Abstract
FBD, a Chinese herbal formula, composed of Fu Ling [the sclerotium of the fungus of Poria cocos (Schw.) Wolf (Polyporaceae)], Bai Zhu [the rhizome of Atractylodes macrocephala Koidz.(Compositae)], and Danggui [the root of Angelica sinensis (Oliv.) Diels (Umbelliferae)], has been found beneficial in treating brain ischemia-reperfusion (I/R). In this work, oral pretreatment with supercritical CO2 extract (FBD-CO2, 37. 5 mg/kg) twice daily for 3.5 days, significantly attenuated brain infarction (p < 0.05), blocked neuron specific enolase (NSE) efflux (p < 0.05) in mice subjected to repetitive 10 min of common carotid artery occlusion following 24 h reperfusion, whether coupled with aqueous extract (FBD-H2O, 150 mg/kg) or not. Except atractylenolide I and pachymic acid, atractylenolide II, III, levistolid A, and ferulic acid isolated from FBD-CO2 alone protected neuron-like PC12 cells obviously and concentration-dependently against I/R-like insults (p < 0.05 ~ 0.01) induced by sodium dithionite (10 mM), monosodium glutamate (2 mM), KCl (0.2 M), or hydrogen peroxide (0.2 mM) at 1-100 μM. Even though at sub-active concentrations (< 1 μM), the combination of the six components contained in FBD-CO2 (10 μg/mL), protected markedly PC12 cells (p < 0.01), in a synergistic fashion. Moreover, intraperitoneal treatment with dual doses of 37. 5 mg/kg FBD-CO2 and 1. 2 mg/kg combination reduced both infarct size (p < 0.01 and p > 0.05, respectively) and NSE efflux (p < 0.05 and p > 0.05, respectively). Therefore, neuroprotection by FBD on I/R induced neuronal injury originated partially from the synergies of its compounds atractylenolide II, atractylenolide III, levistolid A and ferulic acid.
Introduction
Traditional Chinese medicine (TCM) has a long history in stroke therapy, and more than 100 herbs are in ordinarily clinical use. As documented in Pharmacopeia of the People’s Republic of China, Fu Ling (Poria, or the sclerotium of the fungus of Poria cocos (Schw.) Wolf (Polyporaceae)), Bai Zhu [white atractylodes rhizome, or the rhizome of Atractylodes macrocephala Koidz. (Compositae)], and Danggui [Chinese angelica root, or the root of Angelica sinensis (Oliv.) Diels (Umbelliferae)] are three essential Chinese medicinal plants, which are clinically used to modulate psychoneural, gastrointestinal, and gynecological disorders, respectively (CitationAdams et al., 2007). Recently, research has focused upon their neuroprotective actions, because the three herbs were being frequently applied to cure stroke in Asia (CitationGong & Sucher, 1999).
Pharmacological investigation implied that the combination of Fu Ling, Bai Zhu, and Danggui (FBD) had a predominant potential for stroke treatment. Total extracts of FBD prevented ischemia-reperfusion (I/R) induced brain injury in rodents, in part through antineurotoxic and antioxidant activities (CitationLin et al., 2005; CitationDong et al., 2006; CitationZhou et al., 2006; CitationWu et al., 2007). Neuroprotection of FBD originated principally from its liposoluble factions, because in vitro I/R-like insults in neurons, astrocytes and neuron-like PC12 cells were ameliorated by the total extracts of FBD or its supercritical carbon dioxide extract (FBD-CO2) (CitationWang et al., 2004; CitationZhang et al., 2005; CitationLiu et al., 2007).
