Abstract
The present study assessed the different biological activities of the methanol extract of Thymus serpyllum L. (Labiateae) using bench top bioassays including brine shrimp cytotoxic, antitumor, antimicrobial, and phytotoxicity bioassays. Plant extract showed highly significant (ED50 466 ppm < 1000 ppm) impact on percentage death of brine shrimp. Agrobacterium tumefaciens (At-10)-induced tumors in potato disc tissue were inhibited (P < 0.05) significantly by methanol extract with no activity in antibacterial assay against Agrobacterium tumefaciens. EC50 value remained as 28.7 and 261.1 ppm for 12 days and 21 days of incubation respectively. Moderate antifungal activity (41-51%) was seen against five strains of fungus and no activity against any of the bacterial strain tested. Phytotoxicity to Lemna minor L. (P < 0.05) and radish seed germination and growth (P < 0.05) was observed at higher concentrations of the plant extract.
Introduction
The genus Thymus (Labiateae), widely distributed in temperate zones, comprises about 350 species worldwide (CitationDemissew, 1993) and one [Thymus serpyllum L. (wild thyme)] grows naturally in Pakistan. It is known as “Ben ajvain” and “Tumuro” in local languages. Wild thyme is commonly used by local people for various functions such as antiseptic, anthelmintic, carminative, expectorant, sedative, and tonic (CitationKarnick, 1994; CitationPorchezhian et al., 2006). In a study from Turkey, ice produced from wild thyme hydrosol has shown preservative effect against fish storage (Oral et al., 2008), but still little scientific work determining antimicrobial and antioxidant properties of wild thyme has been reported (CitationDursun et al., 2003). There is a need to further evaluate it scientifically to recognize the hidden potential of this plant. The present study, therefore, screened it for various diverse activities by using different bioassays.
Screening programs for biologically active natural products require the right bioassays. They must be simple, inexpensive, rapid, and sensitive enough to detect active principles which are generally present only in small concentrations in crude extracts. Their selectivity should be such that the number of false positives is reasonably small (CitationHostettmann et al., 1995). A number of bench-top assays including brine shrimp cytotoxicity assay, antitumor assay potato disc assay, antifungal assay, antibacterial assay, radish seed phytotoxicity assay, and Lemna assay can be used as major prescreening assays in this regard.
Brine shrimp assay is a rapid and inexpensive assay used routinely for detection of cytotoxic effects of any compound (CitationAhmed et al., 2007; CitationShaheen et al., 2007; CitationHanif et al., 2007). A positive correlation between brine shrimp toxicity and KB (human nasopharyngeal carcinoma) has been reported (CitationMcLaughlin & Rogers, 1998). CitationGalsky et al. (1980) demonstrated that inhibition of crown gall (a neoplastic disease of plants induced by specific strain of Gram negative bacterium, Agrobacterium tumefaciens) initiation on potato discs showed the apparent agreement with the compounds and plant extracts known to be active in 3PS (in mouse leukemia) antitumor assay. CitationCoker et al. (2002) demonstrated that Agrobacterium tumefaciens induced potato disc assay was an effective indicator of tumor activity regardless of mechanism of action.
Antimicrobial assay is another rapid technique to discover new compounds that are effective against fungi and bacteria (CitationAnsari et al., 2005; CitationHanif et al., 2007). Inhibition of hyphal growth is an end point for an antifungal drug assay, especially during early drug discovery (CitationBrown & Gow, 1999). For phytotoxic activity studies radish seeds have been used previously for evaluation of plant extracts (CitationMannan et al., 2007; CitationInayatullah et al., 2007). Radish seeds are easily available, and assay does not require special training. Similarly, Lemna bioassay is also used for quick measurement of phytotoxicity of materials.
