Abstract
Viscum cruciatum Sieber (Viscaceae) is widely used in folk medicine for various gastrointestinal and inflammatory disorders. The crude extract of Viscum cruciatum (VCr), which tested positive for alkaloids, flavonoids, coumarins, saponins, sterols, tannins, and terpenes, caused concentration-dependent (0.01–3.0 mg/mL) relaxation of spontaneous and K+ (80 mM)-induced contractions of isolated rabbit jejunum, similar to that caused by verapamil. VCr shifted the Ca2+ concentration–response curves to the right with suppression of the maximum response, like verapamil. In guinea-pig ileum preparations, VCr caused atropine-sensitive spasmogenic effects. When tested for its effect on human platelets, VCr inhibited the adrenaline and adenosine 5′-diphosphate (ADP)-induced human platelet aggregation at the concentration range of 0.3–1.2 mg/mL. These observations indicate the presence of spasmogenic, spasmolytic, and antiplatelet activities in Viscum cruciatum mediated through cholinergic and calcium channel antagonist activities along with the blockade of adrenergic and ADP receptors, respectively, which explains its medicinal use in gut motility and inflammatory disorders.
Introduction
Viscum cruciatum Sieber (Viscaceae), commonly known as “mistletoe”, is an evergreen hemi-parasitic perennial herb or shrub, characterized by its whorled leaves and red berries, usually epiphyting various dicotyledonous trees and shrubs (CitationAyuso et al., 1988). It is indigenous to the Northern Areas of Pakistan including Swat, Kurram Agency, and Barikot. It has been used in the native system of medicine as an antiseptic, emetic, purgative, anti-inflammatory, anti-arrhythmic, antispasmodic, anti-psychotic, and anti-epileptic, and is also considered useful to treat the liver and spleen enlargement (CitationNadkarni, 1976). Diarylheptanoid isolated from the aerial parts of the plant exhibited inhibitory action against cultured melanocytes, and renal and breast cancer cells (CitationCarmen et al., 2001), and is reported to be effective in prostate disorders (CitationMohammed et al., 2000). Strong antioxidant activity and low phenolic content have been documented in Viscum cruciatum (CitationAlali et al., 2007).
Cuticular wax obtained from Viscum cruciatum has been reported to contain minor proportions of flavonoid aglycones, flavonols, kaempferol, and quercetin, and a series of their methyl derivatives (CitationHaas et al., 2003). The widespread Viscum species has also been known to contain β-carotenes and syringenin (CitationDuke, 1992).
In this investigation we report gut inhibitory and stimulatory along with antiplatelet effects of the aqueous ethanol extract of Viscum cruciatum, thus rationalizing its usefulness in gastrointestinal motility and inflammatory disorders.
Materials and experimental protocol
Materials
Acetylcholine, atropine sulfate, adenosine 5′-diphosphate (ADP), adrenaline, and verapamil hydrochloride were purchased from Sigma Chemical Co., St. Louis, MO, USA. All chemicals used were of the highest grade available. The drugs were dissolved in distilled water and the subsequent dilutions were made fresh in distilled water/normal saline on the day of the experiment.
Animals
Guinea-pigs (500–550 g) and rabbits (1–1.2 kg) of local breed and either sex were used for this study. Animals were housed at the Animal House of The Aga Khan University, maintained at 23–25°C, and given a standard diet and tap water. Animals had free access to water but food was withdrawn 24 h prior to the experiment. Guinea-pigs were sacrificed by cervical dislocation and rabbits by a blow on the back of the head. Experiments complied with the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, CitationNational Research Council (1996) and were approved by the Ethics Committee of The Aga Khan University.
