Abstract
The present study evaluated the aqueous seed extract of Hunteria umbellata K. Schum (Apocynaceae) for hypoglycemic activity in rats. Diabetes was induced by a single dose of streptozotocin (50 mg/kg i.p.). Daily doses of 400, 800, and 1000 mg/kg of extract were orally administered to fasted normal and diabetic rats. Blood glucose levels were monitored after 0, 2, 4, 8, 12 h and on day 14 post treatment. Liver glycogen levels were also estimated on day 14. In normal rats, only 400 mg/kg of the extract produced a significant reduction in blood glucose at the 4 h (P < 0.05) which was 22.15 ± 4.88%. In diabetic rats, the extract, 400, 800 mg/kg, caused significant reduction (P < 0.01), 51.87% ± 5.79% and 43.47% ± 8.06% respectively, with maximum effect at 8 h. This reduction in blood glucose was greater than that of glibenclamide (31.03% ± 8.86%). Diabetic rats administered with 400 mg/kg extract produced a significant reduction (P < 0.01) on day 14 (43.60% ± 8.10%). Liver glycogen levels were significantly increased (P < 0.05) in diabetic rats administered with doses of 400 and 800 mg/kg extracts and these were comparable to glibenclamide. Acute toxicity data showed no mortality in mice up to 17.5 g/kg. We conclude that the extract possesses marked hypoglycemic effects in diabetic rats possibly through increased glycogenesis, thus justifying its use in herbal medicine for the treatment of diabetes.
Introduction
Diabetes mellitus (DM) is one of the common metabolic disorders and it has been estimated that by 2010 the diabetic population will increase to 221 million around the world (CitationCarter, 2004). It is characterized by hyperglycemia and disturbances of carbohydrate and fat metabolisms, secondary to an absolute or relative lack of insulin (CitationAlberti & Zimmet, 1998). Epidemiological studies (CitationKleiv et al., 1994; CitationLiv et al., 1993) and clinical trials (CitationAbaira et al., 1995) strongly support the notion that hyperglycemia is the principal cause of its complications. Alternative strategies to the current modern drug treatment of diabetes are urgently needed due to inability of existing modern therapies to control all pathological aspects of the disorder (CitationWHO, 2002). Herbal preparations alone or in combination with oral hypoglycemic agents sometimes produce a good therapeutic response in some resistant cases where modern medicines alone fail (CitationAnturlikar et al., 1995). Available literature shows that there are more than 176 plant species belonging to 84 families having anti-diabetic activity (CitationMohamed et al., 2006).
Hunteria umbellata K.Schum (Apocynaceae) is a shrub about 15-22 m tall growing throughout west and central Africa. In southern Nigeria it is called “osu” (Edo), “nkpokiri” (Ibo), and “erin” (Yoruba). It flowers in November and from March to May. The fruit is about 5.25 cm in diameter, yellow to orange, glabrous and smooth containing twelve or more disc-shaped seeds embedded in a gelatinous pulp (CitationKeay et al., 1964). The seeds and bark are used in the treatment of piles, yaws, diabetes, stomach ulcers, prevention of fetal abortion, arresting acute menstrual pain and helminthic infection (CitationSofowora, 1982; CitationElujoba, 1995; CitationOluwemimo & Usifoh, 2001; CitationRaman & Mallam, 1994). Although the seeds of H. umbellata are used locally in Nigeria for treatment of diabetes by boiling with water and subsequent drinking of the extract, there has been no scientific study in support of this. We therefore undertook the present study to evaluate its hypoglycemic effects.
Materials and methods
Plant material and extraction
The ripe fruits of H. umbellata were collected from Okhoro village in Benin City, Nigeria during September 2006. The plant was first identified by MacDonald Idu of the Department of Botany, Faculty of Life Sciences, University of Benin, Benin City and was later authenticated by the Forest Research Institute of Nigeria (Ibadan, Nigeria) where a herbarium sample with voucher number FHI 107678 has been deposited.