Materials and methods
Plant material was collected from Upper Mahudand Lake (altitude of 2362 m), Kalam valley, District Swat, Pakistan by one of the authors (Abdul Mannan) and identified by Mir Ajab Khan of the Department of Plant Sciences, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, Pakistan. A voucher specimen was deposited in the herbarium (herbarium abbreviation ISL, voucher specimen no. 299). Fresh aerial parts dipped in methanol (HPLC grade) were used for extraction of secondary metabolites. After 4 weeks, methanol was separated, filtered twice, and completely evaporated at room temperature. Residues obtained were used to check different activities of extracted secondary metabolites.
Brine shrimp toxicity assay
The method used for brine shrimp lethality bioassay was as reported by CitationInayatullah et al. (2007). For this experiment, extract was used in three methanol dilutions of 1000, 100, and 10 ppm. Each dilution was taken in small vials. After methanol evaporation, residues were resolubilized in sea salt water (sea salt was obtained from Sigma). Ten shrimps were added to each vial and final volume was raised up to 5 mL with sea salt water. The experiment was repeated three times. The vials were maintained under illumination at room temperature of 25°C and survivors were counted after 24 h. The resulting data were analyzed by Probit analysis for the determination of ED50 value for the extract.
Antitumor assay
The potato disc method was used for antitumor activity of plant extract as reported by CitationInayatullah et al. (2007). A 48 h bacterial culture of At 10 strain of Agrobacterium tumefaciens was used in this experiment. An inoculum with three concentrations of test samples (1000, 100, and 10 ppm) was prepared containing bacterial culture and distilled water. Red-skinned potatoes were surface sterilized in 0.1% HgCl2 solution, washed three times with autoclaved distilled water, and cut into 2 mm thick discs of 8 mm diameter. Autoclaved agar solution (1.5%) was poured into Petri plates and solidified. Ten discs were placed on the agar surface of each Petri plate, and 50 μL of inoculum was placed on the surface of each disc. The plates were sealed with parafilm to avoid contamination and moisture loss and were incubated in the dark at 28°C. The experiment was carried out in strict aseptic condition and repeated three times. After 21 days of incubation, discs were stained with Lugol solution (10% KI and 5% I2) and tumors were counted on each disc by using dissecting microscope. Percentage tumor inhibition was determined. Tumor inhibition of 20% was considered significant. EC50 value was calculated by graph method. Data were statistically analyzed by using ANOVA.
Antibacterial assay of 1000 ppm methanol extract was also performed against At-10 strain of Agrobacterium tumefaciens to check its activity against this strain.
Antifungal assay
The agar tube dilution method was used for antifungal activity of the plant extract as reported by CitationHanif et al. (2007). Nine strains of fungus were used which were Mucor species, Aspergillus niger, Aspergillus flavus, Aspergillus fumigatus, Fusarium moniliforme, Alternaria species, Fusarium solani, Candida albicans, and Candida glabarata. Methanol extract (24 mg/mL) as test sample, standard (12 mg/mL) of terbinafine and clotrimazole as positive control and pure DMSO as negative control were used on seven-day-old fungus culture. Fungus growth was determined by measuring linear growth (mm) on media and growth inhibition was calculated with reference to negative control.
Below 40% inhibition, low activity; 40-60% inhibition, moderate activity; 60-70% inhibition, good activity; 70% and above, significant activity.
Antibacterial assay
The agar-well diffusion method was used to screen for antibiotic activity (CitationPerez et al., 1990). Eight strains of bacteria were used in which three were Gram-positive [Staphylococcus aureus (ATCC 6538), Bacillus subtilis (ATCC 6633) and Micrococcus luteus (ATCC 10240)] and five were Gram-negative [Escherichia coli (ATCC 15224), Salmonella setubal (ATCC 19196), Pseudomonas picketii (ATCC 49129), Bordetella bronchiseptica (ATCC 4617), and Enterobacter aerogens (ATCC 13048)]. These organisms were maintained on nutrient agar medium at 4°C; methanol extract (25 mg/mL) as test sample, standard (2 mg/mL) of roxithromycin and cefixime-USP as positive control and pure DMSO as negative control were used.
