Abstract
Extracts from five Tibetan medicinal plants collected from the Tibetan Plateau were evaluated for antiviral activity against herpes simplex virus type 2 (HSV-2) in vitro and in vivo. Viral plaque reduction assays showed that extracts from four out of five plants inhibited HSV-2 infection significantly with 50% effective concentrations (EC50) values ranging from 0.35 ± 0.11 to 1.83 ± 0.21 mg/mL. The other plant, Swertia mussotii Franch. (Gentianaceae), exhibited activity in inhibiting the viral biosynthesis. In the attachment assay, two plants, Dracocephalum heterophyllum Benth. (Lamiaceae) and Dracocephalum tanguticum Maxim. (Lamiaceae) reduced the attachment of HSV-2 to cell surface. Interestingly, all of the extracts showed virucidal activity. Analyzed by real-time PCR, three extracts showed strong inhibition of HSV DNA replication with Dracocephalum heterophyllum and Dracocephalum tanguticum at the concentration of 4 mg/mL and Lagotis brevituba Maxim. (Scrophulariaceae) at 1 mg/mL. BALB/c mice were used for determining in vivo efficacy. Mice encephalitis herpes models were established by infection with HSV-2. The extracts of Dracocephalum heterophyllum, Dracocephalum tanguticum, and Swertia mussotii at a dose of 1 g/kg per day significantly prolonged the mean survival times and reduced the mortality of HSV-2 infected mice compared with control group (P < 0.05). Taken together, we conclude that the antiviral mechanisms of these plants involve various stages of virus replication. Extracts from three of these plants, Dracocephalum heterophyllum, Dracocephalum tanguticum, and Swertia mussotii, may be possible candidates in developing anti-HSV-2 medicine.
Introduction
In many developing countries, traditional medicine is still the mainstay of healthcare, and most of the drugs and cures used come from plants. Traditional medicines have been employed for use in pharmaceutical products as new and effective remedies, or for use in treating many common and some uncommon conditions. In China, traditional herbal medicine has been frequently used in conjunction with conventional medicine to treat diseases caused by viruses. The anti-HSV activities of some Chinese medicinal plant extracts have been reported (CitationKuo et al., 2001; CitationYang et al., 2005; CitationChiang et al., 2002).
As people increasingly seek alternatives to modern medicine, interest is growing in the ancient system of Tibetan medicine, which has been practiced for over 2,500 years. With the recent fascination in Tibet and Tibetan culture, Tibetan medicine is receiving greater attention from public, scholars, and the media. Dracocephalum heterophyllum Benth. (Lamiaceae), Dracocephalum tanguticum Maxim. (Lamiaceae), Aconitum tanguticum Maxim. (Ranunculaceae), Swertia mussotii Franch. (Gentianaceae), and Lagotis brevituba Maxim. (Scrophulariaceae), are all endemic in the Tibetan Plateau, and distributed mainly in Sitsang, Qinghai, Gansu of China. D. heterophyllum grows in dry and rocky mountain slopes with altitude 1,100-5,000 m, D. tanguticum grows in grasslands and thickets at an altitude of 3,200-4,700 m, while A. tanguticum, S. mussotii and L. brevituba grow in grasslands at altitudes of 3,200-4,800, 2,600-4,000, and 3,000-4,500 m, respectively (CitationAnon, 1996). These medicinal plants are very important in Tibetan folk medicine. The aerial parts of D. heterophyllum, D. tanguticum, A. tanguticum, L. brevituba, and S. mussotii are widely used and well known as Ji-Mei-Qing-Bao, Zhi-Yang-Gu, Bang-Ga, Hong-Lian, Zang-Yin-Chen, respectively, in Tibetan medicine. Furthermore, it is traditionally believed that they can reduce heat, detoxify poison in the body, and calm the liver. D. heterophyllum is habitually used as the therapy for jaundice, hepatitis, gum gall, bleeding, lymphangitis, cough, and oral cavity ulcer. D. tanguticum is widely used for the treatment of liver and stomach diseases, edema, eczema, fester to inflammatory skin disease, arthritis, and hemostasia. A. tanguticum is a rare species for the treatment of fever, pneumonia, inflammation, flu, as well as alimentary toxicosis. S. mussotii is reputed in Tibetan medicine as a good source of medicine for the treatment of jaundice and viral hepatitis. L. brevituba is of value as a remedy for nephritis, pulmonary disease, arteriosclerosis, hypertension, and emmeniopathy. These herbs are also main constituents in many ancient Tibetan herbal formulas (CitationAnon, 1996, Citation1991; CitationLuo, 1997). While the benefits of these herbal remedies are appreciated within communities, most of them lack documented evidence of therapeutic effectiveness.
