Abstract
Besides having lipid-lowering properties, statins (HMG-CoA reductase inhibitors) are known as strong anti-inflammatory agents reducing the expression of inducible matrix metalloproteinases (MMP). Our objective was to evaluate pravastatin effect (a hydrophilic statin) on serum gelatinase activities (MMP-2 and MMP-9) in vitro. Blood samples were obtained from 17 healthy individuals never treated with statins, and gelatinase activities were measured by gelatin zymography. After electrophoresis zymographic gels were incubated with or without pravastatin (5 μg/mL) for 18 h. We noticed that pravastatin enhances gelatinolytic activity at 72 kDa molecular mass (MMP-2) as well as at 130 kDa (MMP-9 heterodimer with neutrophil gelatinase-B associated lipocalin, NGAL) without significant effects on 92 kDa (MMP-9). The comparison of the above finding with previous studies suggests that statins can exert the opposite effects; the inhibition of MMP expression and the augmentation of its activity.
Keywords::
Introduction
Matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9, gelatinases) belong to Zn2+ dependent endopeptidases targeting extracellular proteins such as different types of collagen, elastin, laminin, and moreover, gelatin and casein. They play an important role in numerous physiological and pathological processes such as cardiovascular disorders (CitationBlankenberg et al., 2003), cancer (CitationPolette et al., 2004) as well as neurological diseases: multiple sclerosis, amyotrophic lateral sclerosis, Guillain-Barré syndrome and ischemic stroke (CitationHartung & Kieseier, 2000; CitationCastillo & Rodriguez, 2004). Gelatinases are involved in the destruction of basal membrane, the facilitation of leukocytes migration across the vessel wall and the activation of proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) (CitationOpdenakker et al., 2001).
The classic mechanism of 3-hydroxy-3- methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor (statin) action depends on the decreasing of low-density lipoprotein (LDL) serum level. Beyond lipid-lowering properties, statins are known as anti-inflammatory drugs mainly through the inactivation of nuclear factor kB (NF-kB), the key activator of cytokine transcription and through the resulting decrease in the expression of numerous proinflammatory cytokines which can induce the expression of MMPs (CitationLaws et al., 2004). This is in contrast to previous data suggesting that simvastatin augments the proteolytic activity of gelatinases in vitro evaluated in gelatin zymography (CitationKieseier et al., 2004). The phenomenon that simvastatin reduces MMP secretion and simultaneously increases geltinolytic activity is unexplained, and further investigations in this field are needed. Our purpose was to evaluate pravastatin effects (a hydrophilic HMG-CoA reductase inhibitor) on MMP activity.
Materials and methods
The present work was performed in accordance with the ethical standards of the Helsinki Declaration of 1975, revised in 1983. All individuals gave informed consent. The serum samples were obtained from 17 healthy volunteers (female/male: 10/7, mean age ± SD: 28 ± 3.6 years), without symptoms of either chronic or acute inflammatory disorders. All of them had never been treated with statins in the past. After centrifuging, the serum was stored at -30°C for up to two weeks.
Gelatinase activity was determined by gelatin zymography according to previously described methods (CitationKurzepa & Stryjecka-Zimmer, 2007). Briefly; the samples consisted of 9 μL of diluted serum (1/50 with redistilled water) + 3 μL of sample buffer with 10% sodium dodecyl sulfate (SDS, Bio-Rad 161-0301, Richmond, CA) were applied to a 10% standard, 0. 75 mm thick, polyacrylamide gel with 0.05% gelatin type A from porcine skin (Sigma-Aldrich G2500, St. Louis, MO). The gels were run for 1.5 h with 30 mA/gel in room temperature. After electrophoresis, washing was carried out for two 30-min periods with buffer 50 mM Tris-HCl, pH 7.2 (Bio-Rad 161-0716, Hercules, CA), containing 10 mM CaCl2 (Sigma-Aldrich C5080), 0.02% NaN3 (Sigma-Aldrich 13412), and 2.5% Triton X-100 (Sigma-Aldrich 93443). Incubation with or without 5 μg/mL pravastatin (Sigma-Aldrich P4498) was performed for 18 h at 37°C in the above buffer but with 1% Triton X-100. Gels were stained (3 h) with 0.1% Coomassie Blue R-250 in 30% ethanol and 10% acetic acid and destained (2 × 15 min) in 30% ethanol and 10% acetic acid. MMP-2 and MMP-9 activity was detected as unstained bands on the blue background. Enzymes were identified by co-localization with molecular mass standard (Fermentas SM0441, St. Leon-Rot, Germany). Quantification of zymograms was done using a computer scanner (1200 dpi) and ZERO-Dscan Image Analysis System v.1.0 (Scanalytics). MMP-2 and MMP-9 activities were expressed as the optical density (OD) of the substrate lysis zone.