Phytotoxicity assays
Radish seed bioassay
The experiment was conducted according to the standard procedure described by CitationInayatullah et al. (2007). Two different parameters, namely percentage of seed germination and root length were observed. For seed germination, 5 mL each concentration of 7.5 and 1 g/L of methanol extracts was used in separate Petri plates having sterilized filter paper. Methanol was evaporated and 5 mL distilled water was added. In this experiment, 100 seeds were surface sterilized with 0.1% HgCl2 and placed in each plate. Plates were properly sealed with parafilm to avoid contamination and moisture loss and incubated at 25°C in dark. In control 5 mL methanol was used and evaporated. Seed germination was observed on days 1, 3, and 5. For root length, 5 mL each dilution of 10 and 1 g/L of methanol extracts were used in separate Petri plates by using the same methodology as above. Twenty radish seeds were placed in each Petri plate. Root length was measured on days 1, 3, and 5 of incubation. Each experiment was repeated three times and results were statistically analyzed using ANOVA and Duncan’s multiple range test.
Lemna bioassay
Standard Lemna bioassay method for inhibitors and promoters of plant growth reported by CitationRahman et al. (2001) is used in this experiment. Lemna minor L. plants were used in this experiment grown in E-medium (CitationRahman et al., 2001), and the experiment was performed in working E-medium (10% E-medium, 90% distilled H2O). In this experiment we used methanol extract (0.02 g/L) as a test sample, paraquat (0.02 g/L) as positive control, and methanol as negative control. Three flasks were inoculated by using 10, 100, and 1000 μL of the stock solution of each sample as well as controls. Methanol was evaporated and residue was resolubilized with 40 mL working E-medium. In this way final concentration of each sample in flasks was 10, 100, and 1000 ppm, respectively. Ten plants of Lemna minor, each containing a rosette of two fronds were also placed in every flask. The flasks were placed in a growth chamber for 7 days. Plants were examined daily during the incubation period, and the number of fronds per flask was counted. The percentage inhibition for each concentration was determined by using the following formula:
where ns = numbers of fronds in sample and nc = number of fronds in control. Growth inhibition above 70% was considered significant. Data were statistically analyzed by using ANOVA.
Results and discussion
On the basis of previous reports suggesting that methanol is a better solvent than water or chloroform, methanol was selected for preparation of the plant extract. CitationChandrasekaran and Venkatesalu (2004) proposed that the methanol extract has better activity than that of aqueous extract which may be due to solubility of the different constituents in different solvents. CitationVlachos et al. (1996) also concluded that methanol was the most effective solvent for the extraction of antibacterial compounds from the selected seaweeds.
Brine shrimp assay
Methanol extract of Thymus serpyllum L. tested against brine shrimp exhibited highly significant (ED < 1000 ppm) effect on prcentage death. The extract showed ED50 value of 466 ppm. Although this ED50 value is higher than that of Acer oblongifolium reported by CitationInayatullah et al. (2007) it is better than another scientific report from Brazil, in which 60 medicinal plant species were evaluated for their cytotoxicity to brine shrimp and only 10% plants showed ED50 < 1000 ppm (CitationMaria et al., 2000), while CitationJacques et al. (2003) screened 226 plant extracts against larvae of brine shrimp and identified several cytotoxic plant species.
