Abstract
This study examined the antihyperlipidemic effect of the aqueous extract of Plumbago zeylanica Linn. (Plumbaginaceae) roots in diet-induced hyperlipidemic rats. The oral administration of the aqueous extract at the dose of 20, 40, and 80 mg kg−1 were found to ameliorate the hyperlipidemic condition as evidenced by a reduction of cholesterol and triglyceride levels. The standards fenofibrate (20 mg kg−1) and atorvastatin (8 mg kg−1) were also found to exhibit significant (p < 0.05) cholesterol and triglyceride lowering effect. Further, the aqueous extract at all doses demonstrated a significant (p < 0.05) increase in fecal cholesterol excretion indicating a reduction in intestinal cholesterol absorption. Additionally, the activity of lipogenic enzymes like HMGCoA reductase in the liver remained significantly (p < 0.05) low on treatment of aqueous extract (80 mg kg−1), thus decreasing the cholesterogenesis. The aqueous extract (20, 40 and 80 mg kg−1) also significantly (p < 0.05) reduced the total lipid content in the liver. Moreover, the aqueous extract demonstrated a potential antioxidant capacity in DPPH and TBARS in vitro antioxidant assay. Thus the results suggest a beneficial role of aqueous extract of Plumbago zeylanica roots in ameliorating the hyperlipidemic condition leading to atherosclerosis.
Introduction
The World Health Organization (WHO) has reported cardiovascular disease (CVD) to be the leading cause of disease and death worldwide (CitationYokozawa et al., 2003; CitationFunahashia et al., 2004; CitationScott, 2004). Atherosclerosis, a multifactorial disease, is a single major contributor to this growing burden of CVD (CitationGlass & Witztum, 2001; CitationLibby, 2002). Considerable epidemiological, experimental and clinical trial data has proved hyperlipidemia to be a major risk factor involved in development of atherosclerosis (CitationFrishman, 1998; CitationGoldberg et al., 2000; CitationWarnholtz et al., 2001). The pathophysiology of atherosclerosis has traditionally been thought to involve lipid deposition, oxidative modification and cellular uptake followed by release of inflammatory and growth factors resulting in smooth muscle cell proliferation and collagen matrix production (CitationSingh et al., 2002). The oxidative modification of lipids constitutes a key event in these processes and is often mediated by free radicals (CitationGlass & Witztum, 2001). Further, the proinflammatory properties of oxidized LDL (oxLDL) are attributed mainly to products of LDL lipid peroxidation (CitationKontush & Chapman, 2006). Thus, combined antioxidant and antihyperlipidemic activity has the potential to markedly limit the progression of atherosclerosis.
The conventional therapeutic modalities available for atherosclerosis majorly include lipid lowering drugs like statins and fibrates (CitationFazio & Linton, 2004). However, these drugs exhibit compromised safety profiles and are reported to have serious side effects (CitationKnopp, 1999; CitationSchreiber & Anderson, 2006). Hence, efforts are being directed towards discovering safe and effective agents that will be beneficial in correcting the lipid metabolism and thus preventing cardiac diseases. Recently, there has been a renewed interest in medicinal plants attributed with therapeutic virtues. It is well known that many of these plants contain biologically active components and at least 25% of drugs presently used in modern medicine are derived from plants (CitationMouhssen, 2007; CitationMukherjee et al., 2007). India has a rich heritage of medicinal plants of wide diversity, which are used by the local population and traditional healers for the treatment of several diseases including lipid disorders.
