Abstract
Leaf essential oil of Asimina triloba (L.) Dunal (F. Annonaceae) was found to be active against both human lung carcinoma and breast carcinoma cell lines. From this hydrodistillation extract, 37 components were identified by GC-MS analysis; sesquiterpenes dominated the oil composition (83%), with cadinene derivatives occurring in the greatest abundance (70%). Using the DDPH assay for antioxidant testing, the essential oil displayed moderate activity relative to vitamin E. These findings suggest that A. triloba essential oil may provide leads for active anticancer agents.
Introduction
Herbaceous essential oils are products of plant secondary metabolism and have myriad applications in folk medicine, food flavoring, and the pharmaceutical industry. With respect to potential antitumor activity, the possible efficacy of essential oil has emerged. Anticancer activities have been reported for essential oils from Satureja montana L. (Lamiaceae) and Streblus asper Lour (Moraceae) (CitationLampronti et al., 2006; CitationPhutdhawong et al., 2004).
Asimina triloba (L.) Dunal (Annonaceae), commonly known as the pawpaw tree, is native to temperate eastern North America. Products made from plant leaves exhibit strong anticancer effects against several human tumor cell lines. Literature on anticancer bioactives in A. triloba leaves has focused on its non-volatile fatty acid derivatives, acetogenins (CitationAlali et al., 1999). In the search for essential oils of potential anticancer activity, A. triloba was selected for this study. The leaves when crushed produce an unpleasant odor, which suggests the presence of volatile constituents. Nevertheless, neither its chemical composition nor its anticancer activity has been previously investigated. Our strategy was to isolate the leaf essential oil of A. triloba and select cancer cell lines to assay for anticancer activity. In this study, the chemical composition of A. triloba leaf essential oil and results for its anticancer and antioxidant activities are reported for the first time.
Materials and methods
Chemicals and plant material
Asimina triloba mature leaves (15– 20 cm length) were collected on July 2006 from fruit-producing trees (8–10 years old) growing in the University of Kentucky Arboretum and authenticated by Robert Pratley at the University of Kentucky Herbarium, USA, where the voucher specimen is stored. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Extraction of the oil
Fresh ground leaf tissue sample (100 g) was distilled for 3 h in a modified Clevenger apparatus with distilled water. The distillate was extracted with chloroform (GC grade) and concentrated under nitrogen gas to give a brownish yellow oil in 0.0007% (v/w) yield, based on the fresh weight of the sample. The oil was stored at −20°C until further analysis.
GC-MS analysis and quantification
Gas chromatography-mass spectrometry (GC-MS) analysis was performed on an HP 5890A (Hewlett-Packard, Palo Alto, CA) instrument equipped with an HP-5HS column (Agilent), 30 m × 0.32 mm, with 0.25 μm thick bonded methyl siloxane. Injections were made in the splitless mode for 30 s, and the gas chromotograph was operated under the following conditions: injector 220°C; column oven 40°C for 3 min, then programmed at a rate of 12°C/min to 180°C, kept at 180°C for 5 min, and finally ramped at a rate of 40°C/min to 250°C and kept for 2 min; He carrier gas, linear flow velocity 50 cm/s. The transfer-line and ion-source temperatures were adjusted to 230°C and 190°C, respectively. The HP quadrupole mass spectrometer was operated in the electron ionization mode at 70 eV and a source temperature of 180°C, scan range from m/z 35 to m/z 300. Volatile components were deconvoluted using AMDIS software (www.amdis.net), and identified by their retention indices relative to n-alkanes (CitationAdams, 1995) and mass spectra matching to EPA/NIH (Environmental Protection Agency/National Institutes of Health) and built-in Wiley databases. Authentic standards (when available) and/or data reported in the literature were also used for further identification (CitationFarag, 2008; CitationGoodrich et al., 2006). Peaks were quantified according to selected abundant fragments (m/z) to overcome the problem of co-eluted compounds (CitationBroeckling et al., 2006), and relative volatile abundances were calculated using a custom PEARL script to extract peak areas of individual ions characteristic of each component (CitationBroeckling et al., 2006; CitationFarag et al., 2006).
Cell culture
Human lung cancer A549 (ATCC#CCL-185) and human breast cancer MDA-MB231 (ATCC#HTB-26) cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA: www.ATCC.org). Cells were maintained at 37°C in a 5% CO2 atmosphere. A549 cells were grown in RPMI 1640 medium (MP Biomedicals Inc., Irvine, CA) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT) as well as 0.2% glucose, 2 mM glutamine, 500 μg/mL streptomycin, and 500 IU/mL penicillin. MDA-MB231 breast cancer cells were propagated and treated in the same medium as for A549, except for the addition of 0.25% glucose and 1 mM Na pyruvate.
