Abstract
This study describes the antidiarrheal, antisecretory, and bronchodilatory activities of Hypericum perforatum Linn. (Hypericaceae), commonly known as St. John’s wort, to justify its traditional use in the hyperactivity of the gastrointestinal and respiratory systems. The crude extract of Hypericum perforatum (Hp.Cr) at a dose of 500 mg/kg caused 20% protection against castor oil-induced diarrhea in mice and 60% at 1000 mg/kg (p < 0.05 vs. saline). Hp.Cr at 300 and 1000 mg/kg reduced the castor oil-induced fluid accumulation in mice to 107.0 ± 3.3 g (p < 0.01) and 84.0 ± 4.2 g (p < 0.001) respectively, whereas in the castor oil-treated group, it was 126.9 ± 3.9 g. When tested against carbachol (CCh)-mediated bronchoconstriction in rats under anesthesia, Hp.Cr dose-dependently (3– 30 mg/kg) suppressed the CCh (1 μmol/kg)-induced increase in the inspiratory pressure. Thus this study rationalizes the Hypericum perforatum usefulness in overactive gut and airways disorders, such as diarrhea and asthma.
Introduction
Hypericum perforatum Linn. (Hypericaceae) is commonly known as St. John’s wort. The genus Hypericum consists of more than 370 species (CitationBaquar, 1989; CitationNahrstedt & Butterweck, 1997). Among the various species of Hypericum, the plant Hypericum perforatum is well known for therapeutic efficacy. Euryphon, a Greek physician in 288 bc, first described the medicinal value of Hypericum perforatum, and it was prescribed by Hippocrates himself (CitationMahady et al., 2001). Since the middle of the 19th century, the application of St. John’s wort for mild to moderate depression has become prominent worldwide. Its use in crude form (botanical) for the treatment of mild to moderate depression is a common practice of modern physicians in Germany and other European countries (CitationMiller & Murray, 1998; CitationMuller, 2003).
Hypericum perforatum is used in folk medicine to treat asthma, bronchitis, diarrhea (CitationNadkarni, 1976; CitationMcKenna et al., 2002), excoriations, hemorrhoids, and wounds (CitationBaquar, 1989). It is also considered useful as an anthelmintic, astringent, detersive, diuretic, and emmenagogue. The herb is used to treat burns, eczema, and hysteria (CitationBarnes et al., 2001; CitationMateo et al., 2002). Its oil is taken orally to treat abdominal spasm, gastritis, gastric ulcers, and inflammatory conditions of the colon (CitationWeiss, 1988). The powdered drug or extracts of the plant are used as an antirheumatic, as well as for supportive treatment of nervous excitement and sleep disturbances (CitationWyk et al., 2002). It is known to be a useful herbal remedy for the treatment of neurological disorders, such as coxalgia, menopausal neurosis, headache, hypersensitivity, mental ailments, neuralgia, paralysis, spinal convulsions, stiff neck, and tetanus (CitationOzturk et al., 1996).
Phytochemical studies revealed the presence of multiple chemicals in the plant, such as ascorbic acid, brenzcatechin, cadinine, carotene, carotenoids, catechin, choline, emodinantherol, epicatechin, gurjunene, hyperforin, hypericin, hyperoside, imanin, isohype-ricin, isoquercitrin, limonene, mannitol, myristic acid, pectin, phenol, phlobaphene, phloroglucinol, protohypericin, pseudohypericin, pyrogallol, quercetin, quercitrin, resorcynol, rutin, saponin, tannins (CitationDuke, 1992; CitationBombardelli & Morazzoni, 1995), amino acids, biflavones, essential oils, flavonols, glycosides, naphthodianthrones, phenylpropanes, proanthocyanidins, and xanthones (CitationNahrstedt & Butterweck, 1997; CitationWheatley, 1998).