Six small compounds, including atractylenolide I, II, III, levistolid A, ferulic acid, and pachymic acid have been identified from bio-fluids of rats orally taking FBD-CO2 (CitationCao et al., 2007; CitationLuo et al., 2005; CitationWang et al., 2006). However, active components in FBD or FBD-CO2 remained unclear. Up to now, few psychoneural activities were available on these compounds except ferulic acid. As phospholipase A2 inhibitor (CitationCuella et al., 1996; CitationGiner et al., 2000), and DNA topoisomerases inhibitor (CitationLi et al., 2004), pachymic acid from Fu Ling inhibited tumor (CitationGapter et al., 2005; CitationKaminaga et al., 1996), and free-radical-induced hemolysis (CitationSekiya et al., 2003). Of the three sesquiterpenoids from Bai Zhu, atractylenolide I and III inhibited pro-inflammatory cytokines production and uterine contraction (CitationLi et al., 2007; CitationZhang et al., 2000). Being an N-methyl-d-aspartate (NMDA) receptor antagonist and antioxidant (CitationKanski et al., 2002; CitationOgiwara et al., 2002; CitationZhang et al., 2003), ferulic acid from Danggui protected brain against glutamate or amyloid beta-induced neurotoxicity and cerebral I/R (CitationJin et al., 2005; CitationYu et al., 2006). To identify potential active components and underlie their pharmacological relationships to FBD or FBD-CO2, the work assessed neuroprotective activities of its six components on neuronal injury in vivo and in vitro.
Materials and methods
Reagents and chemicals
Nimodipine, memantine, monosodium glutamate (MSG), N, N-dimethylformamide, triphenyltertrazolium chloride (TTC), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), vitamin E (Vit E) were from Sigma (St. Louis, MO). Dulbecco’s modified Eagle medium (DMEM) was from Gibco-BRL (Gaithersburg, MD). Enzyme-linked immunosorbent assay (ELISA) kit for neuron-specific enolase (NSE) was from Biosource International (Camarillo, CA).
Preparation of extracts of FBD
Three herbal materials used in this work, and the formula of FBD were reported previously (CitationLin et al., 2005). The mixed herbal materials were dried and powdered at 60ºC, macerated in 95% ethanol (v/w, 1:1) for 30 min. FBD-CO2, a brown liquid with density 0.85–0.9 at 60ºC, was obtained at 25. 0 MPa and 38ºC and at a CO2 flow of 110 kg/h, with a yield of 2.75%. The residue was then extracted with water for 30 min to gain dried brown powder (FBD-H2O), with a yield of 9.75%. The total extracts include FBD-CO2 and FBD-H2O.
Isolation of atractylenolide I, II, III, levistolid A, ferulic acid, and pachymic acid
Atractylenolide I, II, III from Bai Zhu, levistolid A and ferulic acid from Danggui, pachymic acid from Fu Ling were isolated from FBD-CO2 and identified as reported previously with purity > 98% (CitationLuo et al., 2005). The contents of the six compounds (3.22% in total) in FBD-CO2 were atractylenolide I 0.55%, II 0.88%, III 0.68%, levistolid A 0.003%, ferulic acid 0.39%, and pachymic acid 0.69%, at a ratio of 1:1.6:1.2:0.005:0.7:1.3, quantified by HPLC ().
I/R insults in mice brain
Male ICR mice (26–32 g) were provided by China Pharmaceutical University (Nanjing, China). Bilateral common carotid artery occlusion (CCAO) was performed as previously described (CitationLin et al., 2005; CitationZhen & Doré, 2007). In brief, bilateral CCA were separated, and occluded for 10 min at 38ºC, with 0.15 mL blood lost from tail. After cerebral blood flow comeback for 10 min, another 10 min occlusion followed. Sham-operated mice as sham-operated control did not undergo CCAO. The animal procedures in the research process were in strict accordance with the China National Institutes of Healthy Guidelines for the Care and Use of Laboratory Animals.
