Abstract
The analgesic activity of the methanol extract of the leaves of Kyllinga monocephala Rottb. (Cyperaceae) was evaluated using the acetic acid-induced writhing test on mice and was found to significantly reduce the number of writhes in mice by half. Following a bioassay-guided fractionation scheme, statistically significant analgesic activity was observed with both the hexane and ethyl acetate partitions. An active fraction was identified after fractionation of the hexane extract, whereas two active fractions were identified after fractionation of the ethyl acetate extract. This is the first report of the analgesic activity of K. monocephala.
Introduction
K. monocephala Rottb. (Cyperaceae) (synonym Cype rus kyllingia Endl.), or “anuang” as it is called in Filipino, is a weed found throughout the Philippines in waste places, open fields and roadsides. The plant is traditionally used for the relief of malarial chills, pruritus of the skin, and thirst due to fever and diabetes (CitationQuisumbing, 1978). In India it is used is as an anti-venom (CitationOudhia, 1999). According to CitationHoughton (1993), victims of envenomation often suffer from pain associated with the experience and it is possible that plants used as anti-venom do not inhibit the action of the venom directly but instead provide symptomatic relief due to their anti-inflammatory, tranquilizing, or analgesic properties. Thus, the in vivo analgesic activity of the extracts and fractions of the leaves of K. monocephala were assessed using the acetic acid-induced writhing test.
In contrast to other members of the genus Cyperus, which have extensively been subjected to various chemical and pharmacological studies including anti-convulsant (CitationHelliön-Ibarrola et al., 1999; CitationNgo et al., 2003; CitationBum et al., 2001), diuretic (CitationSripanidkulchai et al., 2001; CitationNgamrojanavanich et al., 2006), anti- inflammatory (CitationSeo et al., 2001), anti-diabetic (CitationRaut & Gaikwat, 2006), anti-epileptic (CitationBum et al., 1996), antimicrobial (CitationMongelli et al., 1995; CitationDuarte et al., 2005), sedative (CitationRakotonirina et al., 2001; CitationHa et al., 2002), anticariogenic (CitationYu et al., 2007), antidiarrheal (CitationUddin et al., 2006), anti-malarial (CitationWeenen et al., 1990), acetylcholinesterase inhibitory (CitationSharma & Gupta, 2007), antioxidant and free radical scavenging (CitationArdestani & Yazdanparast, 2007), anti-obesity (CitationLemaure et al., 2007) and repellant and anti-feedant (CitationAbubakar et al., 2000; CitationSayed et al., 2007) activities, only a few studies have reported on the bioactivity of K. monocephala. These include allelopathic activity of the undeground parts, which contain essential oils rich in terpenes α-cyperone, β-selinene, and α-humulene (CitationKomai & Tang, 1989), and moderate enterokinase inhibitory activity of the cell-free extracts of the tubers (CitationBhat et al., 1981).
The results of the study on a common weed in the Philippines, Kyllinga monocephala, are reported here.
Materials and methods
Plant material
Samples of K. monocephala were collected during January-March 2007 within the University of the Philippines (UP) Diliman Campus area. These were authenticated by Mr. Leonard Co, at the Dr. Jose Vera Santos Herbarium, Institute of Biology, UP Diliman, Quezon City, Philippines, where a voucher specimen was also deposited and given the specimen number 14515.
Extraction of plant material
Distilled technical grade solvents were used for the extraction of plant material. Air-dried leaves of the plant (582.66 g) were powdered and macerated with methanol (2 L). The resulting methanol extract was filtered and concentrated in vacuo using a rotary evaporator (Heidolph Instruments GmbH & Co., Schwabach, Germany). The methanol extract (95.83 g) was suspended in distilled water (100 mL) and partitioned first with hexane (600 mL) ten times then exhaustively with ethyl acetate (EtOAc). The hexane and EtOAc layers were concentrated in vacuo to give the hexane (16.69 g) and EtOAc extracts (4.87 g), respectively.