Antitumor assay
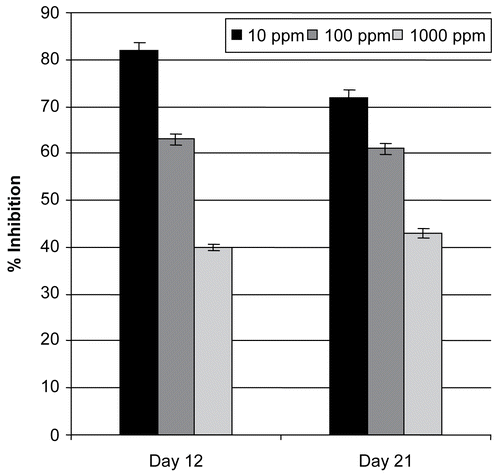
This test discriminates between active and inactive compounds in predicting their in vivo antitumor activity, but not necessarily in a direct linear fashion (CitationMcLaughlin et al., 1993; CitationUllah et al., 2007). The effect of different dilutions of methanol extract, incubation period and interaction between different plant extract dilution and incubation period had highly significant impact (P < 0.05) on tumor inhibition. Percentage inhibitions at 1000, 100, and 10 ppm dilution were 82%, 63%, and 40% after 12 days, and 72%, 61%, and 43%, after 21 days of incubation period respectively (). EC50 value remained as 28.7 and 261.1 ppm for 12 days and 21 days of incubation, respectively. The inhibition of crown gall tumors on discs of potato had shown an apparent correlation with 3PS antitumor compounds and plant extracts (CitationFerrigini et al., 1982). CitationMcLaughlin and Rogers (1998) suggested that crown gall tumors on potato discs could routinely be employed as a comparatively rapid, inexpensive, safe, animal-sparing, and statistically reliable prescreen for in vivo antitumor activity. Many scientists have suggested that tumor inhibition values of more than 20% in two or more independent assays may be considered worthy for further investigation (CitationFerrigini et al., 1982). CitationMcLaughlin and Rogers (1998) used this assay to detect and isolate several dozen novel antitumor compounds from various plant species. Plant extracts that were active during this test were examined for the ability to affect bacterial growth of Agrobacterium tumefaciens by the standard agar-well diffusion method as performed by CitationPerez et al. (1990). Results showed that the methanol extract of wild thyme had no effect on the viability of the bacterium.
Antimicrobial assay
Antifungal assay
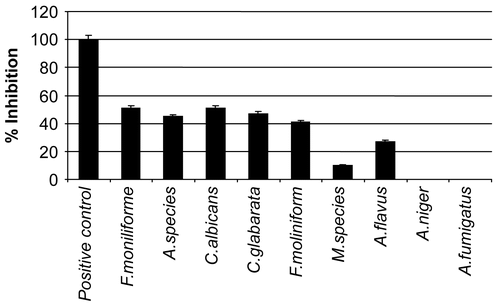
The methanol extract showed an impact on percentage inhibition of test fungi. Moderate activity was seen against Fusarium moniliforme, Alternaria species, Candida albicans, Candida glabarata and Fusarium solani while low activity against Mucor species and Aspergillus flavus (). No effect on linear growth of Aspergillus niger and Aspergillus fumigatus was observed but spore formation inhibition was detected in these fungi. Our results support a previous report that the essential oil of Thymus serpyllum L. has potential to control fungus on citrus fruits (CitationPlaza et al., 2004).
Antibacterial assay
Methanol extracts tested against the eight strains of bacteria showed no activity against any of eight strains tested. In a recent study, Nebahat et al. (2008) has described antimicrobial activity of ice produced from wild thyme hydrosol indicating its importance in fish storage. The difference in results could be an environmental effect or the effect of the solvent used for preparation of the extract.
Radish seed bioassay
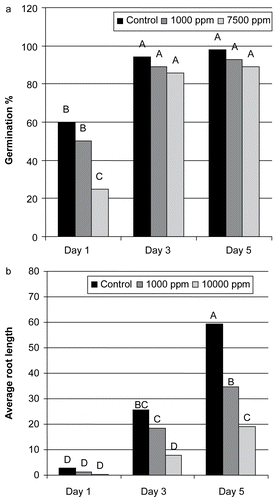
Radish seeds germination and root length was measured on day 1, 3, and 5 of dark treatment. Different dilutions of the plant extract, incubation period and interaction of different plant extracts with incubation period had a highly significant (P < 0.05) effect on radish seeds germination as well as root length (). These results showed that the extract had a dose-dependent phytotoxic effect which increased with increase in incubation period. These results are in agreement with a previous study where CitationDudai et al. (1999) reported that seed germination of several species was strongly inhibited by essential oil from thyme when applied at concentrations of 20 to 80 ppm.
