Abstract
Nerve growth factor (NGF) is one of the well characterized regulators of galanin expression. NGF deprivation can induce galanin expression in dorsal root ganglion (DRG) neurons. Galanin is recognized as one of the DRG injury markers. Norepinephrine (NE) activates alpha-adrenoreceptors usually expressed in sympathetic neuronal membrane. It has been shown that functional alpha-adrenoreceptors are also expressed in primary sensory neurons. Whether NGF association with NE influences the galanin expression in DRG neurons remains unknown. In the present study, we have evaluated the possible regulation of galanin mRNA or galanin peptide expression by co-administration of NGF and NE in primary cultures of rat DRG neurons. After four days incubation with NGF and/or NE in primary cultures of rat DRG neurons, NGF inhibited and NE promoted galanin mRNA and galanin peptide expression as compared with that in control DRG neuron cultures at the same time point. NGF also inhibited the elevation of galanin expression induced by NE when the cultures were incubated with NGF and NE together. NGF but not NE stimulated neurite outgrowth. The results indicate that NE may affect galanin expression rather than stimulating neurite regeneration, whereas NGF stimulates neurite outgrowth and inhibits galanin expression in the absence or presence of NE on cultured DRG neurons.
Introduction
Nerve growth factor (NGF) is a major regulator of inflammatory and homeostatic pain states, influencing both sensory neuron phenotype and physiologic responses (CitationPetruska & Mendell, 2004). It is known that NGF is one of the well characterized regulators of galanin expression (CitationThippeswamy et al., 2007). NGF regulates galanin expression occurring in dorsal root ganglion (DRG) cells after intravesical resiniferatoxin (RTX) application (CitationAvelino et al., 2002). NGF deprivation can induce galanin expression in DRG neurons (CitationKato et al., 2002; CitationThippeswamy et al., 2007). Axonal transection of adult sensory neurons leads to a decrease in the content of target-derived NGF and to dramatic changes in the expression of several neuropeptides including galanin (CitationShadiack et al., 2001).
The 29-30 amino acid neuropeptide galanin is present in a small population of DRG neurons under normal conditions but is strongly up-regulated after nerve injury (CitationZvarova et al., 2004; CitationWilson-Gerwing & Verge, 2006). Axotomy of sensory neurons in DRG increases protein and mRNA levels for galanin (CitationShadiack et al., 2001). Systemic or topical administration of vanilloid substances has been shown to up-regulate galanin in primary sensory neurons (CitationAvelino et al., 2002). There is evidence that this up-regulated galanin has trophic actions, for example promoting neurite outgrowth as well as influencing pain processing (CitationLandry et al., 2005). Furthermore, galanin is recognized as one of the DRG injury markers (CitationShortland et al., 2006).
Norepinephrine (NE) is a classical neurotransmitter which plays a key role in the neuronal response to environmental influences through activation of alpha-adrenoreceptors expressed in sympathetic neurons (CitationHein, 2006). Interestingly, functional alpha- adrenoreceptors are also expressed in primary sensory neurons and regulate neurogenic inflammation and nociceptive responses (CitationPertovaara, 2006; CitationTrevisani et al., 2007). Also, alpha-adrenoreceptors agonists have been reported to increase neurogenic inflammatory responses mediated by capsaicin-sensitive sensory neurons (CitationAndersson, 2000; CitationMilani & Djavan, 2005). Whether NGF association with NE influences the galanin expression in DRG neurons remains unknown. In the present study, we have evaluated the possible regulation of galanin mRNA or galanin peptide expression by co-administration of NGF and NE in primary cultures of rat DRG neurons.