Bioassay-guided fractionation
Single-distilled hexane and EtOAc and analytical grade methanol were used for fractionation of extracts. The hexane extract (6 g) was subjected to vacuum liquid chromatography (VLC) over 85 g silica gel 60G (Merck, Darmstadt, Germany, 5-40 μm) packed on a 15.5 cm × 5.6 cm (length × internal diameter) column and fractionated by gradient elution with hexane and EtOAc with a total eluent volume of 400 mL. Fractions were eluted stepwise initially at 5% (100:0-80:20) then at 10% (80:20-0:100) gradients, and finally washed with 400 mL methanol. The EtOAc extract (4.77 g) was also subjected to VLC using 113 g silica gel 60G (Merck, 5-40 μm) packed on a 15.5 cm × 5.6 cm (length × internal diameter) column. Fractions were eluted stepwise with a total eluent volume of 250 mL initially with hexane:EtOAc (100:0-0:100 at 20% gradient) followed by EtOAc:methanol (100:0–40:60 at 10% gradient, 40:60–0:100 at 20% gradient). Fractions were pooled based on the separation of components as monitored by thin layer chromatography (TLC) using 4.8 × 5.7 cm pre-coated silica gel TLC plates (silica gel 60 F254, Merck). Spots were visualized in daylight then by spraying with vanillin/sulfuric acid solution followed by heating.
Test animals
Swiss Webster mice weighing 16-23 g obtained from the Bureau of Animal Industry (BAI), Quezon City, were used. All animals used in the study were treated in accordance with the rules and regulations of the Philippine Animal Welfare Act of 1998 (Philippine Republic Act Number 8485). The animals were acclimatized for two days in standard colony cages maintained at conditions of room temperature (27°C) and 12 h light/dark cycles. They were fed with standard diet and allowed access to water ad libitum.
Assessment of analgesic activity
The analgesic activities of the extracts and fractions were assessed using the acetic acid-induced writhing test (CitationGuevarra & Recto, 1985). Test samples were dissolved in appropriate solvents. Distilled water was used for the methanol, hexane, and EtOAc extracts, and EtOAc fractions, while corn oil was used for the hexane fractions. Animals were orally treated with the extracts 30 min prior to intraperitoneal (i.p.) injection of 0.7% (v/v) acetic acid. Three mice were treated at a time and placed in separate trays. The animals were observed for abdominal constrictions for 15 min starting 5 min after acetic acid injection. A writhe is defined as constriction of the abdomen followed by elongation of the whole body and extension of the hind limbs of the animal (CitationMiranda et al., 2001). The analgesic activity was expressed as percentage inhibition of the number of writhes in the extract-treated animals relative to the control. Ibuprofen (Skelan Forte®, United Laboratories Inc., Mandaluyong City, Philipines) was used as the positive control in the study.
Statistical analysis
The results are expressed as mean number of writhes ± Standard error of the mean (SEM). These were subjected to one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Differences with p <0.05 between test groups were considered statistically significant.
Results
Administration of the methanol extract at a dosage of 250 mg/kg reduced the number of writhes of the test mice by 50% (). The activity of K. monocephala was found to be statistically significant (P <0.040). Partitioning of the methanol extract yielded the hexane and EtOAc extracts. Both extracts were found to exhibit significant analgesic activity at a dosage of 250 mg/kg (), with the EtOAc extract being more active (71%, P <0.0005) than the hexane extract (53.5%, P <0.001). Both extracts were subjected to vacuum liquid chromatography, and the fractions obtained for each were assayed.
Figure 1. Effects of the methanol, hexane and EtOAc extracts (A), and hexane and EtOAc fractions (B and C, respectively) of K. monocephala leaves administered orally against abdominal constriction induced by 10 mL/kg i.p. injection of 0.7% acetic acid. Unless specified, samples were administered at 250 mg/kg body weight. Ibuprofen was used as positive control, given at 5.7 mg/kg. Points represent the mean number of writhes of 5 animals, with vertical bars indicating SEM. In some cases the SEM is hidden in data points. Asterisks denote significant levels when compared against the acetic acid (*) or corn oil (**) control groups, P <0.05. Percentage analgesic activities of the extracts and fractions are shown in D, where those with significant activities are indicated by asterisks.
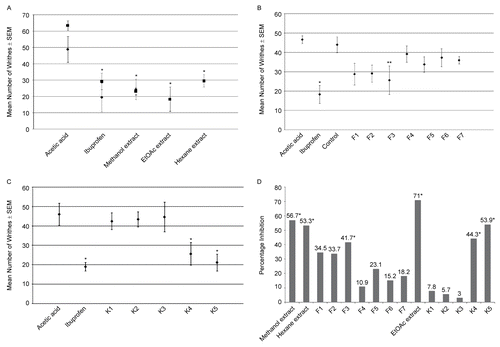
Seven fractions labeled F1-7 were obtained from the fractionation of the hexane extract. summarizes the results of the assessment of analgesic activity of these fractions. F3 exhibited the highest reduction in the number of writhes of the test mice (25.7 ± 7.4, 41.6% activity) when compared against the corn oil group (44.0 ± 4.1). Only the activity of this fraction was found to be statistically significant (P <0.023).