The seeds were removed from the ripe fruits and were sun-dried to a constant weight over a 14-day period. The dried seeds were then powdered using a mechanical grinder. The powdered seeds (400 g) were boiled in 1.5 L of distilled water for 30 min. The material was then filtered, concentrated under pressure in a rotar vapor and dried to a constant weight in an oven set at 40°C for 48 h (yield, 39%). The dried extract was preserved in an air-tight clean glass container and stored at 4°C until required.
Phytochemical screening
The aqueous extract of H. umbellata was subjected to various tests in order to determine the classes of the various chemical constituents present, by using standard methods (CitationTrease & Evans, 1989).
Animals
Adult Wistar rats (180-220 g) of either sex were obtained from the Animal House of the Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Benin, Benin City, Nigeria. The animals were fed with standard rodent cubes obtained from Ladokun Feeds Ltd (Ibadan, Nigeria) and had free access to tap water. Animals were exposed to natural lighting conditions and were handled according to standard experimental protocols approved by the Faculty of Pharmacy Animal Ethics Committee, University of Benin. All animals were fasted overnight before experiments but were allowed free access to water.
Acute toxicity test
Adult Wistar rats of either sex were used separately for the tests. The animals were divided into various test and control groups of five animals per group. Groups A to D received 10, 12.5, 15, and 17.5 g/kg of the extract respectively by use of oro-gastric tube, while the control (group E), received distilled water by the same route. General symptoms of toxicity and mortality in each group were observed within 24 h. In the absence of death, after 24 h, some hematological parameters (white blood cell count, hematocrit and platelet count) were determined in the group of rats that received the highest dose of the extract (17.5 g/kg).
Determination of hypoglycemic effect
Studies in normal rats
Male Wistar rats were divided into five groups (A to E) of five rats each. Basal blood glucose levels were measured after which daily doses of 400, 800, and 1000 mg/kg of the extracts were administered orally by use of an oro-gastric tube to groups A, B and C, respectively. Glibenclamide (suspended in 3% v/v Tween 80), at a dose of 5 mg/kg and 3% v/v Tween 80 (3 mL/kg) were administered orally to animals in groups D and E and served as reference and control, respectively. Treatments were continued for 14 consecutive days. Blood glucose levels were monitored at 0, 2, 4, 8, and 12 h of administering the very first treatment and on day 14.
Studies in diabetic rats
Diabetes was induced by a single intraperitoneal injection of streptozotocin, STZ (Sigma St. Louis, MO, USA) 50 mg/kg in citrate buffer (0.1 M, pH 4.5) to overnight-fasted rats. Blood glucose was measured after 48 h and animals having a blood glucose level above 200 mg/dL were considered diabetic and were used for the experiment (CitationRastogi et al., 1997; CitationChen et al., 2005). The diabetic rats were divided into five groups (A to E) of five rats each. Basal blood glucose levels were measured after which daily doses of 400, 800, 1000 mg/kg of the extracts, 5 mg/kg glibenclamide (in 3% v/v Tween 80) and 3 mL/kg of 3% v/v Tween 80 were administered orally by use of an oro-gastric tube to groups A, B, C, D, and E, respectively, for 14 consecutive days. The blood glucose levels were measured at same time intervals as in the normal rats.
Blood glucose measurement
Blood samples were obtained from the tail tip of the rats and blood glucose levels were determined using a glucometer [ACCU-CHEK® active (Roche, USA)]. The percentage glycemic change at any time point was calculated using the formula:
where: GC is the glucose concentration at different time points and FBG is the fasting blood glucose concentration representing baseline value.
Determination of liver glycogen in normal and diabetic rats
All animals were anesthetized by ether inhalation on day 14 and then sacrificed. The liver of each rat was immediately excised and washed in ice-cold normal saline. Liver glycogen levels were then estimated by a modified anthrone-sulfuric acid method (CitationZhang et al., 1987). Briefly, each rat liver (0.5 g) was homogenized with 2 mL of 30% KOH, the homogenate obtained was transferred into vials and kept at 100°C for 20 min in a water bath. After cooling, 3 mL of anhydrous ethanol was added and the vials were centrifuged at 4,000 g for 15 min. After supernatants were discarded, distilled water, 1 mL and 2 mL of 0.2% anthrone reagent (0.2% of anthrone in 100 mL of 98% H2SO4 (g/mL) prepared freshly within 1 h) were added and the vials were then placed in boiling water for 20 min. After cooling, their absorbances were read at 620 nm. Using the anthrone method for standard glucose estimation, absorbance of glucose at different concentrations of 1, 2, 3, 4, 5, and 6 mg were used to plot a standard calibration curve of absorbance against glucose concentration. The absorbances of the various samples were then read off from the graph to obtain the concentration of glucose in the various samples.