Pretreatment of extracts of FBD
Clinically, a single formula of FBD consisted of 10 g Poria cocos, 5 g Atractylodes macrocephala and 3 g Angelica sinensis. In this work, the dose of 187. 5 mg/kg of the total extracts of FBD twice per day was calculated according to the herbal formula dose of 18 g per day for a person with body weight of 60 kg, and its total extraction yield 12.5%. Extracts of FBD were dissolved with saline (containing 0.5% Tween 80 and 2% alcohol). Mice were randomized into five groups: I/R group and sham-operated group, 10 mL/kg saline, per oral (p.o.); FBD-pretreated groups, 187. 5 mg/kg FBD, 150 mg/kg FBD-H2O, 37. 5 mg/kg FBD-CO2, p.o.; FBD extracts were orally administered twice daily for 3.5 days prior to CCAO.
Measurement of brain infarction and circulating NSE
For TTC staining, 2 pieces of 2-mm thick coronal sections per brain were sliced. After incubation in 2% TTC for 30 min and fixation in 10% formalin for 45 min at 37ºC, brain slices were captured with a digital camera and measured by Zeiss AxioVs 40 image analysis system (Oberkochen, Germany). Brain infarction was presented as following equation:
% brain infarction = white infarct area/slice area × 100%
NSE efflux in serum, another specific biomarker for neuronal injury was assayed by ELISA, with sensitivity of 1 ng/mL.
I/R-like insults in PC12 cells
Neuron-like rat pheochromocytoma PC12 cells were provided by the Institute of Cell Biology (Shanghai, China). The cells were suspended in DMEM supplemented with 10% heat-inactivated newborn calf serum (NCS), benzylpenicillin (100 kU/L) and streptomycin (100 mg/L), and incubated at 37ºC in 5% CO2.
PC12 cells were exposed to: 1) Na2S2O4 (10 mM), an O2 scavenger for 1.5 h to induce hypoxic injury; 2) MSG (2 mM) in a Mg2+ free Earle’s solution for 4 h to induce neurotoxic injury; 3) H2O2 (0.2 mM) for 1 h to induce oxidative injury; or 4) KCl (0.2 M) for 4 h to induce calcium overload injury, then treated with atractylenolide I, II, III, levistolid A, ferulic acid or pachymic acid at 0.1–100 μM, or atractylenolide I (0.24 μM), II (0.38 μM), III (0.27 μM), levistolid A (0.008 μM), ferulic acid (0.20 μM), and pachymic acid (0.13 μM) contained in FBD-CO2 (10 μg/mL) alone or in combination. Memantine (1 μM), an NMDA antagonist, nimodipine (1 μM), an L-type Ca2+ channel blocker, and Vit E (10 μM), a free radical scavenger were set as three positive controls; and the vehicle 0.1% dimethyl sulphoxide (DMSO) artificial cerebrospinal fluid (ACSF) was negative control (CitationLin et al., 2005; CitationCao et al., 2006; CitationGoldshmidt et al., 2001).
After 20 h, MTT colorimetric assay was performed to observe the cell viability in PC12 cells and to calculate the neuroprotection by the six compounds. Briefly, MTT solution (0. 5 mg/mL) was added to each culture well. After incubation for an additional 4 h, the formazan crystals were dissolved by addition of 50 μL DMSO, and measured spectrophotometrically at dual wavelengths, 570 nm/650 nm.
Statistical analysis
SPSS 12.0 software and Origin 7.0 software were applied to analyze experimental data, and results were expressed as mean ± SD. All data were evaluated with analysis of variance (ANOVA) following by Dunnett’s t-test for multiple comparisons and p < 0.05 indicates that the difference was statistically significant.