Plumbago zeylanica Linn. (Plumbaginaceae) is a perennial shrub widely found throughout peninsular region and eastern parts of India. It is commonly known as “chitrak” and is extensively used in preparation of many Ayurvedic formulations (CitationSatyavati & Gupta, 1987). Dried roots of Plumbago zeylanica have profound importance in Ayurvedic medicines used in the alleviation of many diseases like rheumatic fever, dyspepsia, piles, diarrhea, skin disease, leprosy, leucoderma, paralysis, epilepsy and hysteria (CitationSingh et al., 2004). Additionally, the plant is also commonly recommended for obesity – a disorder related to disturbances in the lipid metabolism (CitationSingh et al., 2004). Plumbago zeylanica has been previously investigated for its anti-fertility (CitationGupta et al., 1971; CitationChowdhury et al., 1982), anti-microbial (CitationAhmad et al., 1998), anti-allergic (CitationDai et al., 2004), anti-plasmodial (CitationSimonsen et al., 2001), anti-bacterial (CitationVijver & Lotter, 1971) and central nervous system stimulant activity (CitationBopaiah & Pradhan, 2001). Recently the plant has been reported to be beneficial in arthritis (CitationPoosarla et al., 2007). Plumbagin, a major phytoconstituent of roots of Plumbago zeylanica, has been reported to exhibit anti-bacterial (CitationLemma et al., 2002), anti-fungal, and anti-cancer activity (CitationKrishnaswamy & Purushotaman, 1980). Additionally, plumbagin has been reported to demonstrate anti-hyperlipidemic activity in rabbits (CitationSharma et al., 1991). However, no detailed investigation of the anti-hyperlipidemic activity of the plant extracts has been reported. Thus, it was worthwhile to evaluate the lipid lowering activity of Plumbago zeylanica in high fat diet-induced hyperlipidemic rats.
Materials and methods
Plant material
The roots of Plumbago zeylanica were supplied by Zandu Pharmaceutical, Mumbai, India and authenticated by A.M. Mujumdar, taxonomist, Agharkar Research Institute (Pune, India). A voucher specimen (no R 081) was submitted to the Herbarium of the Institute.
Preparation of aqueous extract of Plumbago zeylanica roots
The weighed quantity (50 g) of Plumbago zeylanica root powder was refluxed with 300 mL of distilled water for 48 h. The aqueous extract was subsequently filtered through Whatman No. 1 filter paper. The filtrate was further concentrated under vacuum (yield 22.07% w/w dry weight basis) to obtain the crude extract. The extract was suspended in 0.1% sodium carboxymethyl cellulose (Na CMC) for experimental studies.
Animals
Healthy male albino rats (250-300 g) of Wistar strain were obtained from Bharat Serum, Thane, India. The animals were housed under controlled conditions of light (12 h) and temperature 25° ± 1°C in the animal house of C.U. Shah College of Pharmacy, India. The animals were allowed to acclimatize for a period of 2 weeks prior to the initiation of experiment. The animals had free access to water and standard laboratory animal diet. The experimental protocol for this study was reviewed and approved by the Institutional Animal Ethics Committee (IAEC) of C.U. Shah College of Pharmacy, India and the pharmacological work was performed as per the Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA) norms.
Acute toxicity study
The acute toxicity was performed according to the OECD 423 guide lines (CitationEcobichon, 1997). The rats (Wistar) were divided into groups of four each containing six animals (n = 6). The aqueous extract at the dose of 5, 50, 300, and 2000 mg kg−1 body weight were administered to rats after overnight fasting. The drug-treated animals were subsequently observed closely for the first 3 h for any untoward symptoms such as tremors, convulsions, exophthalmia, salivation, diarrhea and lethargy followed by observation for a further 14 days. At the end of the experimental period the animals were observed for any changes in behavioral pattern and mortality.
Induction of hyperlipidemia in rats
The hyperlipidemic condition was induced in rats by feeding a high fat diet (HFD) comprising of 2% cholesterol, 1% cholic acid in 1 mL coconut oil along with 30% butter oil by oral gavage for an induction period of 10 days.
Experimental design
The rats were divided into seven groups, each group containing six rats. The group 1 (normal control) animals were retained on standard laboratory animal diet. However, the remaining animals in groups 2 to 7 were fed with a high fat diet for a period of 10 days. The induction period was followed by 15 days of drug intervention period where the animals continued with the high fat diet along with drug treatment. The group 2 animals (high fat diet control) received high fat diet with sodium carboxymethyl cellulose (drug vehicle) for 15 days. Group 3 and group 4 hyperlipidemic animals were orally administered with standard drugs atorvastatin (8 mg kg−1, p.o.) and fenofibrate (20 mg kg−1 p.o.) respectively, for 15 days. The animals of groups 5 to 7 were administered the aqueous extract of Plumbago zeylanica by oral gavage at the doses of 20, 40 and 80 mg kg−1 p.o., respectively, for 15 days.