To compare gelatinase activity with versus without pravastatin, Wilcoxon matched pairs test was used. Statistically significant values were considered when p < 0.05. Data are expressed as median and first, third quartiles. Statistical analysis was performed with the use of the computer assisted statistical program InStat v.3.06. GraphPad Software.
Results and discussion
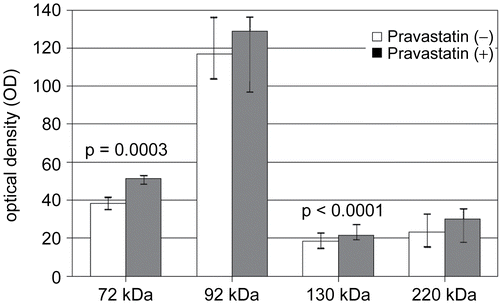
Gelatinolytic activity was detectable at molecular weight of 72 kDa (pro-MMP-2), 92 kDa (pro-MMP-9), 130 kDa (MMP-9 heterodimer with neutrophil gelatinase-B associated lipocalin - NGAL) and approximately 200 kDa (MMP-9 homodimer) in all studied samples (). We noticed that proteolytic activity at 72 kDa in zymograms incubated after electrophoresis with buffer containing pravastatin (5 μg/mL) increased significantly [38.5 (36.0-40.1) versus 51.2 (47.7-53.2), median values (first, third quartiles), p = 0.0003]. Similar results were noted at 130 kDa molecular mass (18.3 (16.1–23.1) versus 21.6 (19.2–26.2), p < 0.0001). The pravastatin effects at 92 kDa and 200 kDa did not reach statistical significance ().
Figure 1. The serum gelatinolytic activity in zymography assay. Zone of clear bands corresponds with protease activity at 72 kDa (pro-MMP-2), 92 kDa (pro-MMP-9), 130 kDa (MMP-9 heterodimer) and 200 kDa (MMP-9 homodimer). Pravastatin line represents zymogram after an 18-h incubation with pravastatin 5 μg/mL versus incubation with conventional buffer only (control).
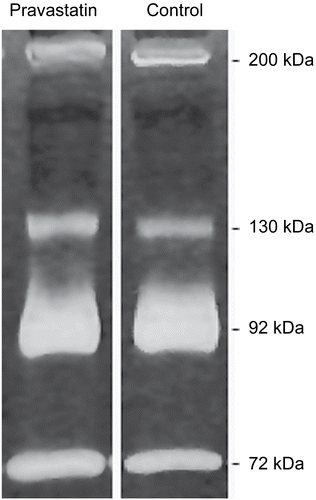
Figure 2. Analysis of serum gelatinase activity. White and gray columns represent the activity of MMP-2 (72 kDa) and MMP-9 (92, 130 and 200 kDa) after incubation without or with pravastatin. Median value and 1st-3rd quartiles. Wilcoxon test.