Results
Neuroprotective effects of extracts of FBD in vivo
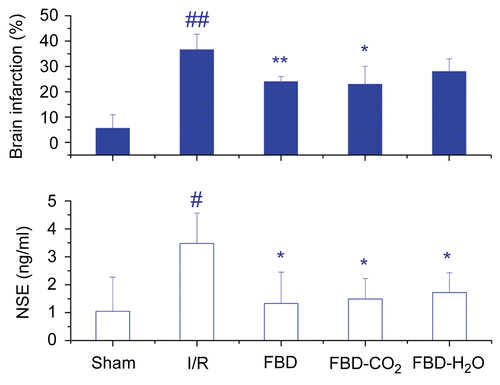
Twenty-four hours after cerebral repetitive I/R, large NSE efflux and diffused brain infarction were observed in I/R mice. As compared with the sham-operated mice, oral pretreatment with 187. 5 mg/kg FBD or 37. 5 mg/kg FBD- CO2, attenuated brain infarction remarkably by 40.6% (p < 0.01) and 43.9% (p < 0.05), respectively, and significantly reduced circulating NSE level (p < 0.05). By contrast, FBD-H2O protected against brain I/R to a lesser extent ().
Figure 2. Effects of FBD, FBD-CO2 and FBD-H2O on brain infarction and circulating NSE efflux in ICR mice subjected to cerebral repetitive I/R. Each column represents mean ± SD of 6 mice. NSE, neuron specific enolase; I/R, ischemia-reperfusion; #p<0.05, ##p<0.01 vs the sham-operated group; *p<0.05, **p<0.01 vs the saline-pretreated I/R group.
Neuroprotective activities of the six compounds of FBD in vitro and in vivo
At concentrations of 1–100 μM, atractylenolide II (), III (), levistolid A () and ferulic acid () except atractylenolide I and pachymic acid, inhibited PC12 cells I/R-like insults with concentration-dependent manners. All the actions of the four compounds were significant, but relatively weak with inhibitions less than 20%, even though at 100 μM, with respect to those of nimodipine 26.6–36.2% ( and ), memantine 38.1% (), and VitE 25.2% (). Herein, the six compounds had no toxicity on innate PC12 cells, at 0.1–100 μM.
Figure 3. Neuroprotective activities of atractylenolide II, atractylenolide III, levistolid A, ferulic acid from FBD-CO2 on PC12 cells I/R-like insults. Each dot represents mean ± SD of 6 wells. I/R, ischemia-reperfusion; *p<0.05, **p<0.01 vs the vechicle-treated PC12 cells.
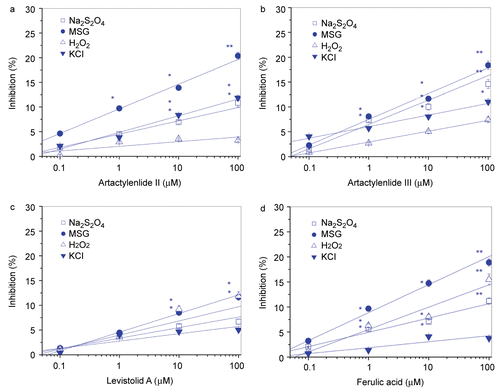
Figure 4. Neuroprotective activities of atractylenolide I, II, III, levistolid A, ferulic acid and pachymic acid combination on PC12 cells I/R-like insults. Values are means ± SD of three different experiments. The A570/650 in control was 0.64 ± 0.02. I/R, ischemia-reperfusion; Ctl, Control; Vh, vehicle; Com, Combination; CO2, FBD-CO2; VE, Vitamin E; ##p < 0.01 vs the control (Ctl); **p<0.01, ***p < 0.001 vs the vehicle (Vh)-treated cells; ††p < 0.01 vs the FBD-CO2-treated cells.
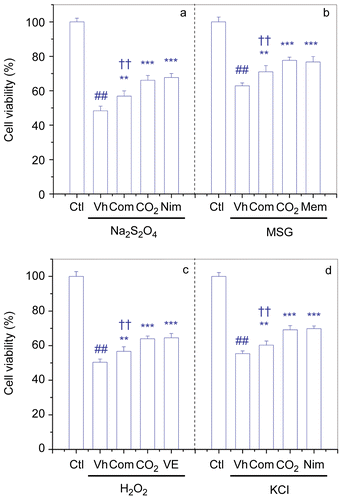
Neither atractylenolide I, II, III, levistolid A, ferulic acid, nor pachymic acid at sub-active concentrations (< 1 μM) had obvious influence on the PC12 cells insults (). However, once in combination the six components protected additively PC12 cells against all the insults (p < 0.01), though their synergies were still weaker than those of FBD-CO2 (p < 0.01) ( and ).