Herpes simplex virus type 2 (HSV-2) is a member of the alphaherpesvirus subfamily called the Herpesviridae. It is extremely widespread in the human population. HSV is responsible for a broad range of diseases, ranging from gingivostomatitis to keratoconjunctivitis, genital disease, encephalitis, and also infects newborn and immunocompromised patients (CitationWhitley et al., 1998). Genital herpes infection is one of the world’s most prevalent sexually transmitted diseases (STDs). It is typically the outcome of infection by HSV-2. It is estimated that several hundred million individuals worldwide are infected with STDs including HSV, human immunodeficiency virus (HIV) and human papillomaviruses (HPV). The development of safe topical microbicides is of great importance in the field of STD prevention (CitationMcGowan, 2006). HSV-2 is also known as an oncogenic virus, which has the ability to transform normal cells into tumor cells (CitationLapucci et al., 1993). Moreover, HSV infections were reported to be a risk factor for human immunodeficiency virus (HIV) infection (CitationHook et al., 1992). In a study to examine the in vivo relationships between HSV-2 seroprevalence (and shedding) and HIV-1 seropositivity in African women in Bangui, Central African Republic, findings indicated a significant seroprevalence of HSV-2 in HIV-1 seropositive than in HIV-1 seronegative women (CitationMbopi-Keou et al., 2000). The studies show that infections due to HSV are therefore a major health problem in regions with high HIV-1 seroprevalence. After the primary infection, HSV tends to persist in the neuron of the ganglia (CitationBaringer & Swoveland, 1973). Reactivation of latent HSV, which is very common during the deficiency of immunity, causes recurrent herpetic infection.
Acyclovir, valaciclovir, famciclovir, and cidofovir have been used for the treatment of HSV infection and associated diseases (CitationCassady & Whitley, 1997). However, resistance of HSV to currently used antiviral agents (CitationEnglund et al., 1990) and the property of latency of HSV infection have limited the efficacy of existing antiviral management (CitationBean, 1992). Viral strains that are resistant to drugs commonly used in the therapy of HSV infection have been increasingly isolated (CitationNugier et al., 1992), particularly from immunocompromised patients (CitationErlich et al., 1989). Therefore, the search for a new antiviral agent emerges as an imperative need.
Previous work focused on the chemical composition research of these medicinal plants (CitationLu & Tian, 1999; CitationZhang et al., 1994; CitationShi & Lu, 2003; CitationWang et al., 2005). However, the pharmacological activity studies have been limited. Some researchers have reported that D. heterophyllum and D. tanguticum possess antioxidation and antianoxia bioactivities (CitationHai et al., 1997; CitationXue et al., 1994). CitationHan et al. (2005) reported that S. mussotii exhibits protective effects on liver and multiple organ injuries by CCL4 in rats. L. brevituba showed anticancer activity (CitationJin & Chen, 2006) and the biological activity of A. tanguticum has not been reported. However, there are no reports about antiviral effects of these medicinal plants. Yet, the traditional usage of these medicinal plants as therapeutic medicine for viral hepatitis and other infectious diseases hinted that they may be antiviral agents. Therefore, it is important to study the antiviral activity of these Tibetan medicinal plants. In this study we investigate the anti-HSV-2 activity in vitro and in vivo using modern techniques. We also addressed the possible mode of action and molecular mechanism of their antiviral activity.
Materials and methods
Plant materials
The aerial parts of D. heterophyllum, D. tanguticum, A. tanguticum, L. brevituba, and S. mussotii were collected in 2006 at the altitude of 3,000 m from the Tibetan Plateau. The plant was authenticated by senior plant taxonomist Jizhou Song, School of Life Science, LanZhou University, Gansu, China. A voucher specimen has been deposited at the herbarium of the School of Life Science, LanZhou University, China.
Preparation of the extracts
Ethanol extracts were prepared from fresh plants listed in . Fresh plants (250 g) were cut into small pieces, immersed in solvent ethanol at room temperature for 24 h, then extracted twice with 95% ethanol under reflux for 2 h. The extract was filtered by gauze and subsequently was concentrated by evaporating the solvent under reducing pressure. The concentrated liquid was finally lyophilized to dryness. The dried extract was sterilized by UV irradiation.
Table 1. EC50 and CC50 determinations for the medicinal plant extracts.
Acyclovir (ACV) was purchased from Zengsheng Pharmaceutical, Wuhan, China. ACV and extracts were dissolved in dimethyl sulfoxide (DMSO) and then diluted with sterile deionized distilled water before use. The final concentration of DMSO was < 0.5% which was non-toxic to Vero cells.
Cell and viruses
All reagents and medium for cell culture were purchased from Gibco (Grand Island, New York). Vero cells (African green monkey kidney cells, ATCC CCR-81), were obtained from the Lanzhou Institute of Biological Products, (Lanzhou, China). Vero cell line is a suitable system for the primary cultivation of herpes simplex viruses. Cells were propagated in Dulbeco’s modified Eagle medium (DMEM) supplemented with 5% fetal calf serum (FCS), 200 U/mL penicillin G sodium, 200 μg/mL streptomycin sulfate and 0.5 μg/mL amphotericin B. Overlay medium for the plaque assay consisted of DMEM plus 2% FCS, 1% methylcellulose and antibiotics as described above. HSV-2 strain 196 was purchased from China Center for Type Culture Collection. Its titer was determined by plaque assay and was expressed as plaque forming units (PFU) per mL. Virus stocks were stored at -80°C until use.
