Abstract
Phoradendron piperoides (Kunth) Trel. (Viscaceae) is a parasitic plant widely distributed in regions of the Brazilian northeast. Different species of Phoradendron are used in folk medicine for the treatment of cough, influenza, gastrointestinal and female disorders, and pain. In order to evaluate the actions of this plant, studies were performed on antinociceptive, anti-inflammatory, and antioxidant activities. The methanol extract (ME) and dichloromethane, ethyl acetate, and methanol partitions of P. piperoides leaves were used in the following experiments. Oral treatment with the ME elicited inhibitory activity (p < 0.01) on the acetic acid effect at 100 (32.08%), 200 (34.46%), and 400 mg/kg (49.50%). P. piperoides ME reduced the formalin effect at the second phase (200 and 400 mg/kg, p < 0.05); however, the ME did not elicit any inhibitory effect on the hot-plate test. Edema formation induced by carrageenan was reduced (p < 0.05) with the ME by 28% (200 mg/kg) and 33% (400 mg/kg). ME, dichloromethane, ethyl acetate, and methanol partitions reacted with the DPPH radical and reduced the DPPH radical by 94.5, 37.2, 77.2, and 95.7%, respectively. ME, ethyl acetate, and methanol partitions exhibited low IC50 values.
Introduction
Mistletoe (Loranthaceae and Viscaceae sp.) represents the most prominent group of angiosperm shoot parasites (CitationNorton & Carpenter, 1998). Extracts of Viscum album L. (Viscaceae), known as “European mistletoe”, have been used for several decades against a variety of diseases, such as arthritis, rheumatism, and hypertension. They have also been used in the treatment of human epithelial tumors (CitationStein et al., 1998; CitationLi et al., 2002b). Viscum album has been used in the indigenous systems of medicine for treatment of headache and some inflammatory diseases (CitationOrhan et al., 2006). The ethyl acetate fraction of Viscum album was shown to possess remarkable antinociceptive and anti-inflammatory activities without inducing any apparent acute toxicity or gastric damage (CitationOrhan et al., 2006).
Most species of Phoradendron (Viscaceae) are used in folk medicine as a substitute of Viscum album in the treatment of high blood pressure due to their external similarity (CitationVarela et al., 2004). The American genus Phoradendron occurs in several tropical and subtropical zones of both hemispheres and comprises a large number of bushy hemiparasitic species. Different species of Phoradendron are used in Brazilian folk medicine in the treatment of cough and influenza, pain, and gastrointestinal and female disorders, and as a vermifuge (CitationAgra et al., 2007; CitationDias et al., 2007).
Phoradendron piperoides (Kunth) Trel. (Viscaceae) is popularly known as “enxerto de passarinho” in the state of Sergipe. The aqueous extract of P. piperoides showed no antinociceptive effect; however, it revealed myorelaxant and antispasmodic activities (CitationDias et al., 2007).
Up to now, few studies have been carried out on P. piperoides, and little is known about the pharmacological and biochemical properties (CitationDias et al., 2007). Conversely, Viscum album has been extensively investigated (CitationHajto, 1986; CitationPortalupi, 1987; CitationJurin et al., 1993; CitationBüssing et al., 1996).
The goal of the present study was to evaluate the antinociceptive, anti-inflammatory, and antioxidant effects of the methanol extract (ME) or partitions of Phoradendron piperoides leaves.
Materials and methods
Plant material
Aerial parts of Phoradendron piperoides were collected in March 2006 at the Federal University of Sergipe campus, São Cristóvão county, Sergipe State (10°559 S, 35°69 W). The plant was authenticated by Professor Carlos Dias da Silva Júnior, Department of Biology, Federal University of Sergipe, and a voucher specimen deposited in the Federal University of Sergipe Herbarium (Av. Marechal Rondon S/N, São Cristóvão-SE 49100-000, Brazil; voucher number 05681). Prior to extraction and isolation, leaves were dried at 40°C in a forced air oven (Marconi MA 037) for 48 h.
Extraction and partitioning of Phoradendron piperoides leaves
The dried leaves of P. piperoides (957 g) were powdered, extracted by maceration at room temperature with methanol (6 L) for 8 days, and filtered. The extraction procedure was repeated three times. All extracts were combined and concentrated to dryness under vacuum to obtain the ME (236 g, 24.6%). The ME (232 g) was further dissolved in methanol (800 mL). Activated charcoal (50 g) was added to the ME. The suspension was stirred for 30 min, filtered, and concentrated under vacuum to obtain the ME without chlorophyll (146 g, 15.2%). The components of the ME were partitioned in a Soxhlet apparatus sequentially with solvents of increasing polarity, specifically with dichloromethane, ethyl acetate, and methanol to obtain the dichloromethane (0.3 g, 0.2%), ethyl acetate (1.9 g, 1.3%), and methanol (135 g, 92.4%) partitions, respectively.