Materials and methods
DRG cell culture preparations
DRG was dissected out from 224 embryonic 15-day-old Wistar rats (male or female not restricted). The animals were obtained from the Experimental Animal Center of Shandong University of China. DRG prior to establishment in culture was digested with 0.25% trypsin (Sigma, St. Louis, MO) in D-Hanks solution at 37°C for 10 min and centrifuged for 5 min at 1 × 103 rpm. The supernatants were removed and the pellets were resuspended in Dulbecco’s modified Eagle medium with F-12 supplement (DMEM/F-12) media (Gibco, Grand island, NY) and triturated using a sterile modified Pasteur glass pipette. Cells were then filtered using a 130 μm filter followed by counting. Dissociated DRG cells were then cultured in flasks (Costar, Corning, NY) for detecting expression of mRNA for galanin by RT-PCR and galanin peptide by Western blot or 24-well clusters (Costar, Corning NY) for measuring the neurite length. DRG cells were plated at a density of 5 × 105 cells/mL in flasks which were precoated with poly-L-lysine prior to plating and at 1 × 105 cells/well in clusters which contained a coverslip precoated with poly-L-lysine in each well. Then DRG cells were cultured in culture media at 37°C with 5% CO2 for 24 h and then maintained in culture media containing cytarabine (ara-C) (5 μg/mL) for another 24 h to inhibit growth of non-neuronal cells, and then cultured in culture media for another 4 days with media change every 2 days. The composition of the culture media is D-MEM/F-12 (1:1) supplemented with 5% fetal bovine serum, 2% B-27 supplement (Gibco, Grand island, NY), insulin (0.25 μg/mL, Sigma, St. Louis, MO), l-glutamine (0.1 mg/mL, Sigma, St. Louis, MO), penicillin (100 U/mL), and streptomycin (100 μg/mL).
Exposure of NGF and NE on DRG neurons
DRG cell cultures were prepared as described above and allowed to grow processes for 2 days, followed by the addition of NGF (10 ng/mL) and/or NE (10−4 mol/L). Cultures were then incubated for an additional 4 days with media change every 2 days. The culture media contained NGF (10 ng/mL), NE (10−4 mol/L) or NGF (10 ng/mL) plus NE (10−4 mol/L) during the 4 days incubation. DRG neurons were cultured continuously in culture media for 6 days as control.
RNA extraction and RT-PCR
The mRNA levels of galanin were analyzed by RT-PCR after treatment with NGF and/or NE on DRG neurons. The expression of β-actin was also determined as an internal control. Total DRG cell RNA of each flask was isolated by TRIzol. cDNA synthesis was performed with M-MLV reverse transcriptase. The gene-specific primers were synthesized by use of the published cDNA sequences for galanin and β-actin. The synthetic oligonucleotide primer sequences for galanin and β-actin were as follows: galanin 5’-ATG CCA ACA AAG GAG AAG AG -3’ (upper primer) and 5’- AGG TGC AAG AAA CTG AGA AA -3’ (lower primer); β-actin 5’-ATC ATG TTT GAG ACC TTC AAC-3’ (upper primer) and 5’-CAT CTC TTG CTC GAA GTC CA-3’ (lower primer).
The predicted size of the amplified galanin and β-actin DNA products were 224 bp and 317 bp, respectively.
PCR amplification was performed for 35 cycles. The cycle profile included denaturation for 45 s at 94°C, annealing for 60 s at 53°C, and extension for 45 s at 72°C. PCR was performed within the range that demonstrates a linear correlation between the amount of cDNA and the yield of PCR products.
The amplified products were analyzed by standard agarose gel electrophoresis and stained with ethidium bromide, visualized by a UV transilluminator and photographed. The photographs were scanned and the electrophoresis gel images were analyzed quantitatively by using a Totallab image analysis software. The levels of galanin mRNA were expressed as the ratio of the gene to β-actin.
Western blot analysis for galanin peptide expression
Galanin peptide expression was analyzed by Western blot. Fresh cultured DRG neurons after treatment with NGF and/or NE were homogenized in 10 mmol/L Tris homogenization buffer (pH 7.4) with protease inhibitors (Sigma). The samples were centrifuged at 12,000 rpm for 20 min and the supernatant collected for Western blot. After determining the protein concentrations of the supernatants (BCA method, standard: BSA), 50 μg protein of each sample was loaded onto the 8% SDS gel, separated by electrophoresis and transferred to polyvinylidene fluoride (PVDF) membrane. The membranes were incubated with goat anti-rat galanin polyclonal IgG (1:1000, Santa Cruz Biotechnology). After being washed three times for 10 min with washing solution, the membranes were incubated with donkey anti-goat IgG-HRP (1:1000, Santa Cruz Biotechnology). The immunoreactive bands were visualized by an ECL Western blotting detection kit (Pierce Biotech) on light sensitive film.