Hypericum perforatum has been known to possess antidepressant (CitationHarrer et al., 1999; CitationJosey & Tackett, 1999; CitationWoelk, 2000), anxiolytic (CitationKumar et al., 2000), antioxidant (CitationTripathi & Pandey, 1999), antibacterial (CitationKeles et al., 2001), antifungal (CitationKhosa & Bhatia, 1982), anti-inflammatory, analgesic (CitationKumar et al., 2001), antiviral (CitationSerkedjieva et al., 1990), hepatoprotective (CitationOzturk et al., 1992), sedative (CitationGirzu et al., 1997), memory enhancing (CitationKhalifa, 2001), and nephroprotective (CitationShibayama et al., 2007) properties.
We have recently reported the antispasmodic and tracheo-relaxant effects of St. John’s wort in isolated tissue preparations, mediated through a combination of Ca2+ antagonist and phosphodiesterase (PDE)-inhibitory mechanisms (CitationGilani et al., 2005a). The present in vivo studies were carried out to validate its effectiveness against the hyperactive state of the gut and airways.
Materials and methods
Chemicals
Atropine sulfate, carbachol (CCh), loperamide, and theophylline were purchased from Sigma Chemical Co., St. Louis, MO, USA. Pentothal sodium (thiopental sodium) and castor oil were respectively obtained from Abbott Laboratories and KCL Pharma, Karachi, Pakistan. All chemicals used were of the analytical grade available.
Animals
Animals used in this study, Sprague-Dawley rats (200–250 g) and Balb-C mice (20–25 g) of either sex, were of local breed, housed at the Animal House of The Aga Khan University, maintained at 23–25°C. Animals were given tap water ad libitum and a standard diet consisting of (g/kg): flour 380, chokar 380, molasses 12, NaCl 5.8, Nutrivet L 2.5, potassium bisulfate 1.2, vegetable oil 38, fish meal 170, and powdered milk 150. Experiments performed complied with the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, CitationNational Research Council (1996) and were approved by the Ethics Committee of The Aga Khan University.
Plant material and preparation of crude extract
The aerial parts (stem and leaves) of Hypericum perforatum were collected from the Northern Areas of Pakistan (Gillyat) in May 2002. The plant was identified with the help of a taxonomist, Dr. M. Ibrar, at the Department of Pharmacy, University of Peshawar, Peshawar, NWFP, Pakistan. A voucher specimen (PUP 012529) was submitted to the Herbarium of the Department of Botany of the same university. The plant material was cleaned, dried, and coarsely ground. The powdered material was extracted with 70% aqueous-ethanol by cold maceration for 3 days with occasional shaking. It was filtered through a muslin cloth and then through a Whatman qualitative grade 1 filter paper (CitationWilliamson et al., 1998). This procedure was repeated twice, and the combined filtrate was evaporated on a rotary evaporator under reduced pressure (–760 mmHg) to a thick, semi-solid mass of dark brown color, i.e., crude extract of Hypericum perforatum (Hp.Cr), yielding approximately 18.52% (w/w). Hp.Cr was completely solubilized in saline (0.9%).
Castor oil-induced diarrhea
Mice were fasted for 24 h before the experiment (CitationDas et al., 1999; CitationGilani et al., 2005b; CitationBorelli et al., 2006). Animals were housed in individual cages and divided into four groups, each containing five mice. The first group received saline (10 mL/kg, p.o.) and served as a negative control. The doses of test extract were selected on a trial basis and two increasing doses were given orally. A group of mice was treated with loperamide ( 10 mg/kg, p.o.), as a positive control. One hour after treatment, each animal received 10 mL/kg of castor oil orally through a feeding needle. Afterward, the cages were inspected for the presence of diarrhea droppings; their absence was noted as a positive result, indicating protection from diarrhea at that time.
Intestinal fluid accumulation
Intestinal fluid accumulation was studied by the enteropooling assay (CitationIzzo et al., 1994; CitationCapasso et al., 2002). Different groups of overnight-fasted mice were treated with increasing doses of extract intraperitoneally through a detachable U-100 insulin syringe with a 25G × 1 in (0.50 × 25 mm) needle, 1 h before the administration of castor oil (10 mL/kg, p.o.). The mice were sacrificed 30 min later by cervical dislocation and the entire intestine was removed and weighed with care, not allowing any intestinal fluid to leak out. The results are expressed as (Pi/Pm) × 1000, where Pi is the weight (g) of the intestine and Pm is the weight of the animal (Gilani et al., Citation2008a).