Determination of serum lipid profile
Blood was withdrawn from the retro-orbital plexus of rats under mild ether anesthesia on day 0 (before starting the induction of hyperlipidemia), day 10 (end of the induction period) and day 25 (end of the drug intervention period) of the study. The animals were fasted for 18 h prior blood withdrawal. The blood was collected and centrifuged to separate serum for estimation of lipid profile. The serum total cholesterol, triglyceride and HDL-C levels were analyzed by enzymatic colorimetric method using commercially available kits. Athero index was calculated as (total cholesterol - HDL–C)/HDL -C (CitationYang et al., 2006).
Estimation of fecal cholesterol excretion
Feces were collected for three consecutive days, i.e, day 23 to day 25 of the study and dried for 1 h at 60°C. The dried fecal matter was powdered and extracted with chloroform:methanol (2:1). The fecal cholesterol content in the extract was estimated by enzymatic method using kits.
Estimation of hepatic hydroxymethyl glutaryl coenzyme (HMGCoA) reductase activity
Animals were sacrificed at the end of the drug intervention period. The hepatic HMGCoA reductase enzyme activity was indirectly measured based on the HMGCoA/mevalonate ratio (CitationRao & Ramakrishnan, 1975). The ratio between HMGCoA and mevalonate is inversely proportional to HMGCoA reductase activity, i.e., an increase in ratio indicates decreased activity.
Estimation of hepatic total lipid content
The total lipids of the liver tissue were extracted with chloroform:methanol (2:1) and quantified gravimetrically (CitationFolch et al., 1957).
Estimation of free radical scavenging activity of aqueous extract by in vitro 1, 1-diphenyl-2-picryl hydrazyl (DPPH) antioxidant assay
DPPH radical is a stable free radical and gives a strong absorption band at 517 nm (CitationSoni et al., 2003). Various concentrations of the aqueous extract were added to DPPH in methanol and incubated for 30 min. Absorbance was read at 517 nm and the percentage inhibition was calculated. The concentration that exhibited a 50% inhibition was considered as the IC50.
Estimation of effect of aqueous extract on lipid peroxidation by in vitro thiobarbituric acid reactive substances (TBARS) antioxidant assay
Malondialdehyde, a product of lipid peroxidation which is associated with hypercholesterolemic atherosclerosis, can be quantified colorimetrically (CitationSoni et al., 2003) following its controlled reaction with thiobarbituric acid. The assay was performed using mouse liver homogenate. Lipid peroxidation was initiated by ferrous sulfate-ascorbic acid system. Various concentrations of the aqueous extract were added and the absorbance was read at 532 nm. The percentage inhibition was calculated and the concentration that exhibited a 50% inhibition was considered as the IC50.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Dunnett’s t-test. Values are expressed as mean ± SEM and p < 0.05 was considered to be significant.
Results
Acute toxicity profile
Oral administration of aqueous extract at the dose of 5, 50, 300, and 2000 mg kg−1 in rats did not demonstrate any untoward behavioral symptoms or mortality. The aqueous extract was found to be safe up to the dose of 2000 mg kg−1 during the 14 days of the observation period.
Effect of aqueous extract on serum lipid profile
Oral administration of high fat diet (HFD) for a period of 10 days resulted in a significant (p < 0.05) rise in serum total cholesterol as well as triglyceride levels ().
Figure 1. Effect of high fat diet on serum lipid profile in rats at the end of 10 days of induction period. Bars represents mean ± SEM of the concentration (mg dL−1) of each of the parameters on day 0 (basal value) and day 10 (induction value) for the 36 rats included in the study. *p < 0.05, induction values compared against basal values. TC, total cholesterol; TG, triglyceride; HDL – C, high density lipoprotein – cholesterol.