Phytochemical screening
The methods of CitationHarborne (1984) were used to screen the ME of P. piperoides leaves used in this study for its chemical constituents. These chemical classes of compounds are speculated to account for the observed pharmacological effects of the plant’s extract.
Animals
Wistar rats (120–180 g) and Swiss mice (20–30 g) of both sexes were obtained from the Central Biotery of the Federal University of Sergipe (São Cristovão, Brazil). Animals were randomly assigned to groups and maintained in plastic boxes at controlled room temperature (25–28°C) with free access to food and water, under a 12:12 h light/dark cycle. All the experimental procedures were carried out during the light period of the day (08:00 a.m. to 05:00 p.m.) and complied with the guidelines on animal care of the Federal University of Sergipe Ethics Committee for Animal Use in Research, conducted in accordance with the internationally accepted principles for laboratory animal use and care. The animals submitted to oral administration of the extract or drugs were fasted for 12 h before the experiments and acclimatized for at least 2 h before the experiments. All efforts were made to minimize the number of animals used and their suffering.
Chemicals and drugs
The following chemicals and drugs were used: aspirin (acetylsalicylic acid, AAS), carrageenan, indomethacin, (–)-epigallocatechin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and morphine hydrochloride from Sigma Chemical Co. (St. Louis, MO, USA); acetic acid from Merck (Damstadt, Germany); formalin from Baker (Santo Amaro, SP, Brazil); solvents from Vetec (Rio de Janeiro, RJ, Brazil). All substances used were dissolved in saline solution, with the exceptions of indomethacin and aspirin which were dissolved in 5% Tween 80 in 0.9% NaCl solution, and DPPH that was dissolved in methanol. The final concentration of Tween 80 did not exceed 5% and did not cause any effect per se.
Nociceptive tests
Acetic acid-induced abdominal writhes
Abdominal writhes consisted of a contraction of the abdominal muscle together with a stretching of the hind limbs (CitationTonos et al., 1999), induced by intraperitoneal (i.p.) injection in mice of acetic acid (0.6% solution, 0.1 mL/10 g), the nociceptive agent (CitationKoster et al., 1959).
The animals were pre-treated with P. piperoides ME (50, 100, 200, or 400 mg/kg) orally (per os, p.o.) 60 min before initiating algesic stimulation, or with indomethacin (10 mg/kg, p.o., 60 min beforehand), used as positive control (n = 9). The abdominal writhes were observed, in separate individual chambers, for a period of 20 min, starting after the administration of acetic acid.
Hot-plate test
Animals were pre-treated with P. piperoides ME (100, 200, and 400 mg/kg, p.o., 60 min beforehand) or with morphine (10 mg/kg, i.p., 30 min beforehand), used as positive control, and afterward they were placed individually on a metallic plate warmed to 55 ± 0.5°C (n = 9). The time that elapsed until the appearance of reactions (latency, in s) to the thermal stimulus, such as lifting or licking of the paws, was recorded as an index of nociception (CitationWoolfe & Macdonald, 1944). Measurements were performed at time 0, 15, 30, and 60 min after the first thermal stimulus. In order to avoid damage to the animal’s paws, the maximal time standing on the plate was limited to 30 s.
Formalin test
The formalin test was applied according to the method of CitationDubuisson and Dennis (1977), modified by CitationHunskaar and Hole (1987).
Mice were pre-treated with P. piperoides ME (100, 200, or 400 mg/kg, p.o., 60 min beforehand), or with morphine hydrochloride (10 mg/kg; i.p., 30 min beforehand), or indomethacin (10 mg/kg, p.o., 60 min beforehand), used as positive controls, before intraplantar injection of 1% formalin solution (20 μL) into the right hindpaw of the animal (n = 9). The control group received vehicle, saline (0. 1 ml/10 g, p.o., 60 min beforehand).
The time that the animal spent licking or biting its paw was measured during the first phase (0–5 min) and the second phase (20–25 min) of the test.
Anti-inflammatory activity
Measurement of paw edema in rats
The anti-inflammatory activity was studied using the paw edema model induced by 1% carrageenan, administered at a volume of 0.1 mL/animal into the subplantar region of the right hindpaw of the rat (CitationWinter et al., 1962).
The volume of the paw was measured by removal of the water column using a hydroplethysmometer (model 7150; Ugo Basile, Varese, Italy), at time 0 and intervals of 1, 2, 3, and 4 h immediately after the subplantar injection of carrageenan (CitationHarris & Spencer, 1962).
The ME of P. piperoides leaves at different doses of 100, 200, and 400 mg/kg, aspirin (acetylsalicylic acid, AAS; 300 mg/kg), and saline control were administered p.o. 1 h before the edematogenic agent to different groups of animals for each treatment (n = 6).