Fluorescent labeling and neurite measurement
After 4 days treated with NGF and/or NE, dissociated DRG cultures were processed for fluorescent labeling. Briefly, the cells on coverslips were fixed in 4% phosphate-buffered paraformaldehyde (pH 7.4) for 20 min at 4°C. The samples were incubated with mouse monoclonal anti-microtubule associated protein 2 (MAP2) (Chemicon) 1:200 overnight at 4°C and then incubated with donkey anti-mouse conjugated Cy3 diluted 1:200 for 1 h at 4°C. The coverslips were mounted with Vectashield anti-fade mounting media (Vecto Laboratories) and stored at 4°C until quantitative analysis. Five samples were selected and 20 neurons were measured per coverslip. One hundred neurons were totally measured in each group (n = 100). Total neurite length per neuron and neurite number per neuron were measured, using computer software (laser scanning microscope LSM 510 Version 2.5 SP2), in an unbiased manner by random focusing.
Statistical analysis
Data of “galanin expression” are expressed as mean ± SD and statistical analysis was evaluated with SPSS software by one-way ANOVA followed by the Student-Newman-Keuls test for significance to compare the differences among various groups. Data of “neurite number per neuron” and “total neurite length per neuron” are presented as median, range, and quartile, and analyzed using non-parametric methods (Mann-Whitney test) in SPSS. Significance was accepted at P < 0.05.
Results
Galanin mRNA and galanin peptide expression with NGF and/or NE treatment
The effects of NGF and/or NE treatment on galanin mRNA and galanin peptide expression in cultured DRG neurons were investigated by RT-PCR () and Western blot (), respectively. NE promoted galanin mRNA and galanin peptide expression in cultured DRG neurons. NGF inhibited galanin mRNA and galanin peptide expression as compared with control at the same time point. NGF also inhibited NE-induced the elevation of galanin mRNA and galanin peptide expression.
Figure 1. Effects of NGF and/or NE on galanin mRNA expression in primary cultured DRG neurons. Galanin and β-actin mRNA was analyzed by RT-PCR. Lane 1: Normal control (galanin mRNA/β-actin mRNA = 0.3209 ± 0.0433). Lane 2: Exposure of NE (galanin mRNA/β-actin mRNA = 0.4213 ± 0.0198). Lane 3: Exposure of NGF (galanin mRNA/β-actin mRNA = 0.2773 ± 0.0247). Lane 4: Exposure of NGF and NE (galanin mRNA/β-actin mRNA = 0.3692 ± 0.0347). Bar graphs with error bars represent mean ± SD (n = 5). *P < 0.05 versus control, **P < 0.05 versus control, #P < 0.05 versus NE, ΔP < 0.0001 versus NGF.
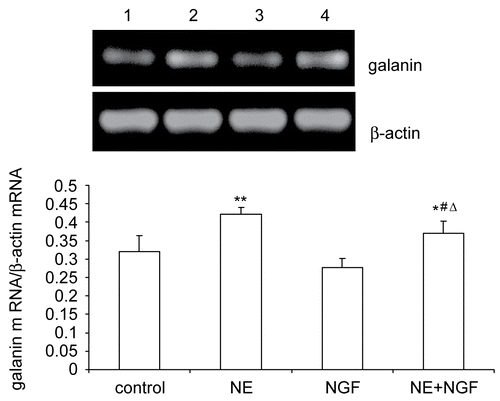
Figure 2. Effects of NGF and/or NE on galanin peptide expression in primary cultured DRG neurons. Galanin peptide expression was analyzed by Western blot. Lane 1: Normal control (galanin/β-actin = 0.2168 ± 0.0414). Lane 2: Exposure of NE (galanin/β-actin = 0.3454 ± 0.0395). Lane 3: Exposure of NGF (galanin/β-actin = 0.1484 ± 0.0454). Lane 4: Exposure of NGF and NE (galanin/β-actin = 0.2807 ± 0.0441). Bar graphs with error bars represent mean ± SD (n = 5). *P < 0.05 versus control, **P < 0.001 versus control, #P < 0.05 versus NE, ΔP < 0.001 versus NGF.