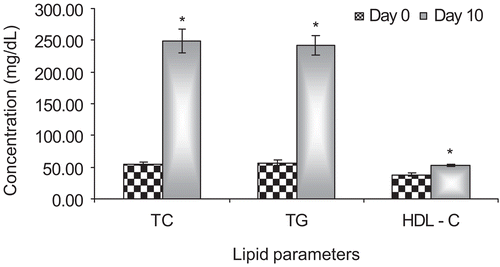
Administration of aqueous extract for a treatment period of 15 days at the dose of 40 and 80 mg kg−1 demonstrated a significant (p < 0.05) reduction in triglyceride and cholesterol levels with respect to the high fat diet control group. However, at the low dose of 20 mg kg−1 the aqueous extract was found to significantly decrease the triglyceride levels, but failed to exhibit significant cholesterol lowering activity. The aqueous extract at 40 and 80 mg kg−1 demonstrated a significant reduction of 54.17% and 64.39%, respectively, in cholesterol levels as compared to the high fat diet group. However, at 20 mg kg−1 the aqueous extract reduced the cholesterol by 44.79% but the decrease was not found to be statistically significant as compared to the high fat diet group. Additionally, the aqueous extract demonstrated a significant triglyceride lowering activity which was evident by a significant reduction ranging from 37.73% to 46.72% exhibited by aqueous extract at all doses. However, the statistical analysis of the data indicated no significant difference between the treatment groups. The standards fenofibrate and atorvastatin demonstrated a significant (p < 0.05) decrease of 80.45% and 55.34% respectively in total cholesterol levels and areduction of 72.99% and 50.83% (p < 0.05), respectively, in triglyceride levels compared to the high fat diet control group ().
Table 1. Effect of aqueous extract of Plumbago zeylanica roots on serum lipid profile (total cholesterol, triglyceride and HDL-C), and athero index in diet-induced hyperlipidemic rats after 15 days of treatment.
The aqueous extract and the standard atorvastatin failed to reduce the athero index significantly, while the standard fenofibrate demonstrated a significant (p < 0.05) reduction at the end of the drug intervention period. However, none of the groups showed a significant effect on serum HDL-C levels ().
Effect of aqueous extract on fecal cholesterol excretion
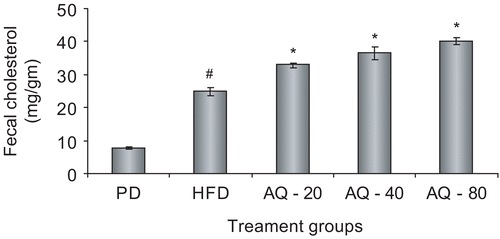
At the end of the drug intervention period the aqueous extract was found to significantly (p < 0.05) increase the fecal cholesterol excretion at the doses of 20, 40 and 80 mg kg−1 with respect to the high fat diet control group ().
Figure 2. Effect of aqueous extract of Plumbago zeylanica roots on fecal cholesterol excretion (mg/g) after 15 days of treatment. Bars represent mean ± SEM from n = 6. #p < 0.05 comparison between pellet diet control and high fat diet control. *p < 0.05 comparison with high fat diet control. PD, pellet diet control; HFD, high fat diet control; AQ – 20, aqueous extract 20 mg kg−1; AQ – 40, aqueous extract 40 mg kg−1; AQ – 80, aqueous extract 80 mg kg−1.
Effect of aqueous extract on HMGCoA reductase activity
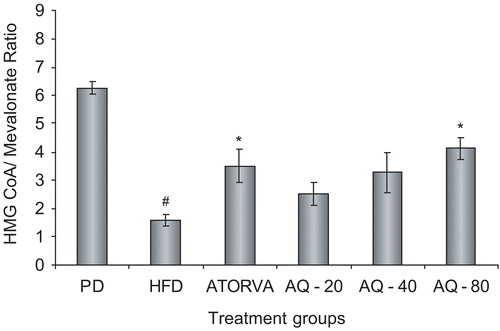
The HMGCoA/mevalonate ratio was significantly (p < 0.05) increased by the standard atorvastatin and aqueous extract at the dose of 80 mg kg−1 as compared to the high fat diet control group ().
Figure 3. Effect of aqueous extract of Plumbago zeylanica roots on hepatic HMGCoA reductase activity after 15 days of treatment. Bars represent mean ± SEM from n = 6. #p < 0.05 comparison between pellet diet control and high fat diet control. *p < 0.05 comparison with high fat diet control. PD, pellet diet control; HFD, high fat diet control; ATORVA, atorvastatin 8 mg kg −1; AQ – 20, aqueous extract 20 mg kg−1; AQ – 40, aqueous extract 40 mg kg−1; AQ – 80, aqueous extract 80 mg kg−1.