Table 1. Neuroprotections (%) of atractylenolide I,II, III, levistolid A, ferulic acid and pachymic acid alone or in combination on PC12 cells I/R-like insults induced by Na2S2O4, MSG, H2O2 and KCl in assay I–IV in vitro.
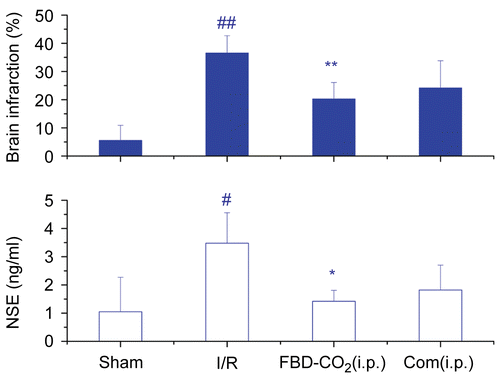
In addition, intraperitoneal treatment with dual doses of 37. 5 mg/kg FBD-CO2 and 1. 2 mg/kg compound combination (atractylenolide I, 0. 21 mg/kg; II, 0. 33 mg/kg; III, 0. 26 mg/kg; levistolid A, 0. 01 mg/kg; ferulic acid, 0. 15 mg/kg; and pachymic acid, 0. 26 mg/kg, i.p.) administered at 30 min prior to and 5 h after CCAO reduced brain infarction by 52.6% and 40%, respectively, and NSE efflux to different extents. No difference existed between the two treatments (p > 0.05, ).
Figure 5. Effects of FBD-CO2 and its six compounds combination on brain infarction and circulating NSE efflux in ICR mice subjected to cerebral repetitive I/R. Each column represents mean ± SD of 6 mice. NSE, neuron specific enolase; I/R, ischemia-reperfusion; #p<0.05, ##p<0.01 vs the sham-operated group; *p<0.05, **p<0.01 vs the saline-pretreated I/R group.
Discussion
In the present work we demonstrated again that total extracts of FBD might prevent and treat I/R-induced neuronal injury. It was likely that neuroprotection of FBD or FBD-CO2 was derived partially from its compounds, atractylenolide II, III, levistolid A and ferulic acid. As evidence, (i) FBD or FBD-CO2 attenuated brain infarction and NSE efflux; (ii) Except atractylenolide I and pachymic acid, atractylenolide II, III, levistolid A, and ferulic acid isolated from FBD-CO2 alone protected PC12 cells against I/R-like insults; (iii) Once in combination, the six compounds may synergistically protected PC12 cells, and ameliorate brain infarction and NSE efflux, similar to FBD-CO2.
It is well documented that repetitive CCAO results in histological and neurochemical abnormality. Our results indicate that pretreatment with FBD or FBD-CO2 offers protection against neuronal damage induced by CCAO, as they reduced the infarct size and NSE release from disrupted neurons (), in harmony with other studies (CitationYan et al., 2007; CitationCho et al., 2007).
The significant protection of FBD-CO2 on brain I/R injury probably at least lay in the synergistic actions of atractylenolide I, II, III, levistolid A, ferulic acid, and pachymic acid, rather than those of single compounds. By contrast, less neuroprotection was elicited by FBD-H2O because none of these components was contained in it, except some ferulic acid. During the early stage of brain I/R, energy depletion, lipid peroxidation, depolarization or excited amino acid release consequently resulted in acute neuronal necrosis and delayed neuronal death (CitationWatanabe et al., 1995). In accordance with the findings in vivo, except atractylenolide I and pachymic acid, atractylenolide II, III, levistolid A or ferulic acid attenuated significantly PC12 cells against I/R-like insults, similar to memantine, nimodipine, or Vit E ( and 4). However, neither atractylenolide I, II, III, levistolid A, ferulic acid, nor pachymic acid might exclusively account for the whole actions of their combination or FBD-CO2, whether in vivo or in vitro. At sub-active concentrations (< 1 μM), no compound had obvious impact on the PC12 cells insults. In contrast, once coupled with one another, the six components contained in FBD-CO2 (10 μg/mL), protected markedly PC12 cells against all the insults (). The actual actions (11%–21.8%) of the combination were 1.7–5.7 times over the predicted sums (2.2%–9.9%, ).