The data obtained for the various groups are reported as mean ± SEM and expressed in milliliters. The percentage inhibition in the edema experiment was calculated based on the area under the curve (AUC) after 4 h.
Quantitative assay of antioxidant activity
The quantitative analysis of antioxidant activity was based on the method described by CitationBrand-Williams et al. (1995) and CitationSánchez-Moreno et al. (1998), with minor modifications. The scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was followed by monitoring the decrease in absorbance at 515 nm, which occurred due to reduction by the antioxidant.
The calibration curve was established by preparing dilutions of a DPPH radical stock solution (40 μg/mL) to obtain final concentrations of 1, 5, 10, 15, 20, 25, 30, and 35 μg/mL. The absorbance of each standard concentration was then monitored in a spectrophotometer (UV BEL Photonics 1105) at 515 nm. The measurements were carried out in triplicate with intervals of 1 min. The equation of the concentration vs. absorbance calibration curve for the DPPH radical was C = 110.547 – 0.02804A, where C is the concentration of the DPPH radical in medium and A is the absorbance at 515 nm. The correlation coefficient was R = 0.9983.
Solutions containing 500 μg/mL of P. piperoides ME and partitions were prepared in methanol, and diluted in concentrations of 4, 8, 17, 25, 33, and 67 μg/mL. The disappearance of DPPH radical was monitored by the decrease in absorbance at 515 nm, which was recorded after 0, 1, 5, and 10 min, and subsequently every 10 min up to 1 h (CitationSousa et al., 2007). The negative control was pure methanol used for dissolving the samples, while the positive control was (–)-epigallocatechin dissolved in methanol at concentrations of 4, 8, 17, 25, 33, and 67 μg/mL. The mixture of methanol and ME was used as a blank.
The concentration of DPPH radical in the reaction mixture was calculated based on the calibration curve, where [DPPH] is expressed in μg/mL. The percentage of remaining DPPH (%DPPHREM) was calculated according to CitationBrand-Williams et al. (1995), as follows: %DPPHREM = [DPPH]T/[DPPH]T0 × 100, where T is the time when absorbance was determined (1–60 min) and T0 is time zero. The amount of antioxidant necessary to decrease the initial concentration of DPPH radical by 50% (IC50) was calculated by plotting the %DPPHREM at time 60 min with 25 μg/mL of each extract. The results are expressed as μg antioxidant/mL DPPH ± standard deviation.
The absorbance values observed in all samples at 60 min (25 μg/ml) were transformed to percentages of inhibition (IP), which were determined using the equation: IP = {[Abscontrol – (Abssample – Absblank)] × 100}/Abscontrol, where Abscontrol is the initial absorbance of the methanol solution of DPPH radical and Abssample is the absorbance of the reaction mixture (DPPH + sample). The results are expressed as % of inhibition.
Toxicity test
The preliminary acute toxicity was evaluated in four groups of mice receiving P. piperoides ME (1–5 g/kg, p.o.) or vehicle (0.9% saline, 0.1 mL/10 g) (CitationLorke, 1983). The mortality was observed during 48 h (n = 9).
Statistical analysis
The results of analgesic and anti-inflammatory activities are presented as the mean ± SEM of n animals per group. The antioxidant activities were determined using Origin version 7.5 software (Microcal, Northampton, MA, USA), and the values are presented as the mean ± standard deviation of three assays. Statistical evaluation of the data was performed using one-way analysis of variance (ANOVA) followed by Tukey’s test, and p values less than 0.05 were considered significant.
Results
Phytochemical screening
Phytochemical screening of the ME of P. piperoides leaves showed that the crude ME, as well as the ethyl acetate partition, contained alkaloids, flavonoids, saponins, tannins, and triterpenes. The dichloromethane partition exhibited large amounts of triterpenes. Furthermore, the methanol partition showed large amounts of alkaloids, flavonoids, saponins, and tannins, and small quantities of triterpenes ().
Table 1. Phytochemical screening of P. piperoides ME.
Acetic acid-induced writhing in mice
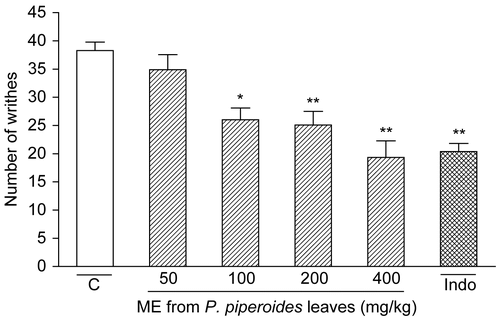
The results in show that P. piperoides ME given p.o. (50– 400 mg/kg, n = 9/dose) 1 h beforehand caused an inhibition of 32.08% (p < 0.01), 34.46% (p < 0.001), and 49.50% (p < 0.001) on acetic acid-induced writhes at doses of 100, 200, and 400 mg/kg, respectively. Indomethacin (10 mg/kg, n = 9) exhibited significant (46.76%, p < 0.001) inhibition of the control writhes in acetic acid-induced writhing.