Effect of aqueous extract on hepatic total lipid content
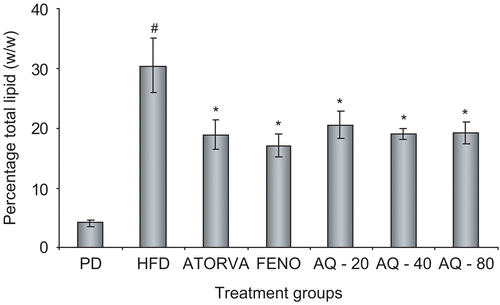
The aqueous extract at 20, 40 and 80 mg kg−1 as well as the standards atorvastatin and fenofibrate at the end of the treatment period exhibited a significant (p < 0.05) reduction in total lipid content of the liver as compared to the high fat diet control group ().
Figure 4. Effect of aqueous extract of Plumbago zeylanica roots on hepatic total lipid content (w/w) after 15 days of treatment. Bars represent mean ± SEM from n = 6. #p < 0.05 comparison between pellet diet control and high fat diet control. *p < 0.05 comparison with high fat diet control. PD, pellet diet control; HFD, high fat diet control; ATORVA, atorvastatin 8 mg kg−1; FENO, fenofibrate 20 mg kg−1; AQ – 20, aqueous extract 20 mg kg−1; AQ – 40, aqueous extract 40 mg kg−1; AQ – 80, aqueous extract 80 mg kg−1.
Free radical scavenging activity of aqueous extract
The aqueous extract demonstrated a potential free radical scavenging effect, by exhibiting IC50 at 63.58 μg mL−1.
Effect of aqueous extract on lipid peroxidation
The aqueous extract demonstrated an appreciable inhibitory effect on lipid peroxidation. The IC50 of aqueous extract was 147.49 μg mL−1.
Discussion
The present study focuses on exploration of the anti-hyperlipidemic activity of aqueous extract of Plumbago zeylanica roots in diet-induced hyperlipidemic rat model.
The rats fed with a high fat diet demonstrated a significant increase in triglyceride and total cholesterol. These elevated lipid levels were significantly reduced by aqueous extract at the dose of 40 and 80 mg kg−1. However, at the low dose of 20 mg kg−1 the aqueous extract failed to significantly reduce cholesterol levels while a significant triglyceride lowering activity was demonstrated.
Further studies were directed towards exploration of the mechanism of action, wherein the effect of aqueous extract on cholesterol biosynthesis and absorption of cholesterol (dietary and cholesterol cleared from the liver through biliary secretion), the two important processes involved in regulation of cholesterol homeostasis was studied. The oral administration of aqueous extract for 15 days was found to reduce the cholesterol absorption from the intestine which was evident by significant (p < 0.05) increase in fecal cholesterol excretion demonstrated by aqueous extract at all three doses employed. However, at the high dose of 80 mg kg−1 the aqueous extract was also found to decrease the cholesterogenesis in liver by significantly (p < 0.05) increasing the HMGCoA/mevalonate ratio. HMGCoA/mevalonate ratio has an inverse relationship to the activity of HMGCoA reductase, a key enzyme in cholesterol biosynthesis (CitationVasu et al., 2005; CitationBahramikia & Yazdanparast, 2008).
The other mechanistic pathway responsible for the cholesterol lowering effect could be via inhibition of acyl-CoA: cholesterol acyltransferase (ACAT) enzyme, a primary enzyme responsible for the intracellular esterification of free cholesterol to cholesterol ester. The esterification of free cholesterol is essential for its intestinal absorption and the assembly of very low density lipoprotein (VLDL). Thus inhibition of ACAT may exhibit cholesterol lowering and anti-atherosclerotic activities by blocking intestinal absorption of dietary cholesterol, inhibiting hepatic secretion of VLDL and preventing formation of foam cells in the arterial wall, thus inhibiting the growth of atherosclerotic lesions (CitationDove et al., 2005; CitationNissen et al., 2006).
The putative mechanism contributing to the triglyceride lowering effect could be via decreased lipogenesis in liver which was consistent with the significant reduction observed in the total lipid content of the liver at doses of 20, 40 and 80 mg kg−1.
Additionally, other mechanisms which would also reduce the triglycerides could be via up-regulating lipoprotein lipase (LPL) activity and inhibition of microsomal triglyceride transfer protein (MTP).
LPL is an enzyme present on the luminal surface of the vascular endothelium in the capillaries of the muscle and adipose tissue. It is responsible for the hydrolysis of triglyceride into free fatty acid (FFA) which is then stored in adipose tissue, thus reducing the plasma triglyceride levels (CitationAnila & Vijayalakshmi, 2002; CitationVasu et al., 2005).