Statistical analysis
All data in the experiments are presented as mean ± SEM (standard error of the mean) and n represents the number of animals per group. Significant differences between each group were determined using one-way analysis of variance (ANOVA) and Tukey-Kramer post hoc tests. P < 0.05 was regarded as significant in all cases.
Results
Phytochemical screening
The phytochemical screening showed the presence of saponins, flavonoids, tannins and steroidal glycosides in addition to alkaloids.
Acute toxicity test
No adverse effect or mortality was observed in rats 24 h after administration of each dose of the extract up to 17.5 g/kg. Measurement of some hematological parameters 24 h following the administration of 17.5 g/kg of the extract in rats did not reveal any significant difference in white blood cell count and hematocrit values (). There was, however, a significant reduction (p < 0.05) in platelet counts when compared to the control.
Table 1. Effect of a single oral high dose of the aqueous seed extract of H. umbellata on some hematological parameters 24 h post-administration in rats.
Hypoglycemic activity
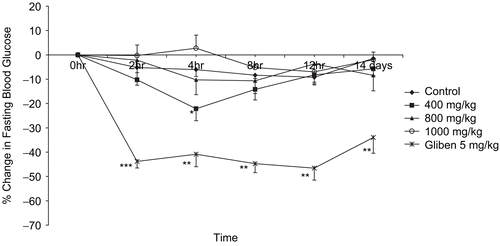
The effects of the extract at specific time points are shown in . Only the dose of 400 mg/kg produced a significant reduction (22.15 ± 4.88%) in blood glucose level at 4 h (p < 0.05). Glibenclamide produced a significant percentage reduction in blood glucose (p < 0.001), which was sustained throughout the period of the experiment.
Figure 1. Percentage change in fasting blood glucose after oral administration of single daily doses of extract to normal rats. Data are mean ± SEM values for five rats in each group.*P < 0.05, **P < 0.01, ***P < 0.001 as compared to the corresponding time point for control.
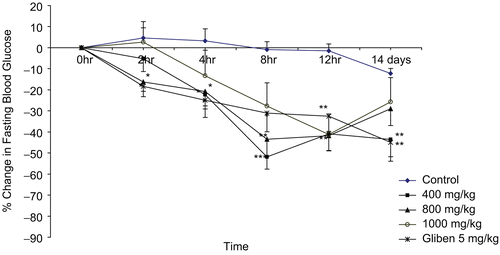
In the diabetic rats, the effects of doses of the extract were time-dependent (). At a dose of 400 mg/kg, there was a significant percentage reduction in blood glucose level from 4 h to 12 h and on day 14 when compared to vehicle-treated rats. The pattern of reduction in blood glucose levels obtained with 800 mg/kg was significant from 2 h to 12 h but not on day 14 when compared to vehicle-treated rats. The dose of 1000 mg/kg of the extract produced a significant reduction in blood glucose level only at 12 h. Blood glucose level was significantly reduced by glibenclamide at all time points.
Figure 2. Percentage change in fasting blood glucose after oral administration of single daily doses of extract in diabetic rats. Data are mean ± SEM values for five rats in each group.*P < 0.05, **P < 0.01, ***P < 0.001 as compared to the control.
shows the effects of the extract on the liver glycogen in both normal and streptozotocin-induced diabetic rats. Liver glycogen was found to be significantly increased (p < 0.05) at 400 mg/kg (57.38 ± 3.86 mg/100 g), 800 mg/kg (61.25 ± 5.24 mg/100 g) of the extract. There was also a significant increase (p < 0.05) in liver glycogen storage with glibenclamide (65.88 ± 7.5 mg/100 g) as compared to the control group. In normal rats when compared with the standard drug, glibenclamide (88.16 ± 3.9 mg/100 g), liver glycogen storage was significantly lower (p < 0.05) in both 800 mg/kg (67.50 ± 3.51 mg/100 g) and 1000 mg/kg (66.38 ± 6.03 mg/100 g) of the extract.