Figure 1. Influence of P. piperoides methanol extract (ME) in nociceptive behavior of mice evaluated in acetic acid-induced abdominal writhing model. Nociception was registered by the number of writhes that the animal presented 20 min following i.p. acetic acid injection. Groups of animals were pre-treated with vehicle (C, control group, open column, n = 9), indomethacin (Indo, 10 mg/kg, cross-hatched column, n = 9), or ME (50– 400 mg/kg, right-hatched columns, n = 9/dose), p.o., 60 min before irritant agent. Each column represents the mean ± SEM. Asterisks denote statistical significance, *p < 0.01 and **p < 0.001, in relation to control group. ANOVA followed by Tukey’s test.
Hot-plate reaction time in mice
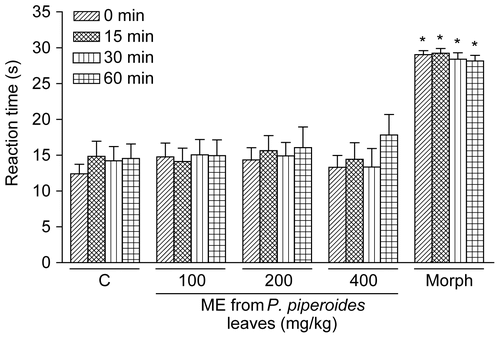
The results in show that the treatment of animals with morphine (10 mg/kg, i.p., 30 min beforehand, n = 9) caused a marked increase in latency (p < 0.001) at all analyzed periods according to assessment in the hot-plate test. As illustrated in , the ME of P. piperoides leaves did not significantly influence the reaction time of mice to the hot-plate at doses of 100, 200, or 400 mg/kg (n = 9/dose).
Figure 2. Influence of P. piperoides ME on nociceptive behavior of mice in the hot-plate test. Nociception was evaluated as the latency (reaction time, in s) for animals to elevate paws from the plate warmed to 55 ± 0.5°C. Groups of mice were pre-treated with vehicle (C, control group, 10 mL/kg, p.o., 60 min beforehand, n = 9), morphine (Morph, 10 mg/kg, i.p., 30 min beforehand, n = 9), or P. piperoides ME (100– 400 mg/kg, p.o., 60 min beforehand, n = 9/dose) and measurements were performed at times 0 (right-hatched columns), 15 (cross-hatched columns), 30 (striped columns), and 60 min (squared columns) after the first thermal stimulus. Each column represents the mean ± SEM. Asterisks denote statistical significance, *p < 0.001 in relation to control group. ANOVA followed by Tukey’s test.
Formalin reaction time in mice
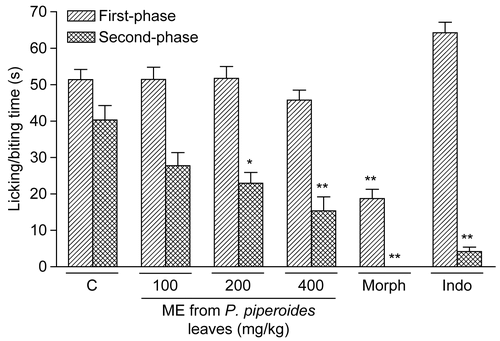
As shown in , P. piperoides ME administered orally (100– 400 mg/kg, 1 h beforehand, n = 9/dose) produced marked inhibition upon intraplantar injection of formalin in mice only against inflammatory pain (second phase) at doses of 200 (p < 0.05) and 400 mg/kg (p < 0.001). Similarly, indomethacin (10 mg/kg, p.o., 60 min beforehand) caused inhibition of the second phase of formalin-induced nociception (p < 0.001, n = 9; ). Morphine (10 mg/kg, i.p., 30 min beforehand) caused significant inhibition of both phases of formalin-induced nociception (p < 0.001, n = 9, ).
Figure 3. Effect of P. piperoides ME in nociceptive behavior of mice evaluated in formalin-induced nociception model. Groups of mice were pre-treated with vehicle (columns C, control groups, 10 mL/kg, p.o., 60 min beforehand, n = 9), indomethacin (Indo, 10 mg/kg, p.o., 60 min beforehand, n = 9), morphine (Morph, 10 mg/kg, i.p., 30 min beforehand, n = 9), or P. piperoides ME (100– 400 mg/kg, p.o., 60 min beforehand, n = 9/dose) against the early phase (0–5 min, right-hatched columns) or late phase (20–25 min, cross-hatched columns) of formalin-induced nociception in mice. Each column represents the mean ± SEM. Asterisks denote statistical significance, *p < 0.05 and **p < 0.001 in relation to control group. ANOVA followed by Tukey’s test.
