta Vet Hung 42: 103–109.
- Kahiya C, Mukaratirwa S, Thamsborg SM (2003): Effects of Acacia nilotica and Acacia karoo diets on Haemonchus contortus infection in goats. Vet Parasitol 115: 265–274.
- Lalchhandama K, Roy B, Dutta BK (2007): In vitro anthelmintic activity of Acacia oxyphylla: Changes in the levels of trace elements and activities of the tegumental enzymes of the cestode, Raillietina echinobothrida. Pharmacologyonline 2: 307–317.
- Mali RG, Mahajan SG, Mehta AA (2007a). In vitro screening of Cleome viscosa extract for anthelmintic activity. Pharm Biol 45: 766–768.
- Mali RG, Mahajan SG, Mehta AA (2007b). In-vitro anthelmintic activity of stem bark of Mimusops elengi Linn. Phcog Mag 3:73–76.
- McDougald LR (2003). Internal Parasites, in: Saif YM, ed., Diseases of Poultry, 11th edn. Ames, Iowa, Iowa State University Press, pp. 931–972.
- Oliveira-Menezes A, Lins R, Norões J, Dreyer G, Lanfredi RM (2007): Comparative analysis of a chemotherapy effect on the cuticular surface of Wuchereria bancrofti adult worms in vivo. Parasitol Res 101: 1311–1317.
- Permin A, Bisgaard M, Frandsen F, Pearman M, Kold J, Nansen P (1999): The prevalence of gastrointestinal helminths in different poultry production systems. Brit Poult Sci 40: 439–443.
- Roy B, Tandon V (1991): Usefulness of tetramethylsilane in the preparation of helminth parasites for scanning electron microscopy. Riv Parassitol 8: 207–215.
- Roy B, Lalchhandama K, Dutta BK (2007): Anticestodal efficacy of Acacia oxyphylla on Raillietina echinobothrida: A light and electron microscopic studies. Pharmacologyonline 1: 279–287.
- Schmahl G, Mehlhorn H, Harder A, Klimpel S, Krieger KJ (2007): Efficacy of a combination of imidacloprid plus moxidectin against larval and adult stages of nematodes (Trichuris muris, Angiostrongylus cantonensis) in rodents. Parasitol Res 101: S85–S92.
- Singh K, Nagaich S (1999): Efficacy of aqueous seed extract of Carica papaya against common poultry worms Ascaridia galli and Heterakis gallinae. J Parasit Dis 23: 113–116.
- Singh K, Nagaich S (2002): Anthelmintic efficacy of the alcoholic extract of Ocimum sanctum against common poultry worms Ascaridia galli and Heterakis gallinae. J Parasit Dis 26: 42–45.
- Stear MJ, Doligalska M, Donskow-Schmelter K (2007): Alternatives to anthelmintics for the control of nematodes in livestock. Parasitology 134: 139–151
- Stepek G, Buttle DJ, Duce IR, Lowe AE, Behnke JM (2005): Assessment of the anthelmintic effect of natural plant cysteine proteinases against the gastrointestinal nematode, Heligmosomoides polygyrus, in vitro. Parasitology 130: 1–9.
- Stepek G, Lowe AE, Buttle DJ, Duce IR, Behnke JM (2006): In vitro and in vivo anthelmintic efficacy of plant cysteine proteinases against the rodent gastrointestinal nematode, Trichuris muris. Parasitology 132: 681–689.
- Stepek G, Lowe AE, Buttle DJ, Duce IR, Behnke JM (2007): Anthelmintic action of plant cysteine proteinases against the rodent stomach nematode, Protospirura muricola, in vitro and in vivo. Parasitology 134: 103–112.
- Tandon V, Pal P, Roy B, Rao HSP, Reddy KS (1997): In vitro anthelmintic activity of root-tuber extract of Flemingia vestita, an indigenous plant in Shillong, India. Parasitol Res 83: 492–498.
- Tippawangkosol P, Choochote W, Na-Bangchang K, Jitpakdi A, Pitasawat B, Riyong D (2004): The in vitro effect of albendazole, ivermectin, diethylcarbamazine, and their combinations against infective third-stage larvae of nocturnally subperiodic Brugia malayi (Narathiwat strain): Scanning electron microscopy. J Vector Ecol 29: 101–108.
- Waller PJ (2003): The future of anthelmintics in sustainable parasite control programs for livestock. Helminthologia 40: 97–102.