Cytotoxicity assay
For the cytotoxicity assay, Vero cells were seeded in 96-well plates at a cell concentration of 2 × 103 cells per well in 100 μL of DMEM medium. After incubation of the cells for 24 h at 37°C, various concentrations of extracts were added, and the incubation was continued for 48 h, and viable cell yield was determined by MTT reduction assay according to reported procedure (CitationMosmann, 1983). In brief, 3-(4,5)-dimethylthiahiazo (-z-yl)-3,5-di-phenytetrazoliumromide (MTT) was dissolved in phosphate buffered saline (PBS) at 5 mg/mL and sterilized by filtration to remove a small amount of insoluble reside present in some batches of MTT. At the times indicated in the following, the MTT solution (25 μL) was added to each well, and plates were incubated again in 5% CO2 at 37°C for 2 h. Ethanol: DMSO (1:1) was added to all wells and mixed thoroughly to dissolve the dark blue crystals. After 20 min at room temperature to ensure that all crystals were dissolved, the plates were read on a Perkin-Elmer ELISA reader, using a test wavelength of 570 nm and a reference wavelength of 620 nm. The concentration of herbal extract reducing cell viability by 50% (CC50) was determined from a curve relating percentage cell viability to the concentration of extract.
Antiviral assay
Viral plaque reduction assays were performed according to the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS). Vero cells were seeded onto 24-well culture plates (Falcon, New Jersey) at density of 105 cells per well and incubated for 48 h to reach at least 95% confluence. The medium was discarded and cell monolayer was infected with 100 PFU HSV-2 in the absence or presence of extract. After 1 h incubation for virus adsorption, cells were overlaid with medium containing 1% methylcellulose. The plate was incubated at 37°C with atmosphere of 5% CO2 for 48 h. Later, the virus plaques formed on Vero cells were stained with 1% crystal violet. The fraction of percentage inhibition in inhibiting HSV replication was determined and the minimal concentration of extract required to suppress the formation of virus plaque numbers by 50% (IC50) was calculated by regression analysis of the dose–response curve generated from data (CitationGong et al., 2005).
Virucidal assay
Virucidal activity of extracts was evaluated as described by CitationCarlucci et al. (1999). Briefly, a virus suspension containing 100 PFU HSV-2 was mixed with or without various concentrations of extracts for 1 h at 37°C. The residual infectivity of the sample was determined by plaque assay. Plaques were counted and the percentage of inhibition of was calculated by the following formula:
Attachment assay
For the attachment assay, confluent Vero cell monolayers in 24-well plates were treated with extracts before virus infection. Extracts were incubated with semi-confluent cells in 24-well tissue culture plates with various concentrations for 1 h at 37°C and 5% CO2. After removal of the unbound extracts the cells were washed with phosphate-buffered saline and then infected with 100PFU/mL of HSV-2. After 1 h incubation the unabsorbed virus was removed, and the cell monolayer was overlaid with medium. After a further 48 h of incubation at 37°C, the cell monolayer was fixed and stained. Plaques were counted and the percentage of inhibition of attachment was calculated. Controls consisted of Vero cells untreated alone and Vero cells infected with HSV-2.
Biosynthesis assay
The biosynthesis assay of herpes simplex virus into Vero cells was performed according to procedures in the literature (CitationCheng et al., 2004) with minor modifications. Vero monolayer was grown in 24-well culture plates. Cell monolayer was then infected with 100PFU HSV-2 and incubated at 37°C for another 1 h to allow the attachment of HSV-2 toward cell monolayer. After 1 h of incubation, Extracts at various concentrations were added. The control group contained no extracts. The infected cell was covered with overlay medium. After a further 48 h of incubation at 37°C, the cell monolayer was fixed and stained. Plaques were counted and the percentage of inhibition of biosynthesis was calculated as previously described.
Real-time PCR for effect on HSV DNA synthesis
Confluent Vero cells were infected with HSV-2 at a multiplicity of infection 100PFU HSV-2 in the absence or presence of extract. At 18 h post-infection (p.i.), the medium was aspirated and the cells were washed with PBS. The lysates from triplicate wells were pooled (CitationGong et al., 2005). The DNA was extracted by using the standard phenol/chloroform method (CitationSambrook et al., 1989).
HSV-2 PCR Fluorescence Quantitative Diagnostic Kit (DaAn Gene, Sun Yat-Sen University, China) was used for quantitation of viral DNA. HSV-2 specific primers and a fluorescent probe directed to the DNA polymerase gene (UL30) were used for real-time PCR. Forward Primer sequence is 59 CGACTTTGCCAGCCTGTACC 39, Reverse primer sequence is 59 AGTCCGTGTCCCCGTAGATG 39. Probe sequence is GGCGTAGTAGGCGGGGATGTCGCG. DNA preparations (2 μL) were mixed with 23 μL of PCR mixtures. The amplification was performed using the Bio-Rad icycler (Milpitas, CA 95035, USA) under the following conditions: incubation for 2 min at 93°C, followed by 45 cycles of 30 s at 95°C and 45 s at 55°C. Each PCR (Applied Biosystems, Courtaboeuf, France) run contained two control groups, (the control-1 group contained Vero cells with HSV-2 infection no extracts, the control-2 group contained only normal cells no extracts, no virus), and 10-fold serially diluted reference DNA offered by Kit in order to generate the standard curve. Fluorescence measurements were performed at each cycle, which allowed determination of the cycle threshold (CT) value for each DNA sample. The detection threshold of the assay was 10 copies per run. Viral load was derived from CT using the standard curve generated in parallel and expressed as the number of copies per volume unit (CitationThi et al., 2006).