Plant material and extraction
The aerial parts of Viscum cruciatum were collected in April, 2004 from the Northern Areas of Pakistan (Swat). The plant was identified with the help of an expert taxonomist, Dr. Altaf A. Dasti, from the Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan. A sample voucher (VC-A-09-05) was submitted to the herbarium of the Department of Biological and Biomedical Sciences, The Aga Khan University, Karachi. The plant material was rendered free of soil and adulterated materials, shade dried at 40°C, and then ground into a coarse powder by an electrically driven device. The powdered material was soaked in aqueous-ethanol (80%) for 72 h with occasional shaking. The soaked material was rendered free of plant debris by passing through a muslin cloth, and the fluid portion was filtered through a fine filter paper (CitationWilliamson et al., 1996). The above-mentioned extraction procedure was repeated twice, and the filtrates were combined and subjected to evaporation under reduced pressure on a rotary evaporator to a thick paste-like mass of dark green color, i.e. the crude ethanol extract (VCr). The extract was transferred to Petri dishes and placed in desiccators to get rid of remaining solvent. The approximate yield of the extract was found to be 7.2%.
Phytochemical screening
Preliminary phytochemical analysis of the plant extract was carried out for the presence of alkaloids, anthraquinones, coumarins, flavonoids, saponins, sterols, tannins, and terpenes as described previously (CitationEvans, 1996; CitationEdeoga et al., 2005), with some modifications. Briefly, alkaloids were detected by treating the plant material with Dragendorff’s reagent, resulting in the appearance of a precipitate at the bottom of the test tube. Plant material was deemed positive for flavonoids when it gave a yellow color with AlCl3 reagent, and for tannins when a green or black color was produced with aqueous FeCl3. The observation of yellow fluorescence under ultraviolet light on examination of the filter paper previously exposed to vapors from the boiling plant material indicated the presence of coumarins. For the detection of sterols and terpenes, plant material was treated with petroleum ether and subsequently extracted with CHCl3. The gradual appearance of green to pink (for sterols) and pink to purple colors (for terpenes) was then noted after treatment of the CHCl3 layer with acetic anhydride and concentrated HCl in succession. The presence of saponins was based on the appearance of froth upon vigorous shaking of diluted samples. Lastly, anthraquinones were screened, by treatment of the plant material with NH4OH reagent added after dissolving it in 1% HCl and purifying with benzene. The appearance of a pink, violet, or red color demonstrated their presence.
Preparation of rabbit jejunum
The jejunum was dissected out, kept in Tyrode’s solution, and cleaned of mesenteries. Each segment of about 2 cm length was suspended in a 10 mL tissue bath containing Tyrode’s solution, maintained at 37°C, and aerated with a mixture of 95% oxygen and 5% carbon dioxide (carbogen). The composition of the Tyrode’s solution in mM was: KCl 2.68, NaCl 136.9, MgCl2 1.05, NaHCO3 11.90, NaH2PO4 0.42, CaCl2 1.8, and glucose 5.55. Intestinal responses were recorded isotonically using Bioscience transducers and an oscillograph. Each tissue was allowed to equilibrate for at least 30 min before the addition of any drug, and then stabilized with a sub-maximal concentration of acetylcholine (ACh) with a 3 min interval until constant responses were recorded. Under these experimental conditions, rabbit jejunum exhibits spontaneous rhythmic contractions, allowing the testing of relaxant (spasmolytic) activity directly without the use of an agonist (CitationGilani et al., 2005).
High K+ (80 mM) was used to depolarize the preparations (CitationFarre et al., 1991) in order to elucidate the Ca2+ channel blocking (CCB) effects. High K+ was added to the tissue bath, which produced a sustained contraction. Test materials were then added in a cumulative fashion to obtain concentration-dependent inhibitory responses (CitationVan Rossum, 1963).
To confirm the Ca2+ antagonist action of the test substance, the tissue was allowed to stabilize in normal Tyrode’s solution, which was then replaced with Ca2+-free Tyrode’s solution containing ethylenediaminetetraacetic acid (EDTA; 0.1 mM) for 30 min in order to remove Ca2+ from the tissues. This solution was further replaced with K+-rich and Ca2+-free Tyrode’s solution, having the following composition (mM): KCl 50, NaCl 91.04, MgCl2 1.05, NaHCO3 11.90, NaH2PO4 0.42, glucose 5.55, and EDTA 0.1. Following an incubation period of 30 min, control concentration–response curves (CRCs) of Ca2+ were obtained. When the control Ca2+ CRCs were found to be superimposable (usually after two cycles), the tissue was pretreated with the plant extract for 60 min to test the possible CCB effect. The CRCs of Ca2+ were reconstructed in the presence of different concentrations of the test material.