Anti-inflammatory effect of the ME of P. piperoides leaves
The anti-inflammatory effect of the ME of P. piperoides leaves was evaluated in the paw edema model (n = 6/dose). As observed in , the single oral treatment of rats with the ME of P. piperoides leaves at 400 mg/kg (p.o., 1 h beforehand) was capable of reducing (p < 0.05) the edema formation induced by carrageenan (1%, 100 μL/paw), an effect observed at 2, 3, and 4 h after the administration of this phlogistic agent. Additionally, P. piperoides ME at 200 mg/kg (p.o., 1 h beforehand) reduced (p < 0.05) the edema formation induced by carrageenan at 3 h. Likewise, aspirin (300 mg/kg, p.o., 1 h beforehand, n = 6) significantly inhibited (p < 0.001) the edematogenic response evoked by carrageenan in rats, at 2, 3, and 4 h.
Figure 4. Effect of P. piperoides ME on rat paw edema induced by carrageenan. Groups of rats were pre-treated with vehicle (control group, 10 mL/kg, p.o., n = 6), aspirin (AAS, 300 mg/kg, p.o., n = 6), or P. piperoides ME in concentrations of 100, 200, and 400 mg/kg (p.o., n = 6/dose) 60 min before carrageenan-induced paw edema. Measurements were performed at times 0, 1, 2, 3, and 4 h after the subplantar injection of carrageenan (1%, 100 μL). Each value represents the mean ± SEM. Asterisks denote statistical significance, *p < 0.05 and **p < 0.001 in relation to control group. ANOVA followed by Tukey’s test.
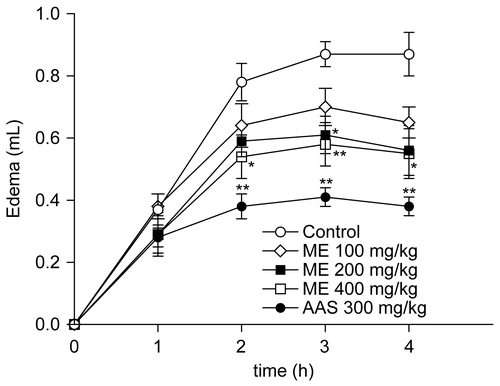
The mean AUC found in carrageenan-treated rats was 1.47 ± 0.09 mL/4 h (n = 6). Based on AUC values, the ME at 200 and 400 mg/kg caused 28% (p < 0.05) and 33% (p < 0.01) of inhibition on the edema response, respectively (n = 6/dose). Aspirin at 300 mg/kg (n = 6) caused an inhibition of 53% (p < 0.001).
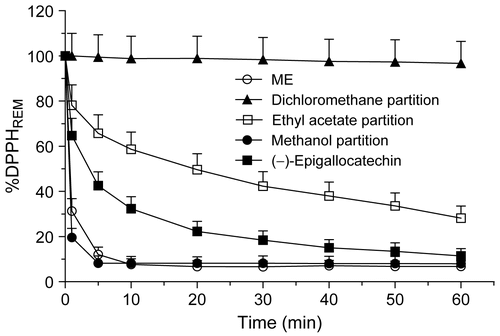
Antioxidant activity
The amount of DPPH radical that reacted with dichloromethane (25 μg/mL, 60 min) was low (37%), while for the ethyl acetate partition (25 μg/mL, 60 min) the amount of DPPH radical used was 77% (). The ME of the leaves and the methanol partition (25 μg/mL, 60 min) consumed 95% and 96% of the DPPH radical, respectively. The ME and methanol partition presented a response similar to that of the positive control (–)-epigallocatechin (25 μg/mL, 60 min, n = 3; ).
Table 2. Radical scavenging activities of P. piperoides extract and partitions determined by the reduction of DPPH free radical.
According to the IC50 values, the antioxidant concentration needed to decrease the initial concentration of DPPH radical by 50% was highest for the dichloromethane partition. The IC50 values for ME, ethyl acetate, and methanol partitions were similar, but lower than the IC50 value of the reference compound (–)-epigallocatechin (n = 3, ).
As shown in , steady state for the ME and methanol partition of P. piperoides leaves was reached within 10 min, and the antioxidant efficiency decreased in the order shown. For the ethyl acetate partition, steady state was not reached within 60 min. In contrast, the dichloromethane partition reacted very slowly with the DPPH radical.
Toxicity test
P. piperoides ME exhibited low toxicity when administered acutely to mice, with an LD50 estimated to be greater than 5 g/kg (n = 9).
Discussion
The present study demonstrates that Phoradendron piperoides ME displays antinociceptive, anti-inflammatory, and antioxidant properties, and provides some evidence on the mechanisms implicated in these effects.