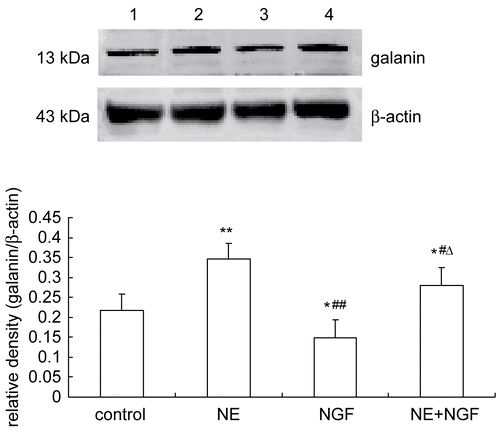
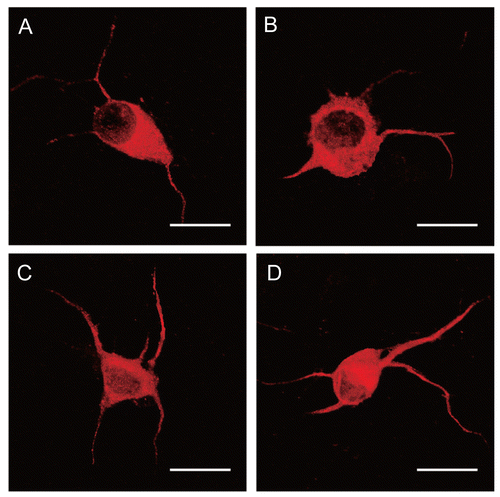
Figure 3. Dissociated DRG neuronal cultures at 2 days of culture were then treated with NGF and/or NE for an additional 4 days and then immunostained with anti-sera to MAP2. Panel A: Normal control. Panel B: Exposure of NE (10−4 mol/L). Panel C: Exposure of NGF (10 ng/mL). Panel D: Exposure of NGF (10 ng/mL) and NE (10−4 mol/L). The number and length of neurites increased in NGF-treated DRG neurons. Scale bar = 20 μm.
Neurite length and neurite number changes with NGF and/or NE treatment
After 4 days incubation with NGF, the neurite length and neurite number increased as compared with that in the control group (DRG neurons continuously cultured in culture media). Neurite length and neurite number in NGF plus NE-treated neurons were not significantly changed as compared with NGF-treated alone. NE did not have any effect on neurite outgrowth as compared with that in control neurons (, and ).
Table 1. Neurite number per neuron after NGF and/or NE treatment (n = 100).
Table 2. Total neurite length per neuron after NGF and/or NE treatment (n = 100).
Discussion
Both in vitro and in vivo studies have revealed that NGF withdrawal additively enhanced the expression of galanin which may use common transcriptional regulation machinery. Although functional correlation of galanin remains unclear, its up-regulation within DRG neurons following peripheral nerve injury may provide more successful protection for injured neurons (CitationKiryu-Seo, 2006). In the present study, NE promoted galanin mRNA and galanin peptide expression rather than stimulating neurite regeneration, whereas NGF inhibited galanin mRNA and galanin peptide expression and stimulated neurite outgrowth which may be not involved in the inhibition of galanin expression in the absence or presence of NE in DRG cell cultures.
The neuropeptide galanin is normally expressed at low levels in sensory neurons (CitationHolmberg et al., 2001) and is present at high levels within the DRG during development (CitationHolmes et al., 2005). Galanin is markedly up-regulated within DRG neurons following peripheral nerve injury and inflammation in the adult (CitationMacdonald et al., 2001; CitationHolmberg et al., 2005; CitationHolmes et al., 2005; CitationHobson et al., 2006; CitationZvarova & Vizzard, 2006). Galanin is rapidly up-regulated 120-fold after peripheral nerve section in the adult (CitationBacon et al., 2002; CitationWynick et al., 2001). DRG cultures seem to represent a suitable model for this study, since the neurons are axotomized during culture preparation and are known to survive independently of added neurotrophic factors (CitationKerekes et al., 1997; CitationShadiack et al., 2001). In the present study, galanin expression was detectable in primary cultured DRG neurons. Activation of alpha-adrenoreceptors may regulate neuropeptide expression or release (CitationSupowit et al., 1998; CitationMelnikova et al., 2006; CitationTrevisani et al., 2007). NE promoted both galanin mRNA and galanin peptide expression in cultured DRG neurons. This result implicated that NE-induced up-regulation of galanin expression may be through the activation of alpha-adrenoreceptors which is expressed in DRG neurons. Axonal transection of adult sensory neurons leads to a decrease in their content of target-derived NGF and to dramatic increases in the expression of neuropeptide galanin (CitationShadiack et al., 2001). Our results showed that the levels of neuropeptide and mRNA for galanin were decreased in the presence of NGF. Interestingly, NGF also inhibited NE-induced up-regulation of galanin expression. NGF-decreased galanin expression may involve transcriptional mechanisms.