Bronchodilatory activity
Rats were anesthetized with sodium thiopental (Pentothal; 80– 100 mg/kg, i.p.), then intubated with a tracheal tube and ventilated with a volume ventilator (Miniature Ideal pump; Bioscience, UK) adjusted to a rate of 70–80 strokes/min to deliver 7–10 mL/kg of room air (CitationDar & Channa, 1997; CitationChanna et al., 2005). A polyethylene catheter was inserted into the jugular vein for drug administration. Changes in airway resistance (mmHg) were measured by a pressure transducer (MLT-1199) connected to the side arm of a tracheal cannula and recorded by a PowerLab 4/25 with running chart software via a Quad bridge amplifier (ADInstruments, Bella Vista, NSW, Australia). Bronchoconstriction was induced with CCh (1 μmol/kg), which was reversed within 7–10 min. The test drug was given to the animals 5–8 min prior to administration of CCh. The responses are expressed as percent reduction of the CCh-induced bronchospasm (Gilani et al., Citation2008b).
Acute toxicity test
Animals were divided into groups of five mice each. The test was performed using increasing doses of test extract, given orally, in a 10 mL/kg volume to different groups serving as test groups (CitationSanmugapriya & Venkataraman, 2006). Another group of mice was administered saline (10 mL/kg, p.o.) as negative control. The mice were allowed food ad libitum during the 24 h test and kept under regular observation for mortality.
Statistical analysis
The data are expressed as mean ± standard error of the mean (SEM) and were analyzed using the GraphPad program (GraphPad, San Diego, CA, USA). The statistical parameter applied was Student’s t-test except in the case of antidiarrheal study, where the χ2 test was used. p < 0.05 was noted as significantly different.
Results
Effect on castor oil-induced diarrhea
Hp.Cr exhibited a dose-dependent (500– 1000 mg/kg) protective effect against castor oil-induced diarrhea in mice. The negative control treatment (saline) did not protect the animals from diarrhea. Pretreatment of animals with Hp.Cr produced 20% protection from diarrhea at 500 mg/kg and 60% protection at 1000 mg/kg (p < 0.05 vs. saline group). Loperamide ( 10 mg/kg), a standard antidiarrheal agent, showed complete protection (100%, p < 0.01 vs. saline group) from diarrhea in the positive control group ().
Table 1. Effect of the crude extract of Hypericum perforatum (Hp.Cr) on castor oil (C.oil, 10 mL/kg)-induced diarrhea in mice.
Effect on intestinal fluid accumulation
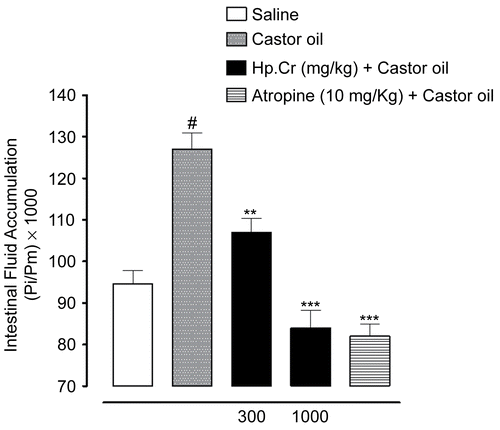
When tested against castor oil-induced intestinal fluid accumulation in mice, Hp.Cr exhibited a dose-dependent (300– 1000 mg/kg) antisecretory effect. Intestinal fluid accumulation in the saline treated group was 94.6 ± 3.2 g (mean ± SEM, n = 5), whereas in the castor oil-treated group, it was 126.9 ± 3.9 g (p < 0.001 vs. saline group). Hp.Cr at the doses of 300 and 1000 mg/kg reduced the castor oil-induced fluid accumulation to 107.0 ± 3.3 g (p < 0.01 vs. castor oil group) and 84.0 ± 4.2 g (p < 0.001 vs. castor oil group), respectively (). Atropine at the dose of 10 mg/kg decreased the intestinal fluid accumulation to 82.0 ± 3.0 g (p < 0.001 vs. castor oil group) as shown in .