For the first time, this work shows that P. piperoides ME p.o. produces significant antinociception according to assessment of the abdominal writhes elicited by acetic acid, a model used to evaluate the potential analgesic activity of drugs. It has been suggested that acetic acid acts by releasing endogenous mediators that stimulate the nociceptive neurons (CitationCollier et al., 1968). This method is sensitive to non-steroidal anti-inflammatory drugs (NSAIDs) and to narcotics and other centrally acting drugs (CitationCollier et al., 1968; CitationSantos et al., 1998; CitationReichert et al., 2001).
P. piperoides ME showed inhibition similar to indomethacin for the acetic acid-induced visceral nociceptive response when given orally (400 mg/kg).
CitationRibeiro et al. (2000) have demonstrated that the nociceptive activity of acetic acid may be due to the release of cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-8, by resident peritoneal macrophages and mast cells. Thus, the previous findings and the results presented herein might indicate that the antinociceptive action of the ME of P. piperoides leaves in the acetic acid-induced writhing test could be due to inhibition of the release of TNF-α, IL-1β, and IL-8 by resident peritoneal cells. However, this possibility remains to be tested in future studies.
Interestingly, Phoradendron piperoides ME at doses that inhibited the nociception caused by acetic acid has no effect on the hot-plate test. Although the hot-plate test is commonly used to assess narcotic analgesics, other centrally acting drugs, including sedatives and muscle relaxants or psychotomimetics, have shown activity in this test (CitationEddy & Leimbach, 1953). However, indomethacin and other NSAIDs are not responsive to the hot-plate test (CitationYamamoto & Nozaki-Taguchi, 1996; CitationSantos et al., 1998).
The results of the present study have also shown that morphine is largely effective in preventing both the first and second phases of formalin-induced pain. It is well known that NSAIDs (such as aspirin, acetaminophen, and diclofenac), known to inhibit cyclo-oxygenase (COX) activity, are largely ineffective or cause very weak inhibition at the first phase of the formalin test (CitationHunskaar & Hole, 1987; CitationMalmberg & Yaksh, 1992; CitationSantos et al., 1998). In addition, NSAIDs can attenuate, in a dose-related manner, the second-phase of formalin-induced licking (CitationHunskaar & Hole, 1987; CitationMalmberg & Yaksh, 1992; CitationSantos et al., 1998). Previous studies have shown that formalin releases various inflammatory mediators (CitationHunskaar et al., 1986; CitationHunskaar & Hole, 1987; CitationSantos & Calixto, 1997). Our results show, however, that P. piperoides ME p.o. produces inhibition in the second phase (inflammatory nociception) of the formalin test in mice. These data suggest that the ME can produce antinociceptive action through inhibition of COX and consequently prostaglandin synthesis. However, the possibility that P. piperoides acts on COX remains to be tested in future studies.
The anti-inflammatory effect of Phoradendron piperoides ME was evaluated in carrageenan-induced paw edema, an animal model widely employed for the screening of anti-inflammatory compounds, and has frequently been used to assess the anti-edematous effect of natural products. The experimental model exhibits a high degree of reproducibility. In rats, the inflammatory response induced by carrageenan is characterized by a biphasic response, with marked edema formation resulting from the rapid production of several inflammatory mediators such as histamine, serotonin, and bradykinin (first phase), which is subsequently sustained by the release of prostaglandins and nitric oxide (second phase) with a peak at 3 h, produced by inducible isoforms of cyclo-oxygenase (COX-2) and nitric oxide synthase (iNOS), respectively (CitationSeibert et al., 1994; CitationNantel et al., 1999). In the present work, previous oral treatment with the ME of Phoradendron piperoides leaves was effective in reducing the edematogenic response evoked by carrageenan in rats between 2 and 4 h after injection. This evidence allows us to suggest that anti-inflammatory actions of the ME of P. piperoides leaves are related to the inhibition of one or more intracellular signaling pathways involved in the effects of several inflammatory mediators.
The DPPH test is a very convenient method for screening small antioxidant molecules, because the intensity of the reaction may be analyzed by a simple spectrophotometric assay (CitationSánchez-Moreno et al., 1998; CitationSoler-Rivas et al., 2000). The DPPH radical is scavenged by antioxidants through the donation of hydrogen to form the stable reduced DPPH molecule. The radicals are stabilized through the formation of non-radical products by reaction with antioxidant agents (CitationArgolo et al., 2004).
The capacity for scavenging free radicals was evaluated for the ME and dichloromethane, ethyl acetate, and methanol partitions. IP and IC50 values are considered to be good measures of the antioxidant efficiency of extracts and pure compounds. Typical IP values for plant materials with acknowledged potent antioxidant activities are around 70–90%, as observed for the stem bark (93%) and leaves (75%) of Cassia fistula, a traditional Indian medicine (CitationSiddhuraju et al., 2002), and for the edible seeds (52–80%) of Rosa rubiginosa (CitationMoure et al., 2001). Less potent materials show IP values in the range of 9–14%, as for example the seeds of Gevuina avellana (CitationMoure et al., 2001). Our results showed that the polar fractions (ME and ethyl acetate and methanol partitions) of P. piperoides have the highest antioxidant potential, since the antioxidant efficiency observed (over 75%) is in accordance with the literature.