Determination of acute toxicity (LD50 )
Acute toxicity of the extract was estimated by the intraperitoneal route, using the procedure reported by CitationLorke (1983). This method estimates the dose of the extract that will kill 50% of a reduced sample of animals by a given route. Balb/C mice of both sexes, each weighing 18-22 g, were kept at room temperature and room relative humidity, the animals were randomly distributed into groups of 10 animals per dose per cage, with free access to water and pellet feed. In a first phase, testing the dose of 0% and 100% and then selecting five doses between them. The extracts were given to mice by the i.p route. Mice were kept under observation for 14 days. The weights and mortality were used for the determination of acute toxicity.
Efficacy of plant extracts in an encephalitis mouse HSV-2 infection model
Balb/C mice purchased from Lanzhou Institute of Biological Products, China, were used for determination of in vivo efficacy of the extract. The animals were handled according to guidelines laid down by the animal care and maintenance committee of the Kenya Medical Research Institute that follows internationally acceptable standards on animal care and use in laboratory experimentation.
Balb/C mice 7 weeks old weighing about 20 g were housed for one day to acclimatize. The mice were intracerebrally inoculated with 106 PFU/50 μL /mouse HSV-2 to induce encephalitis. The mice were randomly divided into five groups each comprised of 10 mice. The first group, control-1 group, was not infected and received no treatment. The second group, control-2 group, was infected but received no treatment. The third group, control-3 group, was infected and received 5% DMSO in distilled water treatment. The fourth group, the treatment test group, was infected and received the plant extracts treatment. The final fifth group was the reference drug group. In this group, mice were infected and received ACV treatment. Acyclovir was used as the positive reference drug to evaluate the antiviral capacity of the extracts. Intraperitoneal injection of the test sample and reference drug was given once a day for seven consecutive days. The control mice were administered with 5% DMSO in distilled water at the same time. The mice were fed and observed for 30 days to determine their mortality.
Statistical analysis
The data were expressed as mean ± SD. The statistical significance of the difference between mean values was determined by Student’s t-test. Data were test. Data were considered different at a significance level of P < 0.05.
Results
Cytotoxic effect of the extracts on viability of Vero cells
The concentrations of extracts that did not affect the viability of Vero cells were investigated by MTT assay. Results showed that each extract had a different magnitude of toxicity on Vero cells. D. heterophyllum, D. tanguticum, and S. mussotii extracts had no cytotoxic effect up to the concentration of 2 mg/mL. A. tanguticum was less toxic at 1 mg/mL and L. brevituba extract was not deadly at 0.5 mg/mL. The CC50 value of D. heterophyllum, D. tanguticum, A. tanguticum, S. mussotii, and L. brevituba extracts was 8.79 ± 0.5, 9.09 ± 1.49, 5.25 ± 0.59, 12.77 ± 0.61 and 1.40 ± 0.23 mg/mL, respectively. We, therefore, examined the anti-HSV activity of D. heterophyllum, D. tanguticum, A. tanguticum, and S. mussotii extracts at concentrations of 4 mg/mL or lower, and of L. brevituba extracts at concentrations of 1 mg/mL or lower ().
The antiviral activity of extracts against HSV
Antiviral activity was determined by plaque reduction assay and the EC50 is summarized in . The EC50 value of D. heterophyllum, D. tanguticum, A. tanguticum, S. mussotii, and L. brevituba extracts was 0.99 ± 0.12, 1.43 ± 0.13, 1.83 ± 0.21, 3.55 ± 0.13 and 0.35 ± 0.11 mg/mL, respectively, whereas the EC50 value of the positive control, acyclovir was 1.42 ± 1.17 mg/mL. The inhibition rate (data not showed) of 2 mg/mL of D. heterophyllum, D. tanguticum, A. tanguticum, S. mussotii, and 1 mg/mL of L. brevituba extracts was 88.9, 82.2, 83.3, 10.9, and 31.5% respectively. Even the concentration of extracts decreased 0.25 mg/mL except that of L. brevituba was 0.06 mg/mL, the inhibition rate of extracts was higher than 10% in addition S. mussotii has no effect. Results showed that D. heterophyllum, D. tanguticum, and A. tanguticum extracts exhibited significant inhibitory effect in suppressing HSV-2 replication, whereas L. brevituba and S. mussotii possessed slight effect. The selectivity index (SI = CC50/EC50) of D. heterophyllum, D. tanguticum, A. tanguticum, S. mussotii, L. brevituba extracts and ACV was 8.91, 6.36, 2.81, 3.55, 4.04, and 7.64, respectively, the SI of D. heterophyllum and even higher than ACV, which is commonly known to be active only in affecting the HSV replication.
In a plaque reduction assay, the extracts exhibited significant anti-HSV-2 inhibitory effect. To confirm these findings, a CPE inhibition assay was performed. The cells were infected with HSV-2 and then covered with medium containing no or increasing concentrations of extracts and cultured until the CPE was 100% in control wells (no drug). The viability of protected cells was measured using an MTT assay. The results showed a concentration-dependent protection of cells from viral lysis (data not shown). The results are in accordance with those of the plaque reduction assay.
Effect of the extracts on HSV-2 infectivity
The effect of extracts on viral residual infectivity was investigated with various concentrations. They were mixed directly with HSV-2, 37°C for 1 h and then quantitated by plaque assay on Vero cells. displays the virucidal ability of the extracts on HSV-2. The five extracts completely diminished HSV-2 infectivity. They remained active even when low concentration (0.25 or 0.06 mg/mL) was applied. D. heterophyllum, D. tanguticum, and L. brevituba extracts (at the concentration of 4 mg/mL) showed higher effect than ACV at the concentration of 0.5 mg/mL ( and ). In general, all five extracts significantly reduced HSV-2 infectivity.