Gelatinases are secreted in a latent form as pro-MMP-2 (72 kDa) and pro-MMP-9 (92 kDa) but two additional gelatinolytic activities at 130 kDa (MMP-9 heterodimer with NGAL) and 200 kDa (MMP-9 homo dimer) are often observed. During zymography, pro-forms of gelatinases are converted to the active enzymes by the presence of sodium dodecyl sulfate (SDS) in the SDS-PAGE electrophoresis (CitationSnoek-van Beurden & Von den Hoff, 2005). Pravastatin, in contrast to simvastatin and lovastatin, is an active form of the drug which does not require a conversion to active metabolites in the liver in vivo or an activation with heat NaOH in ethanol treatment in vitro (CitationDe Angelis, 2004). Moreover, pravastatin is a hydrophilic compound, well soluble in water. These features make pravastatin very useful and accessible to examination. Our observation showed that pravastatin can enhance the gelatinolytic activity of MMP-2, and to a lesser degree of MMP-9 (130 kDa form). The high glycosylation ratio can stabilize MMP-9 and protect the enzyme against proteolytic cleavage by other proteases from self-cleavage (CitationMatuu et al., 2000). MMP-2 is free of carbohydrate residues and the enzyme is potentially more sensitive for proteolytic destruction. In a previous study CitationKieseir et al. (2004) showed that simvastatin can enhance the MMP-2 activity in a post-translational manner. Moreover, the authors suggest that statin probably does not affect the active site of gelatinase. The above findings are in variance with general known anti-inflammatory properties of statins, however previously statins have been reported to decrease the expression of interleukin-10, an anti-inflammatory cytokine (CitationNeuhaus et al. 2002). The concentration of pravastatin in our experiment was approximately 100-fold higher than the concentration present in serum during therapy with pravastatin (45-55 ng/mL) (CitationDe Angelis, 2004), therefore, these results should be related carefully to in vivo conditions. Because statins simultaneously inhibit the expression of MMPs, it is difficult to assess the biological role of the augmentation of gelatinase activity.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L, Atherogene investigators (2003): Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 107: 1579–1585.
- Castillo J, Rodriguez I (2004): Biochemical changes and inflammatory response as markers for brain ischemia: Molecular markers of diagnostic utility and prognosis in human clinical practice. Cerebrovasc Dis 17: S7–18.
- De Angelis G (2004): The influence of statin characteristics on their safety and tolerability. Int J Clin Prac 58: 945–955.
- Hartung HP, Kieseier BC (2000): The role of matrix metalloproteinases in autoimmune damage to the central and peripheral nervous system. J Neuroimmunol 107: 140–147.
- Kieseier BC, Archelos JJ, Hartung HP (2004): Different effects of simvastatin and interferon beta on the proteolytic activity of matrix metalloproteinases. Arch Neurol 61: 929–932.
- Kurzepa J, Stryjecka-Zimmer M (2007): The effect of interferon beta-1a on MMP-2 and MMP-9 proteolytic activity. Folia Biol (Praha) 53: 220–221.
- Laws PE, Spark JI, Cowled PA, Fitridge RA (2004): The role of statins in vascular disease. Eur J Vasc Endovasc Surg 27: 6–16.
- Matuu TS, Royle L, Langridge J (2000): O-Glycan analysis of natural human neutrophil gelatinase B using a combination of normal phase-HPLC and online tandem mass spectrometry: Implications for the domain organisation of the enzyme. Biochemistry 39: 15696–15704.
- Neuhaus O, Strasser-Fuchs S, Fazekas F, Kieseier BC, Niederwieser G, Hartung HP, Archelos JJ (2002): Statins as immunomodulators: Comparison with interferon-beta 1b in MS. Neurology 59: 990–997.
- Opdenakker G, Van den Steen PE, Van Damme J (2001): Gelatinase B: A tuner and amplifier of immune functions. Trends Immunol 22: 571–579.
- Polette M, Nawrocki-Raby B, Gilles C, Clavel C, Birembaut P (2004): Tumor invasion and matrix metalloproteinases. Clinical Rev Oncol/Hematol 49: 179–186.
- Snoek-van Beurden PA, Von den Hoff JW (2005): Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 38: 73–83.