The kinetic behavior showed that the polar extract and partitions reacted with the DPPH radical. The ME and methanol partition showed quick kinetic behavior with a percentage of remaining DPPH radical smaller than 50%. For the ethyl acetate partition, steady state was not reached within 60 min, but consuming approximately 40% of the DPPH radical in less than 5 min might be considered an antioxidant efficiency. However, the dichloromethane partition reacted very slowly with the DPPH radical, which is a poor source of antioxidants. Since the extract and partitions contain several compounds, the antioxidant concentration in samples and their antagonistic–synergetic effects may explain these behaviors (CitationSánchez-Moreno et al., 1998; CitationArgolo et al., 2004).
The phytochemical study of P. piperoides leaves detected large amounts of phenolic compounds in ME, as well as the ethyl acetate and methanol partitions. Several studies have highlighted the importance of these substances as antioxidant agents, once their aromatic carbon skeletons are adequate for stabilizing free radicals, such as DPPH. However, the composition and hydroxylation degree are also important factors for their antioxidant activity (CitationLarrauri et al., 1996).
The antioxidant capacity of flavonoids confers a therapeutic potential with antinociceptive and anti-inflammatory properties (CitationRajendran et al., 2000; CitationLi et al., 2002a). It has been demonstrated that flavonoids are able to inhibit both COX-2 and iNOS enzymes, as well as other mediators of the inflammatory process (CitationGonzález-Gallego et al., 2007).
Phoradendron piperoides ME shows antinociceptive, anti-inflammatory, and antioxidant activities. The identification and isolation of such bioactive components are in progress, which could elucidate the analgesic and anti-inflammatory properties of P. piperoides.
Declaration of interest: This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Apoio à Pesquisa e à Inovação Tecnológica do Estado de Sergipe (FAPITEC/SE).The authors report no conflicts of interest.
References
- Agra MF, Freitas PF, Barbosa-Filho JM (2007): Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Rev Bras Farmacogn 17: 114–140.
- Argolo ACC, Sant’Ana AEG, Pletsch M, Coelho LCBB (2004): Antioxidant activity of leaf extracts from Bauhinia monandra. Bioresour Technol 95: 229–233.
- Brand-Williams W, Cuvelier ME, Berset C (1995): Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28: 25–30.
- Büssing A, Suzart K, Bergmann J, Pfeiller U, Schietzel M, Schuweizer K (1996): Induction of apoptosis in human lymphocytes treated with Viscum album L. is mediated by the mistleloe lectins. Cancer Lett 99: 59–72.
- Collier HO, Kinneen LC, Johnson CA, Schneider C (1968): The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol 32: 295–310.
- Dias KS, Almeida DS, Silva ABL, Marques MS, Menezes IAC, Santos TC, Mello ICM, Carvalho ACS, Antoniolli AR, Marçal RM (2007): Avaliação dos efeitos miorelaxante, antiespasmódico e antinociceptivo do extrato aquoso da Phoradendron piperoides (Kunt.) Trel. (Viscaceae). Rev Bras Farmacogn 17: 373–377.
- Dubuisson D, Dennis SG (1977): The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4: 161–174.
- Eddy NB, Leimbach D (1953): Synthetic analgesics. II. Dithienylbutenyland dithienylbutylamines. J Pharmacol Exp Ther 107: 385–393.
- González-Gallego J, Sánchez-Campos S, Tuñón MJ (2007): Anti-inflammatory properties of dietary flavonoids. Nutr Hosp 22: 287–293.
- Hajto T (1986): Immunomodulatory effects of Iscador: A Viscum album preparation. Oncology 43: 51–65.
- Harborne JB (1984): Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. London, Chapman and Hall, p. 324.
- Harris JM, Spencer PSJ (1962): A modified plethysmographic apparatus for recording volume changes in rat paw. J Pharm Pharmacol 14: 464–466.
- Hunskaar S, Berge OG, Hole K (1986): Dissociation between antinociceptive and anti-inflammatory effects of acetylsalicylic acid and indomethacin in the formalin test. Pain 25: 125–132.
- Hunskaar S, Hole K (1987): The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 30: 103–114.
- Jurin M, Zarkovic N, Hrzenjak M, Ilic Z (1993): Antitumorous and immunomodulatory effects of the Viscum album L. preparation isorel. Oncology 50: 1–6.
- Koster R, Anderson N, Debber EJ (1959): Acetic acid for analgesic screening. Fed Proc 18: 418–420.