MTT cytotoxicity assay
Exponentially growing cells were plated on 96-well microplates (Costar, Corning Inc.) at a density of 3 × 103 cells per well in 100 μL of culture medium, and allowed to adhere for 16 h before treatment. Increasing concentrations of essential oil in ethanol were then added (100 μL per well). The final concentration of ethanol in the culture medium was maintained at 0.5% (v/v) to avoid solvent toxicity. Selenite was used as positive control at a concentration range from 0.5 to 100 μM. The cells were incubated for 48 h in the presence and absence of essential oil dilute solutions. Cytotoxicty was assessed using the 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) assay according to the vendor’s protocol (Promega, Madison, WI). Absor bance was measured on an automated 96-well microplate reader SpectraMax M5 (Molecular Devices, CA) at wavelength 570 nm. Cytotoxicity is expressed as the concentration of essential oil inhibiting cell growth by 50% (IC50 value) relative to cells incubated in the presence of 0.5% ethanol as negative control. Each measurement was performed in triplicate.
Antioxidant activity
The antioxidant activity was assayed using a modified quantitative 2,2-diphenyl-1-picrylhydrazyl (DDPH) assay (CitationShimada et al., 1992). Essential oil was dissolved in 99.9% methanol at a concentration of range of 1–100 mg/mL with 50 μL of each test solution added to 5 mL DDPH solution. Blank samples were run using only 99.9% methanol. After a 30 min incubation period at room temperature, the absorbance was read against a blank at 550 nm. Vitamin E (Sigma-Aldrich) was used as a positive control at a concentration of 1–100 mg/mL. Antioxidant activity is expressed as the concentration of essential oil inhibiting DDPH formation by 50% relative to methanol (IC50 value). Inhibition of the DPPH free radical in percent (I%) was calculated according to this formula: I% = (Ablank – Asample/Ablank) × 100, where Ablank is the absorbance of the control reaction (containing all reagents except the oil), and Asample is the absorbance of the oil. Values are expressed as IC50. Each measurement was performed in triplicate.
Results and discussion
Chemical composition of essential oil
The results obtained by GC-MS analysis of A. triloba essential oil are presented in . Thirty-seven compounds were identified, constituting 92% of the essential oil composition. Sesquiterpenes accounted for 83% of the A. triloba oil composition, with cadinene derivatives the most prominent. α-Cadinene (15.7%), α-cadinol (9.5%), τ-muurolene (9.1%), and τ-cadinol (8.5%) were the major sesquiterpenes of A. triloba oil. The monoterpene portion of the essential oil accounted for only 2.7%. The unpleasant odor of A. triloba crushed leaves might be attributed to nitriles found in the essential oil. Although detected at low levels (0.5%), nitriles are recognized by an offensive odor aside from a low odor threshold (CitationScheuer, 1992). The volatile composition of A. triloba leaf oil most closely resembles that of immature green flowers, the latter producing mono- and sesquiterpenes (CitationGoodrich et al., 2006).
Table 1. Chemical composition of Asimina triloba leaf essential oil.
Anticancer activity
The anticancer activity of A. triloba essential oil was assessed against human lung carcinoma cell line A549 and human breast carcinoma cell line MDA-MB231 along with sodium selenite as positive control (CitationLetavayová et al., 2006). A. triloba essential oil exhibited a strong anticancer effect against both cell lines, with an IC50 value of 29.5 ± 2.0 μg/mL for A549 and 39.0 ± 2.9 μg/mL for MDA-MB231 (). These values were two-fold higher than those of selenite, with an IC50 value of 12.6 ± 3.0 and 18.2 ± 4.0 μg/mL for A549 and MDA-MB231, respectively. The cytotoxic effects in A. triloba essential oil could be attributed to sesquiterpene enrichment, similar to that in sweet gale oil (CitationSylvestre et al., 2005). Anticancer activities of several sesquiterpenes identified in this study have been previously reported, including τ-cadinol, α-cadinol, and α-humulene (CitationTakei et al., 2006).
Figure 1. Anticancer activity of A. triloba leaf essential oil against A549 (human lung carcinoma) and MDA-MB231 (human breast carcinoma) cell lines. The concentration inhibiting cell growth by 50% (IC50 value) is 29.5 ± 2.0 μg/mL for A549 and 39.0 ± 2.9 μg/mL for MDA-MB231. Selenite was used as positive control and exhibited an IC50 value of 12.6 ± 3.0 and 18.2 ± 4.0 μg/mL against A549 and MDA-MB231, respectively (data not shown). Results are expressed as cell growth (% of control) ± standard deviation of three replicates.