Figure 1. Virucidal activity of extracts against HSV-2. 100 PFU HSV-2 was studied on each test extract at various concentration (0.25, 0.5, 1, 2, 4 mg/mL except 0.06, 0.13, 0.25, 0.5, 1 mg/mL for L. brevituba, respectively) for 1 h at 37°C. Each point represents the mean ± SD of three independent experiments.
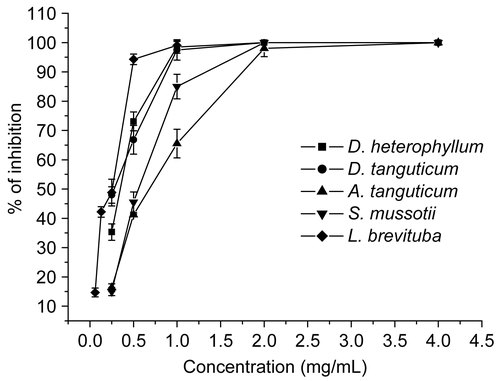
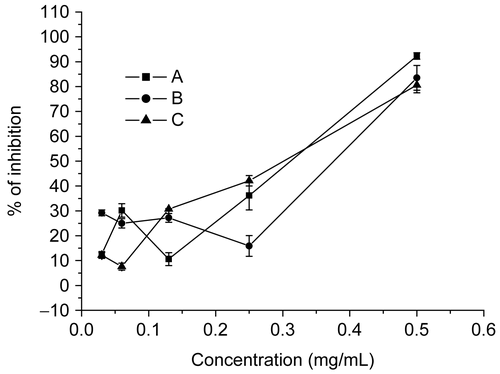
Figure 4. Effect of ACV on HSV-2 infectivity, attachment and biosynthesis to Vero cells. The tested concentration of ACV was 0.03, 0.06, 0.13, 0.25, 0.5 mg/mL. Each point represents the mean ± SD of three independent experiments. A, HSV-2 was mixed with various concentrations of ACV for 1 h at 37°C; B, cells treated with ACV before virus infection; C, cells treated with ACV after virus infection.
Effect of the extracts on the viral attachment and biosynthesis
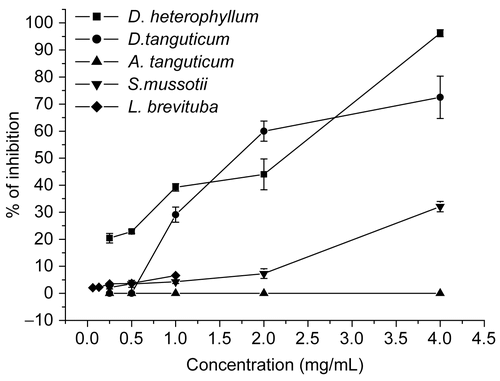
The ability of the extracts to block the HSV entry process was examined in susceptible cells that were incubated with various concentrations of the extracts for 1 h prior to virus infection. Results are shown in and . Results showed that D. heterophyllum and D. tanguticum extracts significantly inhibited HSV-2 attachment with an inhibition rate of 96.2 ± 1.3% and 72.5 ± 7.8% at a concentration of 4 mg/mL, respectively. In contrast, the inhibition rate of ACV was 83.5 ± 5.0% at the 0.5 mg/mL concentration. S. mussotii and L. brevituba extracts had minor effect with inhibition rate lower than 10%, whereas A. tanguticum extracts had no effect. Therefore, it was concluded that the extracts inhibited the attachment between HSV-2 and Vero cells, and its inhibitory effect was dependent to dose levels, except A. tanguticum.
Figure 2. Effect of extracts on HSV-2 attachment to Vero cells. Vero cell monolayer in 24-well plates were treated with extracts before virus infection at various concentration (0.25, 0.5, 1, 2, 4 mg/mL except 0.06, 0.13, 0.25, 0.5, 1 mg/mL for L. brevituba, respectively). Each point represents the mean ± SD of three independent experiments.
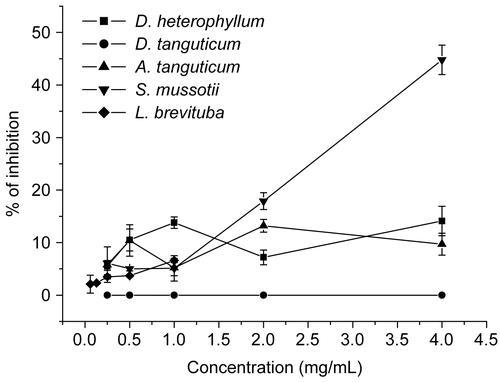
Besides the attachment, Virus protein synthesis of HSV-2 into cells was also investigated. Cells were infected with HSV-2 and incubated for another 1 h to allow the attachment of HSV-2 toward the cell monolayer before extracts were added. Our studies revealed that only S. mussotii extract possessed moderate activity in preventing the biosynthesis of HSV-2 ( and ), with the inhibition percentage 44.8 ± 2.8%. D. heterophyllum, A. tanguticum and L. brevituba extracts showed slight effect with the inhibition rate about 10%. Nevertheless, D. tanguticum completely had no effect.