Figure 1. Effect of the crude extract of Hypericum perforatum (Hp.Cr) and atropine on castor oil-stimulated fluid accumulation in small intestine of mice. Results shown are mean ± SEM for five animals in each experimental group. Intestinal fluid accumulation is expressed as Pi/Pm × 1000 (g) where Pi is the weight of the small intestine and Pm is the weight of the mouse. #p < 0.001 vs. saline group, **p < 0.01 and ***p < 0.001 vs. castor oil group, Student’s t-test.
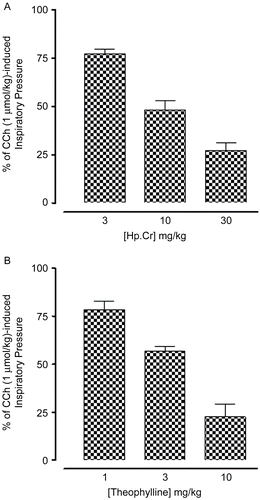
Effect on carbachol-induced bronchoconstriction
Hp.Cr at the doses of 3, 10, and 30 mg/kg caused 23.8 ± 2.3, 51.7 ± 4.9, and 72.7 ± 4.1% (n = 4) respective inhibition of the CCh (1 μmol/kg)-evoked increase in inspiratory pressure of anesthetized rats (). Theophylline was used as a positive control, which suppressed the CCh (1 μmol/kg)-induced bronchoconstriction at 1, 3, and 10 mg/kg by 21.5 ± 4.3, 43.4 ± 2.7, and 77.5 ± 6.6% (n = 4) respectively ().
Acute toxicity study
The two different groups of mice were given Hp.Cr in the graded doses of 5 and 10 g/kg, respectively, and the animals were observed for mortality 24 h after drug administration. The extract did not cause any mortality up to the dose of 10 g/kg.
Discussion
Since Hypericum perforatum has been used in diarrhea (CitationNadkarni, 1976; CitationMcKenna et al., 2002), its extract was tested for a protective effect against castor oil-induced diarrhea in mice. The induction of diarrhea with castor oil results from the action of ricinoleic acid formed by the hydrolysis of oil (CitationIwao & Terada, 1962), which produces changes in the transport of electrolytes and water (CitationGaginella & Phillips, 1975), resulting in the generation of giant contractions of the transverse and distal colon (CitationCroci et al., 1997). Thus, a potential antidiarrheal agent may exhibit its antidiarrheal effect by inhibiting bowel contractions. Hypericum perforatum extract protected the mice against castor oil-induced intestinal fluid secretions, which also contributes to its potential in bowel inflammatory conditions such as gastric ulcers (CitationWeiss, 1988). St. John’s wort was previously observed to suppress irinotecan-induced diarrhea and intestinal lesions through inhibition of proinflammatory cytokines and intestinal epithelial apoptosis (CitationHu et al., 2006), hence accounting for its gastrointestinal protective profile.
Based on the traditional use of Hypericum perforatum in congestive respiratory disorders (CitationMcKenna et al., 2002), it was tested for a possible bronchodilatory effect in anesthetized rats. The extract inhibited CCh-evoked bronchospasm in a dose-dependent manner, like that caused by theophylline, a standard bronchodilator (CitationRabe et al., 1995), thus justifying its application in asthma and bronchitis. Hypericum perforatum is reported to exhibit calcium channel blocking and PDE-inhibitory effects (CitationGilani et al., 2005a); hence the present observations (gut and airways inhibitory activities) are in accordance with the expectation, as both Ca2+ antagonists and PDE inhibitors are considered effective for such actions (CitationReynolds et al., 1984; CitationScratcherd & Grundy, 1984; CitationSopory et al., 2004; CitationBarnes, 2006).