Preparation of guinea-pig ileum
The ileum was dissected out and kept in Tyrode’s solution. The segments, each of about 2 cm in length, were mounted individually in a 10 mL tissue bath, filled with Tyrode’s solution, at 37°C and aerated with carbogen (CitationGilani et al., 1999). An initial load of 0.7 g was applied to the tissue, and isotonic contractions were recorded with a Bioscience transducer coupled to a Harvard oscillograph. An equilibrium period of 30 min was given before administration of drugs. After equilibration, each tissue preparation was repeatedly treated with a sub-maximal concentration of ACh with a 3 min dose cycle until constant responses were recorded. The contractile effect of the test material was assessed as percent of the effect produced by the control drug, ACh (0.3 μM).
Preparation of human platelets
Blood was taken by vein puncture from normal human volunteers reported to be free of medications for 1 week. Blood samples were mixed with 3.8% (w/v) sodium citrate solution (9:1) and centrifuged at 260 × g, 20°C for 15 min to obtain platelet rich plasma (PRP). The platelet count was determined by phase-contrast microscopy, and all aggregation studies were carried out at 37°C, with PRP having platelet counts between 2.5 and 3.0 × 1011/L of plasma (CitationSaeed et al., 1997). All experiments were performed within 2 h of PRP preparation.
Measurement of platelet aggregation
Aggregation was monitored using a dual channel Lumi aggregometer (Model 400; Chronolog Corp., Chicago, IL, USA) using 0.45 mL aliquots of PRP (CitationShah & Saeed, 1995). The final volume was made up to 0.5 mL, with the test drug dissolved in either normal saline or appropriate vehicle known to be devoid of any effect on aggregation. Platelet aggregation was induced with the agonists adrenaline and ADP. The concentration-dependent anti-aggregatory effect was studied by pretreatment of PRP with plant extract for 1 min followed by the addition of adrenaline or ADP. The resulting aggregation was recorded for 5 min by the change in light transmission as a function of time.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM, n = number of experiments) and the median effective concentrations (EC50 values) with 95% confidence intervals (CIs). The statistical parameter applied was Student’s t-test. p < 0.05 was considered statistically significant. The CRCs were analyzed by non-linear regression using the GraphPad program (GraphPad, San Diego, CA, USA).
Results
Phytochemical analysis
The crude extract of Viscum cruciatum (VCr) was found to contain alkaloids, coumarins, flavonoids, saponins, sterols, tannins, and terpenes, while it tested negative for anthraquinones.