Figure 3. Effect of extracts on HSV-2 biosynthesis to Vero cells. Vero cell monolayer in 24-well plates were treated with extracts after virus infection at various concentration (0.25, 0.5, 1, 2, 4 mg/ml except 0.06, 0.13, 0.25, 0.5, 1 mg/mL for L. brevituba, respectively). Each point represents the mean ± SD of three independent experiments.
Effect of the extracts on HSV DNA synthesis
The effect of the extracts on HSV DNA synthesis was analyzed quantitatively using a real-time PCR assay (the Bio-Rad iCycler technology). The infected cells were treated with extracts for 20 h. Total DNA was extracted from the cells and quantitated using the iCycler assay. A significant reduction in HSV DNA synthesis was detected in extract-treated HSV infected cells (). Comparing the DNA copy numbers present in the experimental and control groups, D. heterophyllum, D. tanguticum, A. tanguticum, S. mussotii, and L. brevituba extracts produce 104, 105, 102, 103, 103 fold reduction, respectively, in virus titer in comparison to the virus control.
Table 2. The effect of extracts against HSV DNA synthesis quantitated using a quantitative real-time PCR.
Acute toxicity study (LD50)
The acute toxicity of extracts was evaluated by median lethal dose (LD50) of intraperitoneal injection in mice. The LD50 of D. heterophyllum, D. tanguticum, A. tanguticum, S. mussotii, and L. brevituba extracts, was 14.99, 5.62, 0.85, 19.13, 1.31 g/kg (), respectively. After intraperitoneal injection of extracts, the mice of low dosage groups just showed slight reaction: the animals were found to be listless and crouching unmoved in 3-5 min after administration. Those of high dosage groups became comatose after a very short excitation, spiritless, dyspnea, and some of them walked staggeringly. No differences in acute toxicity in relation to sex were recorded.
Table 3. The effect of extracts against HSV-2-induced encephalitis in mice.
The effect of extracts against HSV-2 induced encephalitis in mice
shows the results of the determination of therapeutic effect of the extracts in Balb/C mice intracerebrally infected with HSV-2 after being given an oral dose per day; 90% of the mice developed encephalitis symptoms at the second day after inoculated with 100 LD50 HSV-2. The encephalitis mice showed hyperirritability and were sensitive to irritation. Then the animals became subject to listlessness, piloerection and anorexia, and finally died. The survival time of each animal was recorded to calculate the average survival time of each group, and the mortality of each group within 14 days after HSV infection was recorded as well (). The results demonstrated D. heterophyllum, D. tanguticum and S. mussotii extracts have an obvious effect against HSV-2 induced encephalitis, whereas L. brevituba and A. tanguticum only show minor effect to prolong mean survival times. D. heterophyllum, D. tanguticum, and S. mussotii extracts prolonged mean survival times as compared with control-3 (5% DMSO in distilled water treatment). The reduced mortality rate of mice treated with the extracts of D. heterophyllum, D. tanguticum, and S. mussotii was 70%, 80%, and 90% at the dose of 1 g/kg, respectively, as opposed to 100% mortality for the infected untreated mice (control-2).The extract delayed the onset of HSV-2 infection in the infected treated mice by 1 day when compared with untreated infected control. There was no significant difference between ACV and three plant extracts in prolonged mean survival times and reduced mortality rate.
Discussion
In this study, five Tibetan medicinal plants were found to inhibit HSV-2 infection efficiently in vitro. The therapeutic index (TI) of D. heterophyllum was even higher than ACV, which is commonly known to be only active in affecting the HSV replication. The extract inhibitory effect was dependent on dose levels. Three extracts (D. heterophyllum, D. tanguticum and S. mussotii) were found to be effective in encephalitis mice induced by HSV-2 infection. Many Chinese traditional medicinal plants showing anti-HSV activity were reported by CitationLi et al. (2005) and CitationCheng et al. (2004). However, there is no report of anti-HSV activities for these Tibetan plant extracts in vitro and in vivo. Therefore, our results are the first evidence demonstrating that these five extracts of the plants showed antiviral activity in vitro and three of them (D. heterophyllum, D. tanguticum, and S. mussotii) exhibited therapeutic antiviral efficacy in vivo. Our findings on antiviral activity could justify some ethnopharmacological usage of these medicinal plants as therapy for virus-caused hepatitis and other infectious diseases (CitationAnon, 1996).
In a plaque reduction assay, the extracts exhibited significant anti-HSV-2 inhibitory effect. To confirm these findings, a CPE inhibition assay was performed. The cells were infected with HSV-2 and then covered with medium containing no or increasing concentrations of extracts and cultured until the CPE was 100% in control wells (no drug). The viability of protected cells was measured using a MTT assay. The results showed a concentration-dependent protection of cells from viral lysis (data not shown). The results accorded with those of the plaque reduction assay.
Although results show that the extracts have HSV-2 inhibition effect, they were unable to provide any information on the mode of action of these extracts. Therefore, a series of experiments was carried out to determine the stage in which the extracts affected the viral life cycle. Results of virucidal assay showed that these medicinal plant extracts significantly reduce the HSV-2 infectivity.