The flavonoids are well known for their antidiarrheal, antisecretory, and bronchodilatory activities (CitationDi Carlo et al., 1993; CitationPietta, 1998; CitationKhan & Gilani, 2006; CitationGhayur et al., 2007) and the presence of these types of compounds, such as quercetin, rutin, catechin, and hyperforin, in St. John’s wort is likely to contribute to its gastrointestinal and respiratory effects, though the likely role of tannins present in the plant cannot be ignored, as the tannins are known to have a beneficial role in diarrhea (CitationHeinrich et al., 1992; CitationGilani et al., 2006). In acute toxicity testing, the extract was found safe up to the maximum dose (10 g/kg) tested, which is in line with the wide therapeutic use of St. John’s wort.
In conclusion, the present study, by reporting the antidiarrheal, antisecretory, and bronchodilatory effects of Hypericum perforatum, contributes toward evidence-based phytomedicine.
Acknowledgements
The authors are thankful to Dr. Fazal Subhan (Department of Pharmacy, University of Peshawar, Pakistan) for supply of the plant material.
Declaration of interest: This study was supported by funds made available by the Pakistan Science Foundation and Higher Education Commission, Government of Pakistan.
References
- Baquar SR (1989): Medicinal and Poisonous Plants of Pakistan. Karachi, Printas, pp. 235–236.
- Barnes J, Anderson LA, Phillipson JD (2001): St. John’s wort (Hypericum perforatum): A review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol 53: 583–600.
- Barnes PJ (2006): Drugs for asthma. Br J Pharmacol 147: S297–S303.
- Bombardelli E, Morazzoni P (1995): Hypericum perforatum. Fitoterapia 31: 43–58.
- Borelli F, Capasso F, Capasso R, Ascione V, Aviello G, Longo R, Izzo AA (2006): Effect of Boswellia serrata on intestinal motility in rodents: Inhibition of diarrhoea without constipation. Br J Pharmacol 148: 553–560.
- Capasso R, Izzo AA, Borelli F, Russo A, Sautebin L, Pinto A, Capasso F, Mascolo N (2002): Effect of piperine, the active ingredient of black pepper on intestinal secretion in mice. Life Sci 71: 2311–2317.
- Channa S, Dar A, Ahmed S, Atta-ur-Rahman (2005): Evaluation of Alstonia scholaris leaves for broncho-vasodilatory activity. J Ethnopharmacol 97: 469–476.
- Croci T, Landi M, Elmonds-Alt X, Le Fur G, Maffrand JP, Manara L (1997): Role of tachykinins in castor oil-induced diarrhea in rats. Br J Pharmacol 121: 375–380.
- Dar A, Channa S (1997): Bronchodilatory and cardiovascular effects of an ethanol extract of Bacopa monniera in anaesthetized rats. Phytomedicine 4: 319–323.
- Das AK, Mandal SC, Banarjee SK, Sinha S, Das J, Saha BP, Pal M (1999): Studies on antidiarrheal activity of Punica granatum seed extract in rats. J Ethnopharmacol 68: 205–208.
- Di Carlo G, Autore G, Izzo AA, Maiolino P, Mascolo N, Viola P, Diurno MV, Capasso F (1993): Inhibition of intestinal motility and secretion by flavonoids in mice and rats: structure-activity relationships. J Pharm Pharmacol 45: 1054–1059.
- Duke JA (1992): Handbook of Phytochemical Constituents of GRAS Herbs and Other Economical Plants. London, CRC Press, pp. 302–303.
- Gaginella TS, Phillips SF (1975): Ricinoleic acid: Current view of ancient oil. Dig Dis Sci 20: 1171–1177.
- Ghayur MN, Khan H, Gilani AH (2007): Antispasmodic, bronchodilator and vasodilator activites of (+)-catechin, a naturally occurring flavonoid. Arch Pharm Res 30: 970–975.
- Gilani AH, Khan A, Subhan F, Khan M (2005a): Antispasmodic and bronchodilator activities of St. John’s wort are putatively mediated through dual inhibition of calcium influx and phosphodiesterase. Fund Clin Pharmacol 19: 695–705.
- Gilani AH, Ghayur MN, Khalid A, Zaheer-ul-Haq Q, Choudhary MI, Atta-ur-Rahman (2005b): Presence of antispasmodic, antidiarrhoeal, antisecretory, calcium antagonist and acetylcholinesterase inhibitory steroidal alkaloids in Sarcocca saligna. Planta Med 71: 1–6.