The exact mechanism by which FBD alleviated neuronal injury was not very clear in this work, because the neuroprotective actions of the compounds combination accounted for 40%–50% of those of FBD-CO2 in vitro, but 76%–80% of those of FBD-CO2 in vivo ( and ). It was strongly attributed to the presence of multiple activities by multiple components. On one hand, various biological activities of the six compounds were implicated in the neuroprotections of their combination or FBD-CO2. Antiplatelet activity of ferulic acid (CitationWang et al., 1988), and anti-inflammatory activities of atractylenolide I, III and pachymic acid could contribute to the effects of their combination or FBD-CO2 on thrombus or inflammation-induced neuronal injury (CitationGiner Larza et al., 2000; CitationLi et al., 2007; CitationSekiya et al., 2003). On the other hand, some other active compounds from FBD might further be additive to the combination. Recently, more than ten prototype components with psychoneural properties, including butylphthalide (CitationXu & Feng, 2000), ligustilide (CitationKuang et al., 2006), and butylidenephthalide (CitationKo et al., 1997), were identified in rabbit orally receiving Danggui (CitationLin et al., 1998; CitationLiu et al., 2004; CitationWang et al., 2005). More bioanalytical and pharmacological tests are needed to uncover the mode and mechanism of the Chinese herbal formula.
To sum up, neuroprotection of FBD or FBD-CO2 on neuronal I/R injury originated partly from the synergies of its neuroprotective compounds atractylenolide II, III, levistolid A and ferulic acid. This helped to better understand its clinical application for ischemic cerebrovascular disorders.
Acknowledgements
The authors thank Yi Cao and Lan Luo for experimental assistance.
Decleration of interest: This work was supported by various grants from the National New Drug Foundation of China (969010538), from the National Natural Science Foundation of China (30271604 & 30500683), and from the Doctoral Innovative Foundation of Jiangsu Province of China (200593).
References
- Adams M, Gmünder F, Hamburger M (2007): Plants traditionally used in age related brain disorders–A survey of ethnobotanical literature. J Ethnopharmacol 113: 363–381.
- Cao LL, Du GH, Wang MW (2006): The effect of salidroside on cell damage induced by glutamate and intracellular free calcium in PC12 cells. J Asian Nat Prod Res 8: 159–165.
- Cao Y, Zhu DN, Lin ZH, Xiao ZB, Yan YQ, Yu BY (2007): Serum pharmacochemistry of FBD (I). J China Pharm Univ 38: 519–522.
- Cho KO, Kim SK, Cho YJ, Sung KW, Kim SY (2007): Regional differences in the neuroprotective effect of minocycline in a mouse model of global forebrain ischemia. Life Sci 80: 2030–2035.
- Cuella MJ, Giner RM, Recio MC, Just MJ, Manez S, Rios JL (1996): Two fungal lanostane derivatives as phospholipase A2 inhibitors. J Nat Prod 59: 977–979.
- Dong WX, Ruan KF, Gu FH, Shen PN, Li PY, Lin ZH (2006): FBD used to improve learning and memory function of animals. Chin Trad Herbal Drugs 37: 1831–1835.
- Gapter L, Wang Z, Glinski J, Ng KY (2005): Induction of apoptosis in prostate cancer cells by pachymic acid from Poria cocos. Biochem Biophys Res Commun 332: 1153–1161.