Five fractions labeled K1-5 were obtained from the fractionation of the EtOAc extract. The result of the assessment of the analgesic activity of these fractions is summarized in . Fraction K5 showed the largest reduction in the number of writhes of the test mice (21.2 ± 4.3, 53.9%), followed by K4 (25.6 ± 5.8, 44.3%), when compared against the acetic acid control (46 ± 5.6). Only the activities of these two fractions were found to be significant (p <0.020 for K4, and p <0.005 for K5).
Discussion
This study reports for the first time the analgesic activity of extracts from the leaves of K. monocephala, a persistent and invasive weed found throughout the Philippines (CitationQuisumbing, 1978). The observed analgesic activity of K. monocephala may explain its use as antivenom in India (CitationOudhia, 1999). A review of plants used for the treatment of envenomation (CitationHoughton & Osibogun, 1993) has shown that several of the plants included possess potent analgesic, tranquilizing or anti-inflammatory activities such as Papaver somniferum and Rauwolfia spp. Victims of envenomation often suffer from inflammation, pain or panic that the experience induces, and it has been suggested that symptomatic relief, rather than direct inhibition of venom action, is provided by some of the plants used as antivenom (CitationHoughton, 1993).
The study adds to the known bioactivities of K. monocephala (CitationBhat et al., 1981; Komai & Tang 1988) and contributes to the increasing recognition of the potential medicinal value and use of weeds (Citationde Albuquerque, 2006; CitationStepp & Moerman, 2001).
Results of the current work suggest that K. monocephala contains more than one constituent exhibiting analgesic activity. At least one of the constituents is present in fraction F3, which elutes at 50:50 EtOAc:hexane when the hexane extract is subjected to vacuum liquid chromatography. One or more are present in the EtOAc extract, which elutes at 40:60-50:50 EtOAc:methanol (K4) and 60:40-0:100 EtOAc:methanol (K5) when the EtOAc extract is subjected to vacuum liquid chromatography. Bioassay-guided isolation of these constituents is being done to identify and elucidate their structures. Future work may involve the elucidation of the mechanism of action by which it exerts its analgesic action.
Declaration of interest: E.C. Amor is grateful to the Natural Sciences Research Institute of the College of Science, UP Diliman, Quezon City for the funding support. The authors alone are responsible for the content and writing of the paper.
References
- Abubakar MS, Abdurahman EM, Haruna AK (2000): The repellant and antifeedant properties of Cyperus articulatus against Tribolium casteneum Hbst. Phytother Res 14: 281–283.
- Ardestani A, Yazdanparast R (2007): Cyperus rotundus suppresses AGE formation and protein oxidation in a model of fructose-mediated protein glycoxidation. Int J Biol Macromol 41: 572–578.
- Bhat PG, Jacob RT, Pattabiraman TN (1981): Enzyme inhibitors from plants: Enterokinase inhibitors in tubers and seeds. J Biosci 3: 371–378.
- Bum EN, Meier CL, Urwyler S, Wang Y, Herrling PL (1996): Extracts from rhizomes of Cyperus articulatus (Cyperaceae) displace [3H]CGP39653 and [3H]glycine binding from cortical membranes and selectively inhibit NMDA receptor-mediated neurotransmission. J Ethnopharmacol 54: 103–111.
- Bum EN, Schmutz M, Meyer C, Rakotonirina A, Bopelet M, Portet C, Jeker A, Rakotonirina SV, Olpe HR, Herrling P (2001): Anticonvulsant properties of the methanolic extract of Cyperus articulatus (Cyperaceae). J Ethnopharmacol 76: 145–150.
- de Albuquerque UP (2006): Re-examining hypotheses concerning the use and knowledge of medicinal plants: a study in the Caatinga vegetation of NE Brazil. J Ethnobiol Ethnomed 2: 30–39.
- Duarte MC, Figueira GM, Sartoratto A, Rehder VL, Delarmelina C (2005): Anti-candida activity of Brazilian medicinal plants. J Ethnopharmacol 97: 305–311.
- Guevarra BQ, Recto BV (1985): Phytochemical, Microbiological and Pharmacological Screening of Medicinal Plants. University of Santo Tomas, Manila, Philippines.