- Gilani AH, Khan A, Ghayur MN, Ali SF, Herzig JW (2006): Antispasmodic effect of Rooibos tea (Aspalathus linearis) is mediated predominantly through K+ channel activation. Basic Clin Pharmacol Toxicol 99: 365–373.
- Gilani AH, Khan A, Raoof M, Ghayur MN, Siddiqui BS, Vohra W, Begum S (2008a): Gastrointestinal, selective airways and urinary bladder relaxant effects of Hyoscyamus niger are mediated through dual blockade of muscarinic receptors and Ca++ channels. Fund Clin Pharmacol 22: 87–99.
- Gilani AH, Khan A, Ali T, Ajmal S (2008b): Mechanisms underlying the antispasmodic and bronchodilatory properties of Terminalia bellerica fruit. J Ethnopharmacol 116: 528–538.
- Girzu M, Carnat A, Privat AM, Fialip J, Carnat AP, Lamaison JL (1997): Sedative activity in mice of a hydroalcohol extract of Hypericum perforatum. Phytother Res 11: 395–397.
- Harrer G, Schmidt U, Kuhn U, Biller A (1999): Comparison of equivalence between the St. John’s wort extract LoHyp-57 and fluoxetine. Arzneimittelforschung 49: 289–296.
- Heinrich M, Rimpler H, Barrera NA (1992): Indigenous phytotherapy of gastrointestinal disorders in a low and mix community (Oaxaca, Mexico): Ethnopharmacologic evaluation. J Ethnopharmacol 36: 63–80.
- Hu ZP, Yang XX, Chan SY, Xu AL, Duan W, Zhu YZ, Sheu FS, Boelsterli UA, Chan E, Zhang Q, Wang JC, Ee PL, Koh HL, Huang M, Zhou SF (2006): St. John’s wort attenuates irinotecan-induced diarrhea via down-regulation of intestinal pro-inflammatory cytokines and inhibition of intestinal epithelial apoptosis. Toxicol Appl Pharmacol 216: 225–237.
- Iwao I, Terada Y (1962): On the mechanism of diarrhea due to castor oil. Jpn J Pharmacol 12: 137–145.
- Izzo AA, Mascolo N, Carlo GD, Capasso F (1994): NG-nitro-l-arginine methyl ester modulates intestinal secretion and motility produced by carbachol. Eur J Pharmacol 271: 31–35.
- Josey ES, Tackett RL (1999): St. John’s wort: A new alternative for depression. Int J Clin Pharmacol Ther 37: 111–119.
- Keles O, Bkirel T, Ak S, Alpmar A (2001): The antibacterial activity of some plants used for medicinal purposes against pathogens of veterinary importance. Fol Veterinaria 45: 22–25.
- Khalifa AE (2001): Hypericum perforatum as a nootropic drug: enhancement of retrieval memory of a passive avoidance conditioning paradigm in mice. J Ethnopharmacol 76: 49–57.
- Khan A, Gilani AH (2006): Selective bronchodilatory effect of Rooibos tea (Aspalathus linearis) and its flavonoid: chrysoeriol. Eur J Nutr 45: 463–469.
- Khosa RL, Bhatia N (1982): Antifungal effect of Hypericum perforatum. J Sci Res Plants Med 3: 49–50.
- Kumar V, Jaiswal AK, Singh PN, Bhattacharya SK (2000): Anxiolytic activity of Indian Hypericum perforatum: an experimental study. Indian J Exp Biol 38: 36–41.
- Kumar V, Singh PN, Bhattacharya SK (2001): Anti-inflammatory and analgesic activity of indian Hypericum perforatum. Indian J Exp Biol 39: 339–343.
- Mahady GB, Fong HS, Fransworth NR (2001): Botanical Dietary Supplements: Quality, Safety and Efficacy. Lisse, Swets and Zeitlinger, pp. 245–261.
- Mateo SCC, Prado B, Rabanal RM (2002): Antidepressant effects of the methanol extract of several Hypericum species from the Canary Islands. J Ethnopharmacol 79: 119–127.