- Larrauri JA, Goñi I, Martín-Carrón N, Rupérez P, Saura-Calixto F (1996): Measurement of health-promoting properties in fruit dietary fibres: Antioxidant capacity, fermentability and glucose retardation index. J Sci Food Agric 71: 515–519.
- Li DW, Lee EB, Kang SS, Hyun JE, Whang WK (2002a): Activity guided isolation of saponins from Kalopanax pictus with anti-inflammatory activity. Chem Pharm Bull 50: 900–903.
- Li S-S, Gullbo J, Lindholm P, Larsson R, Thunberg E, Samuelsson G, Bohlin L, Claeson P (2002b): Ligatoxin B, a new cytotoxic protein with a novel helix–turn–helix DNA-binding domain from the mistletoe Phoradendron liga. Biochem J 366: 405–413.
- Lorke D (1983): A new approach to practical acute toxicity testing. Arch Toxicol 54: 275–287.
- Malmberg AB, Yaksh TL (1992): Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther 263: 136–146.
- Moure A, Franco D, Sineiro J, Dominguez H, Nunes MJ, Lema JM (2001): Antioxidant activity of extracts from Gevuina avellana and Rosa rubiginosa defatted seeds. Food Res Intern 34: 103–109.
- Nantel F, Denis D, Gordon R, Northey A, Cirino M, Metters KM, Chan CC (1999): Distribution and regulation of cyclooxygenase-2 in carrageenan induced inflammation. Br J Pharmacol 128: 853–859.
- Norton D, Carpenter M (1998): Mistletoes as parasites: Host specificity and speciation. Trends Ecol Evol 13: 101–105.
- Orhan DD, Küpeli E, Yesilada E, Ergun F (2006): Anti-inflammatory and antinociceptive activity of flavonoids isolated from Viscum album ssp. album. Z Naturforsch [C] 61: 26–30.
- Portalupi E (1987): Il vischio nella terapia dei tumori: Valutazione critica dell’impiego dell’Iscador nella terapia anti tumorale. Svizzera, Istituto Hiscia, pp. 1–247.
- Rajendran NN, Thirugnanasambandam P, Viswanathan S, Parvathi V, Ramasamy S (2000): Antinociceptine pattern of flavone and its mechanism as tested by formalin assay. Indian J Exp Biol 38: 182–185.
- Reichert JA, Daughters RS, Rivard R, Simone DA (2001): Peripheral and preemptive opioid antinociception in a mouse visceral pain model. Pain 89: 221–227.
- Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato ABP, Poole S, Ferreira SH, Cunha FQ (2000): Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol 387: 111–118.
- Sánchez-Moreno C, Larrauri JA, Saura-Calixto F (1998): A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 76: 270–276.
- Santos ARS, Calixto JB (1997): Further evidence for the involvement of tachykinin receptor subtypes in formalin and capsaicin models of pain in mice. Neuropeptides 31: 381–389.
- Santos ARS, Vedana EMA, Freitas GAG (1998): Antinociceptive effect of meloxicam, in neurogenic and inflammatory nociceptive models in mice. Inflamm Res 47: 302–307.
- Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P (1994): Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA 91: 12013–12017.
- Siddhuraju P, Mohan PS, Becker K (2002): Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem 79: 61–67.
- Soler-Rivas C, Espín JC, Wichers HJ (2000): An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochem Anal 11: 1–9.
- Sousa CMM, Silva HR, Vieira-Jr GM, Ayres MCC, Costa CLS, Araújo DS, Cavalcante LCD, Barros EDS, Araújo PBM, Brandão MS, Chaves MH (2007): Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim Nova 30: 351–355.
- Stein GM, Schietzel M, Bussing A (1998): Mistletoe in immunology and the clinic. Anticancer Res 18: 3247–3250.
- Tonos MP, Sáenz MT, Garcia MD, Fernández MA (1999): Antinociceptive effects of the tubercles of Anredera leptostachy. J Ethnopharmacol 68: 229–234.
- Varela BG, Fernández T, Ricco RA, Zolezzi PC, Hajos SE, Gurni AA, Alvarez E, Wagner ML (2004): Phoradendron liga (Gill. ex H. et A.) Eichl. (Viscaceae) used in folk medicine: Anatomical, phytochemical, and immunochemical studies. J Ethnopharmacol 94: 109–116.
- Winter CA, Risley EA, Nuss GW (1962): Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111: 544–547.
- Woolfe G, Macdonald AD (1944): The evaluation of the analgesic action of penthidine hydrochloride (Demerol). J Pharmacol Exp Ther 80: 300.
- Yamamoto T, Nozaki-Taguchi N (1996): Analysis of the effects of cyclooxygenase (COX)-1 and COX-2 in spinal nociceptive transmission using indomethacin, a non-selective COX inhibitor, and NS-398, a COX-2 selective inhibitor. Brain Res 739: 104–110.