Table 2. Effect of the aqueous seed extract on liver glycogen in normal and diabetic rats.
Discussion
Streptozotocin-induced hyperglycemia in rodents is a widely used preliminary screening model for antidiabetic agents (CitationIvorra et al., 1989; CitationSzkudelski, 2001). Although the experimental STZ diabetic rat model usually involves type 1 diabetes, however, in the present study, at the dose of STZ used, the model probably involved type 2 diabetes because when treated with intermediate doses of STZ (50 to 60 mg/kg), rats fail to gain weight and develop blood glucose levels 3-4 times higher than normal, survive without insulin supplementation and do not develop ketosis (CitationRastogi et al., 1997).
The present study has showed that the aqueous extract of H. umbellata seeds at doses of 400 and 800 mg/kg significantly reduced blood glucose in diabetic rats in a time-dependent manner when compared to the control. The maximal reduction in blood glucose was produced by 400 mg/kg of the extract at the 8 h. The extract at 1000 mg/kg only produced a significant reduction in blood glucose at the 12 h, which shows that the reduction was not dose-dependent. Glibenclamide, a sulfonylurea also reduced blood glucose in a sustained manner for 12 h and at the end of 14 days, but this effect, however, was lower when compared to 400 mg/kg of the extract.
In normal rats only 400 mg/kg of the plant extract significantly reduced blood glucose at 4 h after oral administration, unlike the standard drug glibenclamide which produced a very significant and sustained reduction in blood glucose for the entire period of the experiment. The above results both in normal and diabetic rats thus showed that though the plant extract, at a dose of 400 mg/kg may act like the sulfonylureas by stimulating insulin release from residual pancreatic β-cells (CitationMoller, 2001) as seen in normal rats, the results in diabetic rats show that the plant extract (400 and 800 mg/kg) produced a greater reduction in blood glucose than glibenclamide, therefore suggesting that there could be other mechanism(s) of action other than stimulation of insulin release.
Liver glycogen level may be considered as the best marker for assessing hypoglycemic activity of any drug because it indicates the peripheral free glucose that is being stored in the liver in the form of glycogen by increasing glycogenesis. The extract produced a significant increase in liver glycogen at 400 and 800 mg/kg in diabetic rats when compared to the control, while in normal rats increase in liver glycogen was not significant in 800 and 1000 mg/kg of extract compared to glibenclamide indicating that the extract maybe enhancing glycogen storage with more pronounced effect at lower doses. The apparent increase in liver glycogen by glibenclamide in the normal rats maybe due to the insulin released as a result of the pancreatic β-cells stimulation leading to insulin-induced glycogenesis.
The presence of flavonoids in the plant extract may also contribute to its hypoglycemic effect. Due to their phenolic structure, many flavonoids are antioxidants, since they are known to be involved in the healing process of free radical-mediated diseases including diabetes (CitationCarini et al., 2001; CitationCzinner et al., 2000).
In conclusion, we have demonstrated that the extract produced a more potent hypoglycemic effect at 400 and 800 mg/kg than at 1000 mg/kg in diabetic rats and that the hypoglycemic activity maybe due to increased glycogenesis, thus supporting the traditional usage in Nigeria for the treatment of diabetes.
Acknowledgements
This study was supported by a postgraduate research grant to Ighodaro Igbe by Steven Oluwole Awokoya Foundation for Science Education, Nigeria.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the contents and writing of this paper.
References
- Abaira C, Colwell JA, Nuttall FQ, Sawin CT, Nagel NJ, Comstock JP, Emmanuele NV, Levin SR, Henderson W, Lee HS (1995): Veterans Affairs cooperative study on glycemic control and complications in type II diabetes (VA CSDM): Results of feasibility trial. Diabet Care 18: 1113–1123.