- McKenna DJ, Jones K, Hughes K, Humphrey S (2002): Botanical Medicines: The Desk Reference for Major Herbal Supplements, 2nd ed. New York, Haworth Herbal Press, pp. 923–986.
- Miller LG, Murray WJ (1998): Herbal Medicinals: A Clinician’s Guide. Binghamton, Pharmaceutical Products Press, pp. 219–220.
- Muller WE (2003): Current St. John’s wort research from mode of action to clinical efficacy. Pharmacol Res 47: 101–109.
- Nadkarni KM (1976): Indian Materia Medica, 3rd ed. Bombay, Popular Prakashan, pp. 670–673.
- Nahrstedt A, Butterweck V (1997): Biologically active and other chemical constituents of the Hypericum perforatum. Pharmacopsychiatry 30: 129–134.
- National Research Council (1996): Guide for the Care and Use of Laboratory Animals. Washington, National Academy Press, pp. 1–7.
- Ozturk Y, Aydin S, Baser KHC, Kirmer N, Ozturk KN (1992): Hepatoprotective activity of Hypericum perforatum alcoholic extract in rodents. Phytother Res 6: 44–46.
- Ozturk Y, Aydin S, Beis R, Baser KHC, Berberoglu H (1996): Effects of Hypericum perforatum and Hypericum calycinum extracts on the central nervous system in mice. Phyotmedicine 3: 139–146.
- Pietta P (1998): Flavonoids in medicinal plants. In: Rice-Evans CV, Packer L, eds., Flavonoids in Health and Disease. New York, Marcel Dekker, pp. 61–110.
- Rabe KF, Magnussen H, Dent G (1995): Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants. Eur Resp J 8: 637–642.
- Reynolds IJ, Gould RJ, Snyder SH (1984): Loperamide: blockade of calcium channels as a mechanism for antidiarrheal effects. J Pharmacol Exp Ther 231: 628–632.
- Sanmugapriya E, Venkataraman S (2006): Toxicological investigations on Strychnos potatorum seeds in experimental models. J Health Sci 52: 339–343.
- Scratcherd T, Grundy D (1984): The physiology of intestinal motility and secretions. Br J Anesthesiol 56: 3–18.
- Serkedjieva J, Manolova N, Zgorniak-Nowosielska I, Zawilinsata B, Grzybek J (1990): Anti-viral activity of the infusion (SHS 174) from flowers of Sambucus nigra, aerial parts of Hypericum perforatum and roots of Saponaria officinalis against influenza and herpes simplex virus. Phytother Res 4: 97–100.
- Shibayama Y, Kawachi A, Onimaru S, Tokunaga J, Ikeda R, Nishida K, Kuchiiwa S, Nakagawa S, Takamura N, Motoya T, Takeda Y, Yamada K (2007): Effects of pre-treatment with St. John’s wort on nephrotoxicity of cisplatin in rats. J Ethnopharmacol 81: 103–108.
- Sopory S, Kaur T, Visweswariah SS (2004): The cGMP-binding, cGMP-specific phosphodiesterase (PDE5): INTESTINAL cell expression, regulation and role in fluid secretion. Cell Signal 16: 681–692.
- Tripathi YB, Pandey E (1999): Role of alcholic extract of shoots of Hypericum perforatum on lipid peroxidation and various species of free radical in rats. Indian J Exp Biol 37: 567–571.
- Weiss RF (1988): Herbal Medicine. Beaconsfield, Beaconsfield Publisher, p. 318.
- Wheatley D (1998): Hypericum extract potential in the treatment of depression. CNS Drugs 9: 431–440.
- Williamson EM, Okpako DT, Evans FJ (1998): Selection, Preparation and Pharmacological Evaluation of Plant Material, Vol. 1. Chichester, John Wiley & Sons, pp. 15–23.
- Woelk H (2000): Comparison of St. John’s wort and imipramine for treating depression: randomized controlled trial. Br Med J 321: 536–539.
- Wyk BV, Oudtshoorn BV, Gericke N (2002): Medicinal Plants of South Africa. Pretoria, Briza Publications, pp. 48–49.