Galanin is involved in neuronal differentiation and neurite outgrowth during development. Both the mean axonal length and the number of branch points significantly increased in the presence of galanin in dissociated DRG neurons (CitationSuarez et al., 2006). NE treatment alone did not have an effect on neurite outgrowth in the present study. This result indicated that NE itself could not induce neurite outgrowth and the trophic action of the up-regulated galanin induced by NE is limited and is not enough to trigger neurite outgrowth in the absence of NGF on primary cultured DRG neurons. NGF could promote neurite outgrowth although it down-regulated galanin expression in the absence or presence of NE. This result is consistent with the previous study that NGF caused marked increases in the numbers and lengths of outgrowing axons (CitationOzturk & Tonge, 2001). Interestingly, galanin has been established as a neurotrophic molecule with respect to axonal development and regeneration (CitationSuarez et al., 2006). This observation seems to contradict that NGF decreased galanin expression while it promoted axonal regeneration. Actually, up-regulation of galanin within DRG neurons following peripheral nerve injury may provide more successful protection for injured neurons with target-derived NGF deprivation (CitationKiryu-Seo, 2006). In the presence of NGF, although galanin expression decreased, NGF itself plays an important role in axonal regeneration. NGF inhibition of galanin expression yet promoting axonal regeneration may partially depend on an intricate shift from galanin-mediated signaling to NGF-mediated signaling in the progressing course of axonal regeneration in the presence of NGF. The distinct mechanisms of NGF and galanin in axonal regeneration should be clarified.
It is proposed that release of neuropeptides including galanin from DRG neurons can influence pain processing. Galanin may be involved in both pro- and antinociceptive effects, probably relating to activation of different galanin receptors (CitationLandry et al., 2005). Interestingly, in injury or inflammatory conditions, activation or inhibition of alpha-adrenoreceptors may also influence nociceptive efficacy (CitationPertovaara, 2006). In the present study, the question of whether NE-induced up-regulation of galanin expression is related to galanin-related pain processing or noradrenergic pain modulation should be further studied.
In conclusion, NE promoted galanin expression rather than stimulating neurite regeneration, whereas NGF stimulated neurite outgrowth and inhibited galanin expression in the absence or presence of NE. The up-regulated galanin induced by NE is not enough to trigger neurite outgrowth but may be relevant to noradrenergic pain modulation.
Declaration of interest: This work is supported by the National Natural Science Foundation of China (No. 30770888) and the Natural Science Foundation of Shandong Province of China (Z2006D05). The authors alone are responsible for the content and writing of the paper.
References
- Andersson K-E (2000): Mode of action of alpha1-adrenoceptor antagonists in the treatment of lower urinary tract symptoms. Br J Urol 85: S12–18.
- Avelino A, Cruz C, Cruz F (2002): Nerve growth factor regulates galanin and c-jun overexpression occurring in dorsal root ganglion cells after intravesical resiniferatoxin application. Brain Res 951: 264–269.
- Bacon A, Holmes FE, Small CJ, Ghatei M, Mahoney S, Bloom S, Wynick D (2002): Transgenic over-expression of galanin in injured primary sensory neurons. Neuroreport 13: 2129–2132.
- Hein L (2006): Adrenoceptors and signal transduction in neurons. Cell Tissue Res 326: 541–551.
- Hobson SA, Holmes FE, Kerr NC, Pope RJ, Wynick D (2006): Mice deficient for galanin receptor 2 have decreased neurite outgrowth from adult sensory neurons and impaired pain-like behaviour. J Neurochem 99: 1000–1010.
- Holmberg K, Kuteeva E, Brumovsky P, Kahl U, Karlström H, Lucas GA, Rodriguez J, Westerblad H, Hilke S, Theodorsson E, Berge O-G, Lendahl U, Bartfai T, Hökfelt T (2005): Generation and phenotypic characterization of a galanin overexpressing mouse. Neuroscience 133: 59–77.
- Holmberg K, Shi TJ, Albers KM, Davis BM, Hokfelt T (2001): Effect of peripheral nerve lesion and lumbar sympathectomy on peptide regulation in dorsal root ganglia in the NGF-overexpressing mouse. Exp Neurol 167: 290–303.
- Holmes FE, Mahoney SA, Wynick D (2005): Use of genetically engineered transgenic mice to investigate the role of galanin in the peripheral nervous system after injury. Neuropeptides 39: 191–199.