Effect on isolated rabbit jejunum
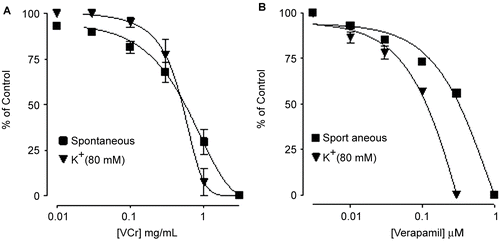
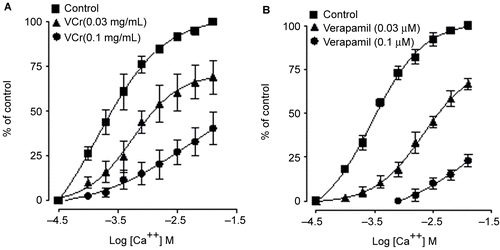
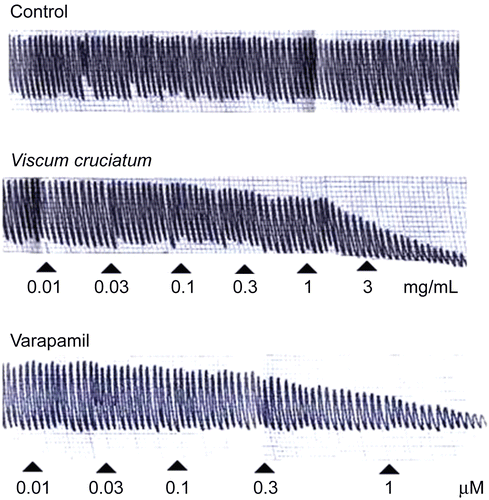
VCr relaxed the spontaneous () and K+ (80 mM)-induced contractions of isolated rabbit jejunum, with respective EC50 values of 0.66 (0.48–0.89, 95% CI, n = 5) and 0.55 mg/mL (0.35–0.85) as shown in . Verapamil inhibited spontaneous () and K+ (80 mM)-induced contractions, with EC50 values of 0.33 (0.24–0.45, 95% CI, n = 6) and 0.09 mg/mL (0.06–0.14), respectively (). VCr (0.03–0.1 mg/mL) caused a rightward shift in the Ca2+ concentration–response curves (CRCs), with suppression of the maximum response, similar to that caused by verapamil ().
Figure 1. Tracing showing the spasmolytic effect of Viscum cruciatum crude extract (VCr) and verapamil on spontaneously contracting isolated rabbit jejunum.
Effect on isolated guinea-pig ileum
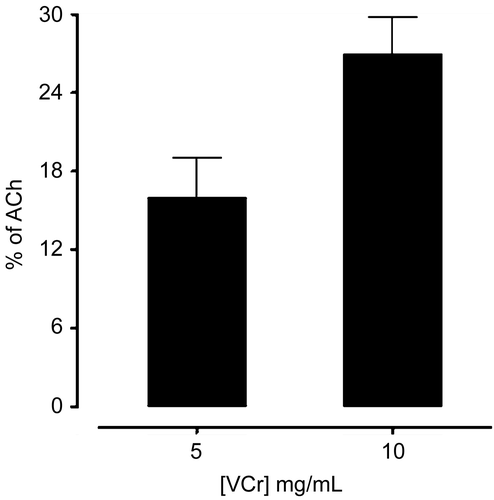
In guinea-pig ileum, VCr produced a concentration-dependent contractile effect. The efficacy of the spasmogenic effect was 16 ± 3.055 and 27 ± 2.752% (n = 5) at 5 and 10 mg/mL respectively, when compared to the response of acetylcholine (ACh) 0.3 μM (). Pretreatment of the tissues with atropine abolished the stimulant effect of VCr (data not shown).
Effect on agonist induced human platelet aggregation
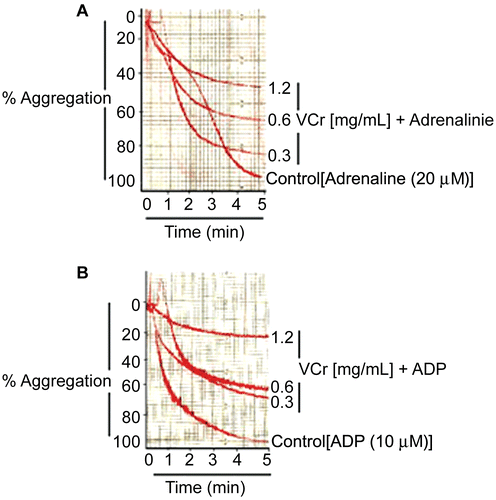
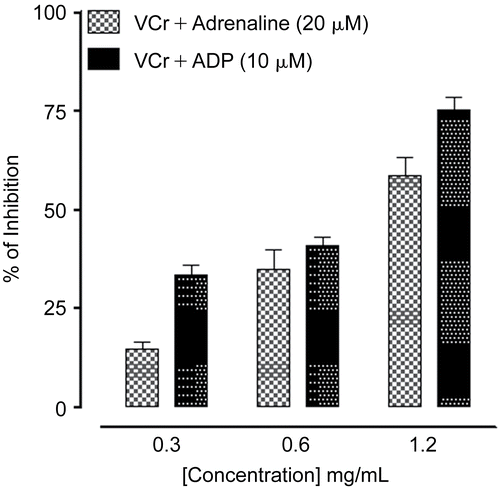
VCr at concentrations of 0.3, 0.6, and 1.2 mg/mL caused 15 ± 1.76, 35 ± 5.04, 59 ± 4.48 and 33 ± 2.60, 41 ± 2.33, 75 ± 3.18% (n = 3) inhibition of adrenaline (20 μM) and adenosine 5′-diphosphate (ADP, 10 μM)-induced human platelet aggregation, respectively. shows typical tracings, while the combined data from different experiments are plotted in . At the maximal tested concentration of 1.2 mg/mL, VCr was more effective against ADP-mediated aggregation than against adrenaline-induced platelet aggregation (p < 0.05).