Studies on the mode of action have shown that D. heterophyllum and D. tanguticum inhibited the attachment and S. mussotii inhibited biosynthesis of HSV-2, efficiently, since these plant extracts have different active compounds, viral differences in sensitivity to the various plant extracts may be due to the different modes of antiviral action of the active compounds in the plant extracts. HSV attachment is mediated by glycoprotein C (gC), (CitationSpear et al., 1992). The stability of attachment between viruses to cells is dependent on the presence of glycoprotein D (gD) (CitationRajcani & Vojvodova, 1998). According to our results on viral attachment assays, the extracts possibly affect the attachment of viruses into cells through the disturbance of viral glycoproteins.
The results of real-time PCR assay demonstrated that all the extracts inhibited HSV DNA replication, especially D. heterophyllum, D. tanguticum which have a significant reduction in HSV DNA copy numbers. Furthermore, there are no significant differences between two tested concentrations. The use of cell DNA after rapid cell lysis undoubtedly adds a level of complexity to the assay when compared to the collection of culture supernatant. However, this permits an earlier read-out, and the use of diluted crude lysate without any need for DNA purification is rather simple. In addition, the correlation of intracellular HSV DNA load with virus infectivity is stronger than that of culture supernatant DNA (CitationThi et al., 2006). Thus, to some extent, intracellular HSV DNA appears to be more relevant than culture supernatant DNA in regard to the process of DNA replication and its inhibition by antiviral drugs.
In the animal experiments using Balb/C mice intracerebrally infected with wild type strains of HSV-2, all the infected untreated mice ultimately died. Intraperitoneal treatment with the extract, however, provided some protection. For instance, there was a delay in the onset of infection in the treated mice when compared to untreated infected control (). And when the infection eventually picked up in the treated mice, the progression was slow and less lethal with an increased mean survival time and a significantly reduced mortality rate (). All this was indicative of the therapeutic effect of the extract. D. heterophyllum and D. tanguticum were found to be effective in encephalitis mice, it is suggested that the plant extracts showed therapeutic efficacy for HSV-2 infection in vivo possibly based on its anti-HSV-2 specific activity observed in vitro. D. heterophyllum and D. tanguticum prolonged the mean survival time and reduced the mortality of infected mice. D. tanguticum showed antianoxic effects on the brains of mice (CitationHai et al., 1997), and the relevant relationship between antianoxic effects and anti-HSV activity of D. tanguticum needs to be further investigated. S. mussotii reduced the mortality of infected mice, although L. brevituba and A. tanguticum did not (). The protective effect of S. mussotii in mice was not related to a direct antiviral effect. This may partly result from an immunomodulatory activity and/or higher bioavailability of S. mussotii which is traditionally believed to have tonic function. Thus, these three plant extracts might contain the active compounds that would be effective in treatment of encephalitis by HSV-2 infection. Although L. brevituba and A. tanguticum exhibited slight protection for encephalitis mice, it did not mean both of them having no antiviral effect in vivo; other animal models such as genital herpes mice need to be established to confirm this. It is worth noting that in all the animal experiments, the normal control mice (control-1 in the experiments) never showed either infection or mortality. This confirmed that the observed deaths in the infected untreated mice were all due to HSV infection. Moreover, the LD50 value of the extracts suggested it was safe to use these extracts at the treatment dosage employed here.
The importance of HSV infection in the dynamics of HIV acquisition, infection and transmission suggests that anti-HSV management is one of the ways to hamper the epidemic of HIV (CitationCelum, 2004; CitationSchacker, 2001). The prevention of HSV-2/HIV shedding and that of genital ulcer formation are the direct approaches.
In summary, the D. heterophyllum and D. tanguticum extracts were concluded to possess effective anti-HSV-2 activity, which are both many times that of Dracocephalum Lamiaceae genus. Therefore, to some extent, the character of antiviral activity of D. heterophyllum and D. tanguticum may possess some of the same active components in their extracts. They inhibit HSV-2 infection primarily through interfering with the early stage of HSV-2 multiplication through diminishing the HSV-2 infectivity and inhibiting HSV DNA replication. Nevertheless, further studies are needed to verify the underlying mechanism of action of the extracts from D. heterophyllum and D. tanguticum in inhibiting HSV-2 infection. We are continuing our studies on the anti-HSV-2 profile of D. heterophyllum and D. tanguticum by purifying the individual components from the effective extracts, and also by examining their inhibitory effect on other RNA and/or non-enveloped viruses. Thus, these Tibetan medicinal plant extracts may be confirmed to be possible candidates for anti-herpes simplex virus-2 infection therapeutics.
Acknowledgements
We sincerely thank researchers Bu Gong and Ben-Jia Leng (Gansu Institute of Tibetan Medicine Research) for providing help collecting the samples.
Declaration of interest: This work was supported by the National Natural Science Foundation of China (No. 30471133).
References
- Anon (1991): Tibetan Medicine Glossary. Xining, Qinghai People’s Press, pp.131–138; 276–289; 384–390; 567–574; 828–932.
- Anon (1996): Chinese Tibetan Medicine. Shanghai, Science and Technology Press of Shanghai, pp. 351–362; 445–450; 634–640; 780–785; 892–898.
- Baringer RJ, Swoveland P (1973): Recovery of herpes-simplex virus from human trigeminal ganglions. New Engl Med 288: 648–650.
- Bean B (1992): Antiviral therapy: Current concept and practices. Clin Microbiol Rev 5: 14–182.