- Kato R, Kiryu-Seo S, Kiyama H (2002): Damage-induced neuronal endopeptidase (DINE/ECEL) expression is regulated by leukemia inhibitory factor and deprivation of nerve growth factor in rat sensory ganglia after nerve injury. J Neurosci 22: 9410–9418.
- Kerekes N, Landry M, Rydh-Rinder M, Hokfelt T (1997): The effect of NGF, BDNF and bFGF on expression of galanin in cultured rat dorsal root ganglia. Brain Res 754: 131–141.
- Kiryu-Seo S (2006): Identification and functional analysis of damage-induced neuronal endopeptidase (DINE), a nerve injury associated molecule. Anat Sci Int 81:1–6.
- Landry M, Liu HX, Shi TJ, Brumovsky P, Nagy F, Hokfelt T (2005): Galaninergic mechanisms at the spinal level: Focus on histochemical phenotyping. Neuropeptides 39: 223–231.
- Macdonald R, Bingham S, Bond BC, Parsons AA, Philpott KL (2001): Determination of changes in mRNA expression in a rat model of neuropathic pain by Taqman quantitative RT-PCR. Brain Res Mol Brain Res 90: 48–56.
- Melnikova VI, Raison D, Hardin-Pouzet H, Ugrumov MV, Calas A, Grange-Messent V (2006): Noradrenergic regulation of galanin expression in the supraoptic nucleus in the rat hypothalamus. An ex vivo study. J Neurosci Res 83: 857–863.
- Milani S, Djavan B (2005): Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Latest update on alpha- adrenoceptor antagonists. BJU Int 95: S29–36.
- Ozturk G, Tonge DA (2001): Effects of leukemia inhibitory factor on galanin expression and on axonal growth in adult dorsal root ganglion neurons in vitro. Exp Neurol 169: 376–385.
- Pertovaara A (2006): Noradrenergic pain modulation. Prog Neurobiol 80: 53–83.
- Petruska JC, Mendell LM (2004): The many functions of nerve growth factor: Multiple actions on nociceptors. Neurosci Lett 361: 168–171.
- Shadiack AM, Sun Y, Zigmond RE (2001): Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J Neurosci 21: 363–371.
- Shortland PJ, Baytug B, Krzyzanowska A, McMahon SB, Priestley JV, Averill S (2006): ATF3 expression in L4 dorsal root ganglion neurons after L5 spinal nerve transection. Eur J Neurosci 23: 365–373.
- Suarez V, Guntinas-Lichius O, Streppel M, Ingorokva S, Grosheva M, Neiss WF, Angelov DN, Klimaschewski L (2006): The axotomy-induced neuropeptides galanin and pituitary adenylate cyclase-activating peptide promote axonal sprouting of primary afferent and cranial motor neurones. Eur J Neurosci 24: 1555–1564.
- Supowit SC, Hallman DM, Zhao H, DiPette DJ (1998): Alpha 2- adrenergic receptor activation inhibits calcitonin gene- related peptide expression in cultured dorsal root ganglia neurons. Brain Res 782: 184–193.
- Thippeswamy T, Haddley K, Corness JD, Howard MR, McKay JS, Beaucourt SM, Pope MD, Murphy D, Morris R, Hokfelt T, Quinn JP (2007): NO-cGMP mediated galanin expression in NGF-deprived or axotomized sensory neurons. J Neurochem 100: 790–801.
- Trevisani M, Campi B, Gatti R, Andre E, Materazzi S, Nicoletti P, Gazzieri D, Geppetti P (2007): The influence of alpha(1)- adrenoreceptors on neuropeptide release from primary sensory neurons of the lower urinary tract. Eur Urol 52: 901–908.
- Wilson-Gerwing TD, Verge VM (2006): Neurotrophin-3 attenuates galanin expression in the chronic constriction injury model of neuropathic pain. Neuroscience 141: 2075–2085.
- Wynick D, Thompson SW, McMahon SB (2001): The role of galanin as a multi-functional neuropeptide in the nervous system. Curr Opin Pharmacol 1: 73–77.
- Zvarova K, Murray E, Vizzard MA (2004): Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury. J Comp Neurol 475: 590–603.
- Zvarova K, Vizzard MA (2006): Changes in galanin immunoreactivity in rat micturition reflex pathways after cyclophosphamide-induced cystitis. Cell Tissue Res 324: 213–224.