Discussion
In view of the well known use of Viscum cruciatum as an antispasmodic, it was tested in spontaneously beating isolated rabbit jejunum. VCr relaxed the spontaneous contractions of jejunum, thus showing its spasmolytic effect. The contraction of smooth muscle preparations, including rabbit jejunum, is dependent upon an increase in the cytoplasmic free calcium [Ca2+], which activates the contractile elements (CitationKaraki & Wiess, 1983). In earlier studies we observed that the spasmolytic effect of medicinal plants is usually mediated through blockade of Ca2+ channels (CitationGilani et al., 2000, Citation2005, Citation2006). To elucidate the Ca2+ antagonist effect, tissues were precontracted with high K+, which is known to cause smooth muscle contractions through opening of L-type Ca2+ channels, thus allowing the influx of extracellular Ca2+ resulting in contraction (CitationBolton, 1979); a substance that relaxes such contraction is considered a Ca2+ antagonist (CitationGodfraind et al., 1986). The extract inhibited high K+-induced contraction, similar to that caused by verapamil, a standard Ca2+ antagonist (CitationFleckenstein, 1977). The Ca2+ antagonist action was further confirmed when VCr, like verapamil, caused a rightward shift in the Ca2+ CRCs. Calcium antagonists constitute an important therapeutic group (CitationTriggle, 1992), and the common characteristic of these drugs is their concentration-dependent inhibition of the slow entry of calcium and their capacity for reversal of this effect by Ca2+ (CitationFleckenstein, 1977). The observed effect of the plant extract to inhibit the induced contractions, followed by a rightward displacement of Ca2+ concentration–response curves, strongly suggests the presence of Ca2+ antagonist(s) in VCr. Thus, the present study provides a pharmacological base for its medicinal use in the hyperactive status of the gut, as Ca2+ antagonists are known to be effective in such conditions (CitationBrunton, 1996).
The plant has also been used to relieve constipation (CitationNadkarni, 1976). It is possible that it contains gut-stimulant constituents, remaining unexpressed in the model of rabbit jejunum, used primarily for the assessment of spasmolytic activity (CitationGhayur & Gilani, 2005). In the guinea-pig ileum, a quiescent preparation considered to be more responsive to stimulants (CitationGilani & Aftab, 1992), VCr showed a moderate contractile effect, as expected. Pretreatment of the tissues with atropine, a muscarinic receptor antagonist (CitationArunlakhshana & Schild, 1959), abolished the spasmogenic effect of VCr, similar to that of ACh, which indicates that the plant extract mediates its gut stimulatory action through a cholinergic pathway. ACh is a neurotransmitter released by the parasympathetic nervous system, causing gut excitation through stimulation of M3 receptor subtypes, and hence plays an important physiological role in regulating the peristaltic movements of the gastrointestinal system (CitationBrown & Taylor, 1996). The observed spasmolytic (Ca2+ antagonist) and spasmogenic (cholinergic) effects of the extract may be due to the presence of flavonoids and saponins, respectively.