Lemna bioassay for inhibitors and promoters of plant growth
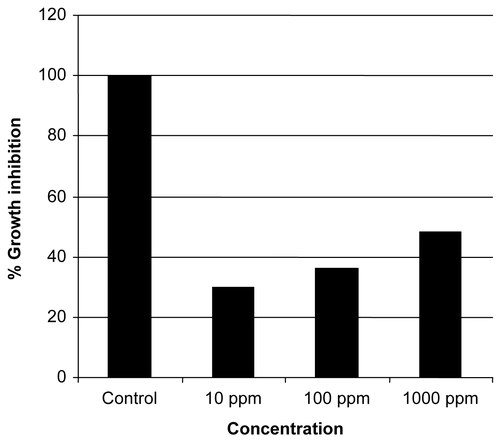
In this assay three dilutions of methanol extract were used and percentage growth inhibition was observed after 7 days that is highly significant (P < 0.05). Mean value of percentage growth inhibition of Lemna minor was 100% in positive control while extract at 10, 100, and 1000 ppm showed 30%, 36%, and 48% growth inhibition respectively (). These results support the phytotoxic potential of this plant extract as showed by radish seed assay too. Previously, CitationTworkoski (2002) reported that essential oil from thyme was the most phytotoxic and caused electrolyte leakage resulting in cell death. These data suggest that the plant extract could be used to inhibit the emergence of weeds.
Conclusion
The obtained findings of Thymus serpyllum L. showing significant activity in brine shrimp lethality assay and antitumor assay provide the evidence for a very strong positive correlation between these two assays and prediction of some valuable anticancerous principles of this plant extract. Furthermore, significant activity in phytotoxicity assays suggests that there is a need to purify the active contents of this plant, which can open a new horizon in the field of herbicides.
Declaration of interest: The authors are thankful to the Higher Education Commission of Pakistan for providing funds throughout the research project. The authors alone are responsible for the content and writing of the paper.
References
- Ahmed MS, Hussain M, Hanif M, Mirza B (2007): Synthesis chemical characterization and biological activities screening for cytotoxicity and antitumor activity of organotin (IV). Derivatives of 3,4-methylenedioxy 6-nitophemylpropenoic acid. Molecule 12: 2348–2363.
- Ansari FL, Nazir S, Nourine H, Mirza B (2005): Combinatorial synthesis and antibacterial evaluation of an indexed chalcone library. Chem Biodivers 2: 1656–1664.
- Brown AJP, Gow NAR (1999): Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol 7: 333–338.
- Coker PS, Redecke J, Guy C, Camper ND (2002): Potato disc tumor induction assay: A multiple mode of drug action assay. Phytomedicine 10: 133–138.
- Chandrasekaran M, Venkatesalu V (2004): Antibacterial and antifungal activity of Syzygium jambulanum seeds. Plant Physiol 91: 105–108.
- Demissew S (1993): The Genus Thymus (Labiatae) in Ethiopia. Opera Bot 121: 57–60.
- Dudai N, Poljakoff-Mayber A, Mayer AM, Putievsky E, Lerner HR (1999): Essential oils as allelochemicals and their potential use as bioherbicides. J Chem Ecol 25: 1079–1089.
- Dursun N, Liman N, Ozyazgan I, Gunes I, Saraymen R (2003): Role of thymus oil in burn wound healing. J Burn Care Rehabil 24: 395–339.
- Ferrigini R, Putnam JE, Andersen B, Jacobsen LB, Nichols DE, Moore DS, McLaughlin JL (1982): Modification and evaluation of the potato disc assay and antitumor screening of Euphorbiaceae seeds. J Nat Prod 45: 679–686.
- Galsky AG, Wilsey JP, Powell RG (1980): Crown gall tumor disc bioassay, a possible aid in detection of compounds with antitumor activity. Plant Physiol 65: 184–185.
- Hostettmann K, Marston A, Wolfender JL (1995): Strategy in the search for new biologically active plant constitutents, in: Hostettmann K, Marston A, Maillard M, Hamburger M, eds, Phytochemistry of Plants Used in Traditional Medicine, Proceedings of the Phytochemical Society of Europe. Oxford, Oxford Science Publications, pp. 18–45.
