Abstract
Context: Standardized myrtol, an essential oil containing primarily cineole, limonene and α-pinene, has been used for treating nasosinusitis, bronchitis and chronic obstructive pulmonary disease (COPD).
Objective: To investigate the effects of standardized myrtol in a model of acute lung injury (ALI) induced by lipopolysaccharides (LPS).
Materials and methods: Male BALB/c mice were treated with standardized myrtol for 1.5 h prior to exposure of atomized LPS. Six hours after LPS challenge, lung injury was determined by the neutrophil recruitment, cytokine levels and total protein concentration in the bronchoalveolar lavage fluid (BALF) and myeloperoxidase (MPO) activity in the lung tissue. Additionally, pathological changes and NF-κB activation in the lung were examined by haematoxylin and eosin staining and western blot, respectively.
Results: In LPS-challenged mice, standardized myrtol at a dose of 1200 mg/kg significantly inhibited the neutrophile counts (from 820.97 ± 142.44 to 280.42 ± 65.45, 103/mL), protein concentration (from 0.331 ± 0.02 to 0.183 ± 0.01, mg/mL) and inflammatory cytokines level (TNF-α: from 6072.70 ± 748.40 to 2317.70 ± 500.14, ng/mL; IL-6: from 1184.85 ± 143.58 to 509.57 ± 133.03, ng/mL) in BALF. Standardized myrtol also attenuated LPS-induced MPO activity (from 0.82 ± 0.04 to 0.48 ± 0.06, U/g) and pathological changes (lung injury score: from 11.67 ± 0.33 to 7.83 ± 0.79) in the lung. Further study demonstrated that standardized myrtol prevented LPS-induced NF-κB activation in lung tissues.
Discussion and conclusion: Together, these data suggest that standardized myrtol has the potential to protect against LPS-induced airway inflammation in a model of ALI.
Introduction
Acute lung injury (ALI) is a life-threatening syndrome characterized by severe lung inflammation and breakdown of the alveolar-capillary barrier, and acute respiratory distress syndrome (ARDS) is the most severe form of ALI (Herridge & Angus Citation2005). Although lung protective ventilatory strategies have been widely applied, the mortality caused by ALI/ARDS is still high (Rubenfeld et al. Citation2005; Galvin et al. Citation2013). Therefore, a novel effective therapy is urgently required.
Standardized myrtol, an essential oil derived from Pinus spp (pine), Citrus aurantifolia (lime) and Eucalyptus globulus, which contains primarily cineole, limonene and α-pinene (Zimmermann et al. Citation1995). This phytomedicine has been widely used in Asian and European for treating nasosinusitis and bronchitis (Meister et al. Citation1999; Matthys et al. Citation2000). Recently, standardized myrtol has also been reported to have positive effect on chronic obstructive pulmonary disease (COPD) in clinical trials and animal studies, mainly due to its secretomotor, secretolytic and mucolytic effects (Rantzsch et al. Citation2009; Cao et al. Citation2011; Begrow et al. Citation2012). Additionally, standardized myrtol showed potent anti-inflammatory and antioxidative activities (Beuscher et al. Citation1998; Grassmann et al. Citation2000; Ying et al. Citation2015). It is unknown; however, whether standardized myrtol has protective effect on acute lung injury.
In the present study, a lipopolysaccharide (LPS) challenged mice model was established for investigating the impact of standardized myrtol on acute lung injury. LPS inhalation can induce rapid influx of neutrophils and apoptosis of lung epithelial cell, resulting in the releases of inflammatory cytokines, reactive oxygen species and chemotactic factors, these effects subsequently cause intra-alveolar and interstitial oedema, alveolar haemorrhage and impairment in the alveolar fluid clearance, which ideally mimic the pathophysiologic events observed in ALI patients (Matute-Bello et al. Citation2008; Chen et al. Citation2010).
Materials and methods
Material
Standardized myrtol (Catalogue No.237124) was obtained from G. Pohl-Boskamp GmbH &Co. (Hohenlockstedt, Germany). Lipopolysaccharide (Escherichia coli 055:B5) was obtained from Sigma-Aldrich (St, Louis, MO). Nuclear and Cytoplasmic Protein Extraction Kit was obtained from Beyotime Institute of Biotechnology (Haimen, China). Antibodies for p65 and β-actin were obtained from Santa Cruz (Santa Cruz, CA). Enzyme linked immunosorbent assay (ELISA) kits for determination of mouse TNF-α and IL-6 were obtained from Dakewe Biotech co. Ltd (Beijing, China). The myeloperoxidase (MPO) activity assay kit was obtained from Nanjing JianCheng Bioengineering Institute (Nanjing, China).