- Giner Larza EM, Manez S, Giner Pons RM, Recio MC, Rios JL (2000): On the anti-inflammatory and anti-phospholipase A-2 activity of extracts from lanostane-rich species. J Ethnopharmacol 73: 61–69.
- Giner EM, Manez S, Recio MC, Giner RM, Cerda-Nicolas M, Rios JL (2000): In vivo studies on the anti-inflammatory activity of pachymic and dehydrotumulosic acids. Planta Med 66: 221–227.
- Goldshmidt Y, Erlich S, Pinkas-Kramarski R(2001): Neuregulin rescue PC12-ErbB4 cells from cell death induced by H2O2: Regulation of reactive oxygen species levels by PI3K. J Biol Chem 276: 46379–46385.
- Gong X, Sucher NJ (1999): Stroke therapy in traditional Chinese medicine (TCM): Prospects for drug discovery and development. TiPS 20: 191–196.
- Jin Y, Yan EZ, Fan Y, Zong ZH, Qi ZM, Li Z (2005): Sodium ferulate prevents amyloid-beta-induced neurotoxicity through suppression of p38 MAPK and upregulation of ERK-1/2 and Akt/protein kinase B in rat hippocampus. Acta Pharmacol Sin 26: 943–951.
- Kaminaga T, Yasukawa K, Kanno H, Tai T, Nunoura Y, Takido M (1996): Inhibitory effects of lanostane-type triterpene acids, the components of Poria cocos, on tumor promotion by 12-O- tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology 53: 382–385.
- Kanski J, Aksenova M, Stoyanova A, Butterfield DA (2002): Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J Nutr Biochem 3: 273–281.
- Ko WC, Sheu JR, Leu YR, Tzeng SH, Chen CM (1997): Stereoselectivity of butylidenephthalide on voltage-dependent calcium channels in guinea-pig isolated ileum. J Pharm Pharmacol 49: 1121–1125.
- Kuang X, Yao Y, Du JR, Liu YX, Wang CY, Qian ZM (2006): Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Res 1102: 145–153.
- Li CQ, He LC, Jin JQ (2007): Atractylenolide I and atractylenolide III inhibit lipopolysaccharide-induced TNF-alpha and NO production in macrophages. Phytother Res 21: 347–353.
- Li G, Xu ML, Lee CS, Woo MH, Chang HW, Son JK (2004): Cytotoxicity and DNA topoisomerases inhibitory activity of constituents from the sclerotium of Poria cocos. Arch Pharm Res 27: 829–833.
- Lin LZ, He XG, Lian LZ, King W, Elliott J (1998): Liquid chromatographic–electrospray mass spectrometric study of the phthalides of Angelica sinensis and chemical changes of Z-ligustilide. J Chromatogr A 810: 71–79.
- Lin Z, Zhu D, Yan Y, Yu B, Wang Q (2005): Protective effects of FBD–an experimental Chinese traditional medicinal formula on memory dysfunction in mice induced by cerebral ischemia-reperfusion. J Ethnopharmacol 97: 477–483.
- Liu J, Lin ZH, Yu BY, Zhu DN, Yan YQ (2007): Study X on substance basis and mechanism of action of Danggui Shaoyao San in the prevention and treatment of senile dementia–Protective effect of FBD and CO2 supercritical fluid extract (P2) on astrocyte anoxic injury. Chin J Exp Trad Med Formul 13: 14–17.
- Liu YM, Zhang JJ, Jiang J, Huang CY, Ren SL, Gao YZ (2004): Observation on clinical effect of Angelica injection in treating acute cerebral infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi 24: 205–208.
- Luo L, Zhu DN, Yan YQ (2005): SFE-CO2 constituents of simplified formula of Danggui Shaoyao powder in the prevention and treatment of vascular dementia. Chin Tradit Patent Med 27: 1251–1254.
- Ogiwara T, Satoh K, Kadoma Y, Murakami Y, Unten S, Atsumi T, Sakagami H, Fujisawa S (2002): Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res 22: 2711–2717.