- Ha JL, Lee KY, Choi HC, Cho J, Kang BS, Lim JC, Lee DU (2002): Modulation of radioligand binding to the GABAA-benzodiazepine receptor complex by a new component of Cyperus rotondus. Biol Pharm Bull 25: 128–130.
- Helliön-Ibarrola MC, Ibarrola DA, Montalbetti Y, Villalba D, Heinichen O, Ferro EA (1999): Acute toxicity and general pharmacological effect on central nervous system of the crude rhizome extract of Kyllinga brevifolia Rottb. J Ethnopharmacol 66: 271–276.
- Houghton PJ (1993): In vitro testing of some West African and Indian plants used to treat snakebites. Actes du 2eColloque Européen d’Ethnopharmacologie et de la 11e Conférence Internationale d’Ethnomédecine, Heidelberg, p. 24–27.
- Houghton PJ, Osibogun IM (1993): Flowering plants used against snakebite. J Ethnopharmacol 39: 1–29.
- Komai K, Tang CH (1989): Chemical constituents and inhibitory activities of essential oils from Cyperus brevifolius and C. kyllingia. J Chem Ecol 15: 2171–2176.
- Lemaure B, Touché A, Zbinden I, Moulin J, Courtois D, Macé K, Darimont C (2007): Administration of Cyperus rotundus tubers extract prevent weight gain in obese Zucker rats. Phytother Res 21: 724–730.
- Miranda HF, Sierralta F, Pinardi G (2001): An isobolographic analysis of the adrenergic modulation of diclofenac antinociception. Anesth Analges 93: 430–435.
- Mongelli E, Desmarchelier C, Coussio J, Ciccia G (1995): Antimicrobial activity and interaction with DNA of medicinal plants from the Peruvian Amazon region. Rev Argent Microbiol 27: 199–203.
- Ngamrojanavanich N, Manakit S, Ponrpakakul S, Petsom A (2006): Inhibitory effects of selected Thai medicinal plants on Na+, K+-ATPase. Fitoterapia 77: 481–483.
- Ngo Bum E, Rakotonirina A, Rakotonirina SV, Herrling P (2003): Effects of Cyperus articulatus compared to effects of anticonvulsant compounds on the cortical wedge. J Ethnopharmacol 87: 27–34.
- Oudhia P (1999): Medicinal weeds in the rice fields of Chattisgarh, India. IRRN 24: 40.
- Quisumbing E (1978): Medicinal Plants of the Philippines. Quezon City, Philippines, Katha Publishing, pp. 116–117.
- Rakotonirina VS, Bum EN, Rakotonirina A, Bopelet M (2001): Sedative properties of the decoction of the rhizome of Cyperus articulatus. Fitoterapia 72: 22–29.
- Raut NA, Gaikwad NJ (2006): Antidiabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia 77: 585–588.
- Sayed HM, Mohamed MH, Farag SF, Mohamed GA, Proksch P (2007): A new steroid glycoside and furochromones from Cyperus rotundus L. Nat Prod Res 21: 343–350.
- Seo WG, Pae HO, Oh GS, Chai KY, Kwon TO, Yun YG, Kim NY, Chung HT (2001): Inhibitory effects of methanol extract of Cyperus rotundus rhizomes on nitric oxide and superoxide productions by murine macrophage cell line, RAW 264.7 cells. J Ethnopharmacol 76: 59–64.
- Sharma R, Gupta R (2007): Cyperus rotundus extract inhibits acetylcholinesterase activity from animal and plants as well as inhibits germination and seedling growth in wheat and tomato. Life Sci 80: 2389–2392.
- Sripanidkulchai B, Wongpanich V, Laupattarakasem P, Suwansaksri J, Jirakulsomchok D (2001): Diuretic effects of selected Thai indigenous medicinal plants in rats. J Ethnopharmacol 75: 185–190.
- Stepp JR, Moerman DE (2001): The importance of weeds in ethnopharmacology. J Ethnopharmacol 75: 19–23.
- Uddin SJ, Mondal K, Shilpi JA, Rahman MT (2006): Antidiarrhoeal actvity of Cyperus rotundus. Fitoterapia 77: 134–136.
- Weenen H, Nkunya MH, Bray DH, Mwasumbi LB, Kinabo LS, Kilimali VA (1990): Antimalarial activity of Tanzanian medicinal plants. Planta Med 56: 368–370.
- Yu HH, Lee DH, Seo SJ, You YO (2007): Anticariogenic properties of the extract of Cyperus rotundus. Am J Chin Med 35: 497–505.