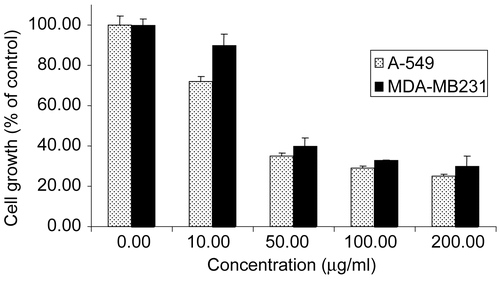
In A. triloba essential oil, cadinene sesquiterpenes accounted for more than 84% of the sesquiterpene fraction, and are likely to mediate its anticancer effect. Nitriles found in the essential oil are unlikely to exert a synergized effect owing to their low abundance (0.5%), lower by ~140-fold than sesquiterpenes.
Antioxidant activity
Free radicals are found to cause cellular damage, a common pathway for cancer and other diseases. A. triloba essential oil was assessed for its capacity to scavenge the DDPH free radical. The oil reduced the concentration of DDPH with an IC50 value of 82 mg/mL, much higher than that of vitamin E, with an IC50 of 1.2 mg/mL. This moderate antioxidant activity is likely associated with the presence of compounds with known antioxidant activity such as farnesol, α-humulene, and linalool (CitationNaderi et al., 2004).
In conclusion, A. triloba essential oil demonstrated promising anticancer activity against two cancer cell lines. These cytotoxic properties may be attributed to the accumulation of sesquiterpenes, and assay of individual components should clarify the role of sesquiterpenes as anticancer agents.
Acknowledgements
The author thanks Robert Pratley, University of Kentucky Herbarium, for plant authentication and Dr. Teresa Fan, University of Louisville, KY, USA for assistance with the anticancer assay.
Declaration of interest: The author reports no conflicts of interest.
References
- Adams RP (1995): Identification of Essential Oil Components by Gas Chromatography–Mass Spectrometry. Carol Stream, IL, Allured, pp. 18–41.
- Alali FQ, Liu XX, McLaughlin JL (1999): Annonaceous acetogenins: recent progress. J Nat Prod 62: 504–540.
- Broeckling CD, Reddy IR, Duran AL, Zhao X, Sumner LW (2006): MET-IDEA: a data extraction tool for mass spectrometry-based metabolomics. Anal Chem 78: 4334–4341.
- Farag MA (2008): Headspace analysis of volatile compounds in leaves from the Juglandaceae (walnut) family. J Essent Oil Res 20: 323–327.
- Farag MA, Ryu CM, Sumner LW, Pare PW (2006): GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67: 2262–2268.
- Goodrich KR, Zjhra ML, Ley CA, Raguso RA (2006): When flowers smell fermented: the chemistry and ontogeny of yeasty floral scent in pawpaw (Asimina triloba: Annonaceae). Int J Plant Sci 167: 33–46.
- Lampronti I, Saab AM, Gambari R (2006): Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int J Oncol 29: 989–995.
- Letavayová L, Vlcková V, Brozmanová J (2006): Selenium: from cancer prevention to DNA damage. Toxicology 227: 1–14.
- Naderi GA, Asgary S, Ani M, Sarraf-Zadegan N, Safari MR (2004): Effect of some volatile oils on the affinity of intact and oxidized low-density lipoproteins for adrenal cell surface receptors. Mol Cell Biochem 267: 59–66.
- Phutdhawong W, Donchai A, Korth J, Pyne SG, Picha P, Ngamkham J, Buddhasukh D (2004): The components and anticancer activity of the volatile oil from Streblus asper. Flav Frag J 19: 445–447.
- Scheuer PJ (1992): Isocyanides and cyanides as natural products. Acc Chem Res 25: 433–439.
- Shimada K, Fujikawa K, Yahara K, Nakamura T (1992): Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40: 945–948.
- Sylvestre M, Legault J, Dufour D, Pichette A (2005): Chemical composition and anticancer activity of leaf essential oil of Myrica gale L. Phytomedicine 12: 299–304.
- Takei M, Umeyama A, Arihara S (2006): T-cadinol and calamenene induce dendritic cells from human monocytes and drive Th1 polarization. Eur J Pharmacol 537: 190–199.