- Carlucci MJ, Ciancia M, Matulewicz MC, Cerezo AS, Damonte EB (1999): Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antivir Res 43: 93–102.
- Cassady KA, Whitley RJ (1997): New therapeutic approaches to the alphaherpesvirus infections. J Antimicrob Chemother 39: 119–128.
- Celum CL (2004).The interaction between herpes simplex virus and human immunodeficiency virus. Herpes 11(Suppl 1): 36A–45A.
- Cheng HY, Lin TC, Yang CM, Wang KC, Lin LT, Lin CC (2004): Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage of herpes simplex virus type 2 infection in vitro. J Antimicrob Chemother 53: 577–583.
- Chiang LC, Chiang W, Chang MY, Ng LT (2002): Antiviral activity of Plantago major extracts and related compounds in vitro. Antivir Res 55: 53–62.
- Englund JA, Zimmerman MK, Swierkosz EM, Goodman JL, Scholl DR, Balfour HH (1990): Herpes simplex virus resistant to acyclovir. Arch Inter Med 112: 416–422.
- Erlich KS, Mills J, Chatis P, Mertz GJ, Busch DF. Follansbee SE, Grant RM, Crumpacker CS (1989): Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. New Engl Med 320: 293–296.
- Gong E, Matthews B, McCarthy T, Chua JH (2005): Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses. Antivir Res 68: 139–146.
- Hai P, Zhou S, Shang H, Zhao G (1997): Antianoxic effects of Dracocephalum tanguticum on brain of mice. Zhong Yao Cai 20: 198–200.
- Han LS, Hu H, Wang F (2005): Protective effects of zang ying chen on liver and multiple organs injury by CCl4. J Med Pharm China Minorities 5: 20–21.
- Hook EW, Cannon RO, Nahmias AJ (1992): Herpes simplex virus infection as a risk factor for human immunodeficiency virus infection in heterosexuals. J Infect Dis 165: 251–255.
- Jin L, Chen Z (2006): Anticancer research of Lagotis Brevituba Maxim. J Qinghai Normal University (Natural Science) 2: 86–87.
- Kuo YC, Chen CC, Tsai WJ, Ho YH (2001): Regulation of herpes simplex virus type 1 replication in Vero cells by Psychotria serpens: Relationship to gene expression, DNA replication, and protein synthesis. Antivir Res 51: 95–109.
- Lapucci A, Macchia M, Parkin A (1993): Antiherpes virus agents: A review. Farmaco, IL. 245–260
- Li SY, Chen C, Zhang HQ, Guo HY, Wang H (2005): Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir Res 67: 18–23.
- Lorke D (1983): A new approach to practical acute toxicity testing. Arch Toxicol 54: 275–287.
- Lu M, Tian X (1999): Analysis of essential oil of Dracocephalum heterophyllum. Acta Pharma Sini. 34: 925–927.
- Luo DS (1997): Zhonghua Tibetan Herbal. Beijing, National Press, pp. 445–448.
- McGowan I (2006): Microbicides: A new frontier in HIV prevention. Biologicals 34: 241–255.
- Nugier F, Collins JN, Langlois M (1992): Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: Report on a two-year sensitivity screening survey. J Med Virol 36: 1–12.
- Mbopi-Keou, FX, Gresenguet Mayaud, P, Weiss, HA, Gopal R, Matta M, Paul, JL, Brown DWG, Hayes RJ, Mabey DCW, Belec L (2000): Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women. J Infect Dis 182: 1090–1096.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 65: 55–63.
- Rajcani J, Vojvodova A (1998): The role of herpes simplex virus glycoproteins in the virus replication cycle. Acta Virol 42: 103–118.
- Shi GY, Lu RH (2003): Study on chemical constituents of essential oil from Lagotis brevituba. Nat Prod Res Dev 15: 23–28.
- Spear PG, Shieh MT, Herold BC, WuDunn D, Koshy TI (1992): Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv Exp Med Biol 313: 341–353.
- Schacker T (2001): The role of HSV in the transmission and progression of HIV. Herpes 8: 46–49.
- Sambrook J, Fritsch EF, Maniatis T (1989): Molecular Cloning: A Laboratory Manual. New York, Cold Spring Harbor Laboratory, pp. 851–862.
- Thi TN, Deback C, Malet I, Bonnafous P, Agut H (2006): Rapid determination of antiviral drug susceptibility of herpes simplex virus types 1 and 2 by real-time PCR. Antivir Res 69: 152–157.
- Whitley RJ, Kimberlin DW, Roizman B (1998): Herpes simplex viruses. Cli Infect Dis 26: 97–109.
- Xue D, Yang F, Bee D (1994): Modification of the rabbit carotid body type I cell mitochondria by high altitude exposure and the effects of Dracocephalum heterophyllum. Adv Exp Med Biol 360: 357–360.
- Wang YB, Huang R, Zhang HB (2005): Diterpenoid alkaloid from Aconitum tanguticum. Helve Chimi Acta 88: 1081–1084.
- Yang CM, Cheng HY, Lin TC, Chiang LC, Lin CC (2005): Acetone, ethanol and methanol extracts of Phyllanthus urinaria inhibit HSV-2 infection in vitro. Antivir Res 67: 24–30.
- Zhang XF, Hu BL, Wang SX (1994): The chemical constituents from Dracocephalum tanguticum Maxim. Acta Bot Sini 36: 645–648.