MTP is essential for the assembly and secretion of VLDL in the liver and chylomicrons in the intestine, thus playing an important role in regulation of plasma triglyceride (CitationCuchel et al., 2007).
The athero index is believed to be an important risk factor of atherosclerosis. The aqueous extract (20, 40, and 80 mg kg−1) and the standard atorvastatin were found to decrease the athero index at the end of the treatment period, but the decrease exhibited was not statistically significant. However, the standard fenofibrate was found to significantly (p < 0.05) reduce the athero index (CitationMehta et al., 2003; CitationKumari & Augusti, 2007).
Oxidative stress is one of the causative factors that link hyperlipidemia with the pathogenesis of atherosclerosis. Oxidized lipids can elicit a wide variety of biological responses that could contribute to atherosclerotic lesion development.
Thus, the pathophysiology of the disease suggests that a combined antioxidant and lipid lowering acticvity would have marked benefits in ameliorating the progression of the atherosclerotic lesion. The present study suggests that the aqueous extract of Plumbago zeylanica roots has potential antioxidant activity as evidenced by the results of the in vitro DPPH scavenging and TBARS antioxidant assays.
Conclusion
The integrated data thus suggest a significant anti- hyperlipidemic activity of the aqueous extract of Plumbago zeylanica roots which was additionally supported by an appreciable antioxidant potential, thus emerging to be beneficial in ameliorating the hyperlipidemic condition and thus prove to be beneficial in the management of atherosclerosis.
Acknowledgement
The authors are thankful to the University Grant Commission (UGC), New Delhi, India for providing financial assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmad I, Mehmood Z, Mohammad F (1998): Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol 62: 183–93.
- Anila L, Vijayalakshmi NR (2002): Flavonoids from Emblica officinalis and Mangifera indica Effectiveness for dyslipidemia. J Ethnopharmacol 79: 81–87.
- Bahramikia S, Yazdanparast R (2008): Effect of hydroalcoholic extracts of Nasturtium officinale leaves on lipid profile in high-fat diet rats.J Ethnopharmacol 115: 116–121.
- Bopaiah CP, Pradhan N (2001): Central nervous system stimulatory action from the root extract of Plumbago zeylanica in rats. Phytother Res 15: 153–156.
- Chowdhury AAK, Sushanta KC, Khan AAK (1982): Antifertility activity of Plumbago zeylanica Linn. Root. Indian J Med Res 76: 99–101.
- Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ (2007): Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med 356: 148–156.
- Dai Y, Hou LF, Chan YP, Cheng L (2004): Inhibition of immediate allergic reactions by ethanol extract from Plumbago zeylanica stem. Biol Pharm Bull 27: 429–432.
- Dove DE, Su YR, Zhang W, Jerome WG, Swift LL, Linton MF, Fazio S (2005): ACAT1 deficiency disrupts cholesterol efflux and alters cellular morphology in macrophages. Arterioscler Thromb Vasc Biol 25: 128–134.
- Ecobichon DJ (1997): The Basis of Toxicology Testing. New York, CRC Press, pp. 43–86.
- Fazio S, Linton MF (2004): The role of fibrates in managing hyperlipidemia: Mechanisms of action and clinical efficacy. Curr Atheroscler Rep 6: 148–157.
- Folch J, Lees M, Sloane-Stanley GH (1957): A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509.
- Frishman WH (1998): Biological markers as predictors of cardiovascular disease. Am J Med 104: 18S–27S.
- Funahashia T, Matsuzawab Y, Kihara S (2004): Adiponectin as a potential key player in metabolic syndrome: Insights into atherosclerosis, diabetes and cancer. Int Congr Series 1262: 368–371.
- Glass CK, Witztum JL (2001): Atherosclerosis: The road ahead. Cell 104: 503–516.
- Goldberg IJ, Kaka Y, Lutz EP (2000): Responses to eating: Lipoproteins, lipolytic products and atherosclerosis. Curr Opin Lipidol 11: 235–241.
- Gupta ML, Gupta TK, Bhargava KP (1971): A study of antifertility effect of some indigenous plants. J Res Indian Med 6: 112–116.
- Knopp RH (1999): Drug treatment of lipid disorders. N Engl J Med 341: 498–512.