Phytochemicals have been reported to exhibit calcium channel blocking (CitationRevuelta et al., 1997) and cholinomimetic (CitationGilani et al., 1994) activities.
Based on the use of Viscum cruciatum as an anti-inflammatory remedy (CitationNadkarni, 1976), the extract was tested for antiplatelet effects in human platelet-rich plasma against adrenaline- and ADP-induced platelet aggregation. In this model, VCr inhibited the platelet aggregation. Adrenaline is known to cause platelet aggregation through activation of α2-adrenergic receptors, while ADP causes activation of P2 Y1 and P2 Y12 receptors, resulting in inhibition of the adenyl cyclase pathway, thus leading to decreased intracellular cAMP (cyclic adenosine monophosphate), which in turn raises the cytosolic free Ca2+ concentration (CitationKimura & Okuda 1994; CitationOury et al., 2006). The plant extract inhibited ADP- and adrenaline-induced platelet aggregation possibly due to an intrinsic Ca2+ antagonist effect, as the Ca2+ channel blockers are known to possess antiplatelet activity (CitationSalam et al., 1991).
This study clearly shows that the crude extract of Viscum cruciatum contains components with spasmolytic (Ca2+ antagonist) and spasmogenic (cholinomimetic) effects, which explains its use in the hyper- and hypoactive status of the gut such as abdominal spasms and constipation. The observed antiplatelet (adrenergic and ADP receptor antagonist) activity validates the Viscum cruciatum effectiveness as an anti-inflammatory agent.
Acknowledgements
This study was carried out during the elective-ship of Malik Hassan Mehmood at The Aga Khan University and Dr. Panjwani Center for Molecular Medicine and Drug Research, University of Karachi, with financial support from the Higher Education Commission, Government of Pakistan.
References
- Alali FQ, Tawaha K, El-Elimat T, Syouf M, El-Fayad M, Abulaila K, Nielsen SJ, Wheaton WD, Falkinham JO, Oberlies NH (2007): Antioxidant activity and total phenolic content of aqueous and methanolic extracts of Jordanian plants: An ICBG project. Nat Prod Res 12: 1121–1131.
- Arunlakhshana O, Schild HO (1959): Some quantitative uses of drug antagonists. BrJ Pharmacol 14: 48–58.
- Ayuso MJ, Martín-Cordero C, Sáenz MT (1988): Activité antimitotique de Viscum cruciatum Sieber parasite de Olea europaea subsp. Europaea et de Retama sphaerocarpa. Fitoterapia 59: 222–226.
- Bolton TB (1979): Mechanism of action of transmitters and other substances on smooth muscles. Physiol Rev 59: 606–718.
- Brown JH, Taylor P (1996): Muscarinic receptor agonists and antagonists. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, eds., Goodman & Gillman’s the Pharmacological Basis of Therapeutics, 9th ed. New York, McGraw-Hill, pp. 141–159.
- Brunton LL (1996): Agents effecting gastrointestinal water flux and motility; emesis and antiemetics; bile acids and pancreatic enzymes. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, eds., Goodman & Gillman’s the Pharmacological Basis of Therapeutics, 9th ed. New York, McGraw-Hill, pp. 917–936.
- Carmen MC, Miguel LL, María AA, Eduardo N, Juan T, María JA (2001): A cytotoxic diarylheptanoid from Viscum cruciatum. Phytochemistry 58: 567–569.
- Duke JA (1992): Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants, 2nd ed. Boca Raton, CRC Press, p. 636.
- Edeoga HO, Okwu DE, Mbaebie BO (2005): Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotech 4: 685–688.
- Evans WC (1996): Trease and Evan’s Pharmacognosy, 14th ed. London, WB Saunders, pp. 161–408.
- Farre AJ, Columbo M, Fort M, Gutierrez B (1991): Differential effects of various Ca++ antagonists. Gen Pharamacol 22: 177–181.