- Sekiya N, Goto H, Shimada Y, Endo Y, Sakakibara I, Terasawa K (2003): Inhibitory effects of triterpenes isolated from Hoelen on free radical-induced lysis of red blood cells. Phytother Res 17: 160–162.
- Wang P, Yu BY, Lin ZH, Yan YQ (2004): Study on basis and mechanism of action of optimized Danggui Shaoyao San in the improvement effect on learning and memory impairment in mice. Chin J Exp Trad Med Formul 10: 18–21.
- Wang R, Wang GJ, Hao HP, Xie HT, Zhang JF, Wu F (2006): Quantitative determination of atractylenolide III in rat plasma by LC-ESI-MS. J Chromatogr B 831: 36–41.
- Wang YL, Liang YZ, Chen BM, He YK, Li BY, Hu QN (2005): LC-DAD-APCI-MS-based screening and analysis of the absorption and metabolite components in plasma from a rabbit administered an oral solution of danggui. Anal Bioanal Chem 383: 247–254.
- Wang Z, Gao YH, Huang RS, Zhu GQ (1988): Sodium ferulate is an inhibitor of thromboxane A2 synthetase. Acta Pharmacol Sin 9: 430–433.
- Watanabe Y, Zhang XQ, Liu JS, Guo Z, Ohnishi M, Shibuya T (1995): Protection of glutamate induced neuronal damages in cultured cerebella granule cells by Chinese herbal medicine, Toki-shakuyaku-san and its comprised six medicinal herbs. J Tradit Med 12: 93–101.
- Wu F, Zhu DN, Lin ZH, Yan YQ (2007): Study on material basis and mechanism of action of Danggui Shaoyao San in the prevention and treatment of senile dementia IX: The anti-oxidative activity of the polysaccharides of FBD. Chin J Exp Trad Med Formul 13: 23–26.
- Xu HL, Feng YP (2000): Inhibitory effects of chiral 3-n-butylphthalide on inflammation following focal ischemic brain injury in rats. Acta Pharmacol Sin 21: 433–438.
- Yan B, Bi X, He J, Zhang Y, Thakur S, Xu H, Gendron A, Kong J, Li XM (2007): Quetiapine attenuates spatial memory impairment and hippocampal neurodegeneration induced by bilateral common carotid artery occlusion in mice. Life Sci 81: 353–361.
- Yu L, Zhang Y, Ma R, Bao L, Fang J, Yu T (2006): Potent protection of ferulic acid against excitotoxic effects of maternal intragastric administration of monosodium glutamate at a late stage of pregnancy on developing mouse fetal brain. Eur Neuropsychopharmacol 16: 170–177.
- Zhang QC, Wang QJ, Kou JP, Zhu DN, Yan YQ, Yu BY (2005): Protective effect of cerebrospinal fluids containing optimized Danggui-shaoyao-san (FBD) on injured PC12 cells. Chin J Exp Trad Med Formul 11: 55–58.
- Zhang YQ, Xu SB, Lin YC, Li Q, Zhang X, Lai YR (2000): Antagonistic effects of 3 sesquiterpene lactones from Atractylodes macrocephala Koidz on rat uterine contraction in vitro. Acta Pharmacol Sin 21: 91–96.
- Zhang Z, Wei T, Hou J, Li G, Yu S, Xin W (2003): Iron-induced oxidative damage and apoptosis in cerebellar granule cells: attenuation by tetramethylpyrazine and ferulic acid. Eur J Pharmacol 467: 41–47.
- Zhen G, Doré S (2007): Optimized protocol to reduce variable outcomes for the bilateral common carotid artery occlusion model in mice. J Neurosci Methods 166: 73–80.
- Zhou SN, Wang QJ, Zhu DN (2006): The effect of optimized Danggui Shaoyao San (FBD) on cerebral ischemia reperfusion. Chin J Exp Trad Med Formul 12: 47–50.