- Kontush A, Chapman MJ (2006): Functionally defective HDL: A new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev 58: 342–374.
- Krishnaswamy M, Purushotaman K (1980): Plumbagin: A study on its anticancer, antibacterial, antifungal properties. Indian J Exp Biol 18: 876–877.
- Kumari K, Augusti KT (2007): Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. J Ethnopharmacol 109: 367–371.
- Lemma H, Debella A, Addis G, Kunert O, Geyid A, Teka F, Yersaw K (2002): Anti-bacterial activity of Plumbago zeylanica L. roots on some pneumonia causing pathogens. Ethiop J Sci 25: 285–294.
- Libby P (2002): Inflammation in atherosclerosis. Nature 420: 868–874.
- Mehta K, Balaraman R, Amin AH, Bafna PA, Gulati OD (2003): Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J Ethnopharmacol 86: 191–195.
- Mouhssen L (2007): Screening of natural products for drug discovery. Expert Opin Drug Discovery 2: 697–705.
- Mukherjee PK, Rai S, Kumar V, Mukherjee K, Hylands PJ, Hider RC (2007): Plants of Indian origin in drug discovery. Expert Opin Drug Discovery 2: 633–657.
- Nissen SE, Tuzcu EM, Brewer BH, Sipahi I, Nicholls SJ, Ganz P, Schoenhagen P, Waters DD, Pepine CJ, Crowe TD, Davidson MH, Deanfield JE, Wisniewski LM, Hanyok JJ, Kassalow LM (2006): Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 354: 1253–1263.
- Poosarla A, Kumar VB, Rao RT, Rao DN, Athota RR (2007): Alleviation of collagen-induced arthritis by Plumbago zeylanica in mice. Pharm Biol 45: 54–59.
- Rao VA, Ramakrishnan S (1975): Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin Chem 21: 1523–1525.
- Satyavati VG, Gupta KA (1987): Medicinal plants of India. Indian Council of Medical Research 2: 471–477.
- Schreiber DH, Anderson TR (2006): Statin-induced rhabdomyolysis. J Emerg Med 31: 177–180.
- Scott J (2004): Pathophysiology and biochemistry of cardiovascular disease. Curr Opin Genet Dev 14: 271–279.
- Sharma I, Gusain D, Dixit VP (1991): Hypolipidemic and antiatherosclerotic effect of plumbagin in rabbits. Indian J Physiol and Pharmacol 35: 10–14.
- Simonsen HT, Nordskjold JB, Smitt UW, Nyman U, Palpu P, Joshi P, Varughese G (2001): In vitro screening of Indian medicinal plants for antiplasmodial activity. J Ethnopharmacol 74: 195–204.
- Singh J, Mishra NP, Jain SP, Singh SC, Sharma A, Khanuja SPS (2004): Traditional uses of Plumbago zeylanica (Chitrak). J Med Arom Plant Sci 26: 795–800.
- Singh RB, Mengi S, Xu YJ, Arneja AS, Dhalla NS (2002): Pathogenesis of atherosclerosis: A multifactorial process. Exp Clin Cardiol 7: 40–53.
- Soni K, Suresh Kumar P, Saraf MN (2003): Free radical scavenging and antilipid peroxidation activity of Tephrosia purpurea Linn. Indian J Pharm Sci 65: 27–30.
- Vasu VT, Modi H, Thaikoottathil JV, Gupta S (2005): Hypolipidaemic and antioxidant effect of Enicostemma littorale Blume aqueous extract in cholesterol fed rats. J Ethnopharmacol 101: 277–282.
- Vijver LM, Lotter AP (1971): The constituents in the roots of Plumbago auriculata Lam. and Plumbago zeylanica L. responsible for antibacterial activity. Planta Med 20: 8–13.
- Warnholtz A., Maollnau H., Oelze M., Wendt M., Munzel T (2001): Antioxidants and endothelial dysfunction in hyperlipidemia. Current Hypertension Report 3: 53–60
- Yang R, Le G, Li A, Zheng J, Shi Y (2006) Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition 22: 1185–1191.
- Yokozawa T, Ishida A, Cho EJ, Nakagawa T (2003): The effects of Coptidis Rhizoma extract on a hypercholesterolemic animal model. Phytomedicine 10: 17–22.