- Fleckenstein A (1977): Specific pharmacology of Ca++ in myocardium cardiac pacemakers and vascular smooth muscle. Rev Pharmacol Toxicol 17: 149–166.
- Ghayur MN, Gilani AH (2005): Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci 50: 1889–1897.
- Gilani AH, Aftab K (1992): Presence of acetylcholine-like substance(s) in Sesamum indicum. Arch Pharmacal Res 14: 3–6.
- Gilani AH, Aftab K, Ahmed S(1994): Cholinergic actions of crude saponins from Castanospermum australe. Int J Pharmacog 32: 209–216.
- Gilani AH, Aziz N, Ahmed M, Alam MT, Riawani GH (1999): Spasmogenic and spasmolytic constituents in Sida pkistanica. Pharm Biol 37: 173–180.
- Gilani AH, Aziz N, Khurram IM, Rao ZA, Ali BA (2000): The presence of cholinomimetic and calcium antagonist constituents in Piper betle Linn. Phytother Res 14: 338–344.
- Gilani AH, Shah AJ, Ghayur MN, Majeed K (2005): Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci 76: 3089–3105.
- Gilani AH, Khan A, Ghayur MN (2006): Presence of calcium antagonist and cholinomimetic constituents explains the medicinal uses of olives in gastrointestinal disorders. Nutr Res 26: 277–283.
- Godfraind T, Miller R, Wibo M (1986): Calcium antagonism and calcium entry blockade. Pharmacol Rev 38: 321–416.
- Haas K, Bauer M, Wollenweber E (2003): Cuticular waxes and flavonol aglycones of mistletoes. Z Naturforsch (7–8): 464–470.
- Karaki H, Wiess G (1983): Mini-review: calcium release in smooth muscles. Life Sci 42: 111–112.
- Kimura Y, Okuda H (1994): Effects of alpha and beta-adrenergic antagonist on epinephrine-induced aggregation and intracellular free calcium concentration in human platelets. Biochem Biophys Res Commun 202: 1069–1075.
- Mohammed S, Ali S, Zohara Y, Jamal M (2000): Ethnobotanical survey in the Palestinian area: a classification of the healing potential of medicinal plants. J Ethnopharmacol 73: 221–232.
- Nadkarni KM (1976): Indian Materia Medica, 3rd ed. Bombay, Popular Prakashan, pp. 560–561.
- National Research Council (1996): Guide for the Care and Use of Laboratory Animals. Washington, National Academy Press, pp. 1–7.
- Oury C, Toth ZE, Vermylen J, Hoylaerts MF (2006): The platelet ATP and ADP receptors. Curr Pharm Des 12: 859–875.
- Revuelta MP, Cantabrana B, Hidalgo A (1997): Depolarization-dependent effect of flavonoids in rat uterine smooth muscle contraction elicited by CaCl2. Gen Pharmacol 29: 847–857.
- Saeed SA, Shah BH, Khan N, Gilani AH (1997): Synergistic interaction of calcium-ionophore, A-23187 and dopamine in human platelet aggregation. Med Sci Res 25: 219–221.
- Salam SR, Saxena R, Saraya AK (1991): Effect of calcium channel blocker (diltiazem) on platelet aggregation. Indian J Exp Biol 29: 484–485.
- Shah BH, Saeed SA (1995): Phosphatidylinositol 3-kinase inhibitor, wortmannin, inhibits 5-hydroxytryptamine-mediated potentiation of platelet aggregation induced by epinephrine. Res Commun Mol Pathol Pharmacol 89: 157–164.
- Triggle DJ (1992): Drugs affecting calcium-regulation and actions. In: Smith GM, Reynard AM, eds., Textbook of Pharmacology. Philadelphia, WB Saunders Co., pp. 453–479.
- Van Rossum JM (1963): Cumulative concentration-response curves. II. Techniques for the making of concentration-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther 143: 299–330.
- Williamson EM, Okpako DT, Evans FJ (1996): Selection, Preparation and Pharmacological Evaluation of Plant Material. Chichester, John Wiley & Sons, pp. 15–23.