- Hanif M, Hussain M, Ali S, Bhatti MH, Ahmed MS, Mirza B, Evans HS (2007): Synthesis, spectroscopic investigation, crystal structure, and biological screening, including antitumor activity, of organotin (IV) derivatives of piperonylic acid. Turk J Chem 31: 349–361.
- Inayatullah S, Irum R, Rehman A, Chaudhary MF, Mirza B (2007): Biological evaluation of some selected plant species of Pakistan. Pharm Biol 45: 397–403.
- Jacques EL, Pohlit AM, Nunomura SM, Da AC, Mustafa EV, Reis SK, Alecrim AM, Brito BR, De CS, Finney EK, Oliveira ED, Santos KD, Pereira LC, Castro LD, Rosha LF, Andrade MM, Henrique MC, Santos MD, Souza PD, Silva SG (2003): Screening of plants in Amazone state for lethality towards brine shrimp. Acta Amaz 33: 93–104.
- Karnick CR (1994): Pharmacopoeial Standards of Herbal Plants. Indological and Oriental Publishers, Delhi, pp. 337–338.
- Mannan A, Shaheen N, Rehman A, Arshad W, Zia M, Mirza B (2007): Production and release of allelopathic compounds by Artemisia hairy root cultures. Allelopathy J 20: 379–386.
- McLaughlin JL, Chang CJ, Smith DL (1993): Simple bench-top bioassay (brine shrimp and potato discs) for the discovery of plant antitumor compounds, in: Kinghorn AD, Balandrin MF, eds, Human Medicinal Agents from Plants. ACS Symposium Series 534, Washington DC, Oxford University Press, pp. 112–137.
- McLaughlin JL, Rogers LL (1998): The use of biological assays to evaluate botanicals. Drug Inf J 32: 513–524.
- Maria T, Silva AF, Brandao M, Mesquita TS, Fatima ED, Junior AS, Zani CL (2000): Biological screening of Brazilian medicinal plants. Mem Inst Oswaldo Cruz 95: 367–373.
- Oral N, Murat G, Leyla V, Abamuslum G (2008): Application of antimicrobial ice for extending shelf life of fish. J Food Prot 71: 218–222.
- Perez C, Pauli M, Bazerque P (1990): An antibiotic assay by the well agar method. Acta Biol Med Experimentalis 15: 113–115.
- Plaza P, Torres R, Usall J, Lamarca N, Vinasa I (2004): Evaluation of the potential of commercial post-harvest application of essential oils to control citrus decay. J Horticult Sci Biotech 6: 935–940.
- Porchezhian E, Dobriyal RM, Narayana DB (2006): Indian spices: An overview of therapeutic applications, in: Ravindran PN, Babu KN, Shiva KN, Kallupurackal JA, eds, Advances in Spices Research, History and Achievements of Spices Research in India Since Independence. Agrobios Publications, Delhi, pp. 139–168.
- Rahman A, Choudhary MI, Thomsen WJ (2001): Bioassay Techniques for Drug Development. Harwood Academic Publisher, Texas, USA, pp. 65–66.
- Shaheen F, Badshah A, Gielen M, Dusek M, Fejfarova K, Vos DD, Mirza B (2007): Synthesis, characterization, antibacterial and cytotoxic activity of new palladium (II) complexes with dithiocarbamate ligands: X-ray structure of bis(dibenzyl-1-S:S´-dithiocarbamate) Pd(II). J Organomet Chem 692: 3019–3026.
- Tworkoski T (2002): Herbicide effects of essential oils. J Weed Sci 4: 425–431.
- Ullah A, Ansari FL, Haq I, Nazir S, Mirza B (2007): Combinatorial synthesis, lead identification, and antitumor study of a chalcone-based-positional scanning library. Chem Biodivers 4: 203–214.
- Vlachos V, Critchely AT, Von HA (1996): Establishment of a protocol for testing antimicrobial activity in Southern African macro algae. Microbios 88: 115–123.