- Alberti KG, Zimmet PZ (1998): Definition, diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539–553.
- Anturlikar SD, Gopumadhavan S, Chauhan BL, Mitra SK (1995): Effect of D-400, a herbal formulation on blood sugar of normal and alloxan-induced diabetic rats. Indian J Physiol Pharmacol 39: 95–100.
- Carini M, Adlini G, Furlanetto S, Stefani R, Facino RM (2001): LC coupled to ion- trap MS for the rapid screening and detection of polyphenol antioxidant from Helichrysum stoechas. J Pharm Biomed Anal 24: 517–526.
- Carter D (2004): Diabetes Mellitus – An Update for Health Professionals. London, British Medical Association Board of Science and Education Publications Unit.
- Chen H, Brahmbhatt S, Gupta A, Sharma AC (2005): Duration of streptozotocin-induced diabetes differentially affects p38-mitogen-activated protein kinase (MAPK) phosphorylation in renal vascular dysfunction. Cardiovasc Diabetiol 4: 3–6.
- Czinner E, Hagymasi K, Blazovics A, Kery A, Szoke E, Lemberkovics E (2000): In vitro antioxidant properties of Helichrysum arenarium (L) Moench. J Ethnopharmacol 73: 437–443.
- Elujoba AA (1995): Female infertility in the hands of traditional birth attendants in South-Western Nigeria. Fitoterapia 66: 239–248.
- Ivorra MD, Paya M, Villar A (1989): A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol 27: 243–275.
- Keay RWJ, Onochie CFA, Stanfield DP (1964): Nigerian Trees, Vol. II: Federal Department of Forest Research, Ibadan, pp. 378–379.
- Kleiv R, Klein BE, Moss SE, Cruickshanks KJ (1994): Relationship of hyperglycaemia to the long-term incidence and progression of diabetic retinopathy. Arch Intern Med 154: 2169–2178.
- Liv QZ, Pettitt DJ, Hanson RL, Charlses MA, Klein R, Bennett PH, Knowler WC (1993): Glycated haemoglobin, plasma glucose and diabetic retinopathy: Cross-sectional and prospective analysis. Diabetologia 36: 428–432.
- Mohamed B, Abderrahim Z, Hassane M, Abdelhafid T, Abdelkhaleq L (2006): Medicinal plants with potential antidiabetic activity-A review of ten years of herbal medicine research(1990-2000). Int J Diabetes Metab 14: 1–25.
- Moller DE (2001): New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414: 821–827.
- Oluwemimo A, Usifoh CO (2001): The anthelminthic activity of Hunteria umbellata K. Schum (Fam. Apocynaceae) extracts. Pak J Sci Ind Res 44: 286–290.
- Raman A, Mallam V (1994): Enhanced in vitro activity of glucokinase enzyme in the presence of extracts of Hunteria umbellata a traditional Nigerian treatment for diabetes. Journal of Pharmacy and Pharmacology 46: (supplement): 1046.
- Rastogi AK, Ramesh C, Arvind KS (1997): Screening of natural products for hypolipidaemic and hypoglycaemic activities. International workshop on medicinal plants, their bioactivity, screening and evaluation: Lucknow, pp 1–17.
- Sofowora A (1982): Medicinal Plants and Traditional Medicine in Africa. John Wiley and Sons Ltd, Chichester pp. 168–171.
- Szkudelski T (2001): The mechanism of alloxan and streptozotocin action in β-cells of the rat pancreas. Physiol Res 50: 537–546.
- Trease GE, Evans MC (1989): A Textbook of Pharmacognosy, 13th edition. London, Bailiere Tindall, pp. 520–521.
- WHO (2002): WHO launches the first global strategy on traditional medicine. Press release WHO/38.
- Zhang CY, Zhu SM, Ren BZ (1987): Laboratory Methods of Biochemistry. Beijing, People’s Health Publishing House, pp. 89–91.