Design
LPS-induced acute lung injury was induced as described by Takano et al. (Citation2011). Male BALB/c mice (8–9 weeks old; 20–25 g; Experimental Animal Centre of Guangdong Province, Guangzhou, Guangdong) were kept in an acrylic chamber (20 × 30 × 40 cm, 24 L) throughout the LPS exposure period. A solution containing 0.5 mg/mL LPS in saline was aerosolized with an ultrasonic nebulizer (NB-150U; Omron Co., Kyoto, Japan) and air pump coupled to a manometer (flow rate was monitored and adjusted to 4.8 L/min) for 30 min. All procedures were performed in accordance with the Declaration of Helsinki of the World Medical Association.
Standardized myrtol dissolved in 0.1% Cremophor EL at 30 and 120 mg/mL or the same volume of dissolvent (0.1 mL/10 g body weight) was administered by gavage 1.5 h before LPS challenge. The dosages of standardized myrtol used in this study were 300 and 1200 mg/kg. Six hours after LPS challenge, mice were anesthetized with an overdose of pentobarbital sodium and then sacrificed by exsanguination (for examination of NF-κB activation, mice were executed at 3 h after LPS challenge).
Bronchoalveolar lavage fluid (BALF) collection and cell count
For the BALF collection, tracheostomy was performed and a cannula was inserted into trachea. The cannula was secured with 3-O surgical silk, and the lungs were lavaged three times with phosphate buffer solution in a total volume of 1.5 ml (0.5 mL ×3). The recovery rate of BALF was above 90%. A 0.1 mL aliquot was used for the total cell count by using a haemocytometer, and the remainder was immediately centrifuged at 500 g for 10 min at 4 °C. Total protein concentration in the supernatant of BALF was quantified by the BCA method with bovine serum albumin as a standard. The rest of supernatant was aliquoted and frozen at −70 °C until the cytokines were assessed. The centrifuged cells were re-suspended in 50 μL PBS, and the cell differentiation was determined for 200 cells by examination of the Wright-Giemsa-stained smears.
Measurement of pro-inflammatory cytokines in BALF
The levels of TNF-α and IL-6 in BALF were measured with mouse TNF-α and IL-6 ELISA kits according to the manufacturer’s protocol (Dakewe Biotech co. Ltd, Beijing, China).
Histological evaluation
After mice were killed, the lungs were excised. The right lobes were fixed with 10% neutral phosphate-buffered formalin and then embedded in paraffin. Sections with 5 μm thick were stained with haematoxylin and eosin. The rest of lung lobes were flash frozen in liquid nitrogen and stored at −70 °C until examination of MPO. Lung injury was scored according to the following criteria (Schingnitz et al. Citation2010): (1) alveolar congestion, (2) haemorrhage, (3) infiltration or aggregation of neutrophils in airspace or vessel wall, and (4) thickness of the alveolar wall. For each subject, a five-point scale was applied: 0, minimal (little) damage; 1+, mild damage; 2+, moderate damage; 3+, severe damage; and 4+, maximal damage. Points were added up and are expressed as median ± range of injury score.
Measurement of MPO
Lung tissues were frozen in liquid nitrogen and then homogenized in PBS. The homogenate was used to determine MPO according to the manufacturer’s instruction. MPO activities were expressed as units per gram of protein.
Western blot
After mice were killed, the whole lung tissues were flash frozen in liquid nitrogen immediately after removing and stored at −70 °C until use. The extraction of nuclear and cytoplasmic proteins from lung tissues was performed by using a Nuclear and Cytoplasmic Protein Extraction Kit. Protein contents in the supernatant of the lysed lungs were determined by the BCA method. Samples were separated on 10% SDS–PAGE and transferred to polyvinylidene difluoride membranes. After blocking with 5% non-fat milk in TBST (0.1%) for 1 h at room temperature, the membranes were incubated with a primary antibody (p65 or β-actin) and then incubated with the conjugated secondary antibodies. Protein bands were detected by an ECL detection kit (Millipore, Billerica, MA). The protein signals were quantified by scanning densitometry using a Tanon 5200 imaging System (Tanon, Shanghai, China).
Statistical analysis
Data are expressed in term of means ± standard error of the mean (SEM). Data were analyzed by using one-way analysis of variance followed by Student-Newman-Keuls test. Two-tailed p values <0.05 were considered statistically significant. Statistical analyses were conducted using SPSS 13.0 (SPSS Inc., Chicago, IL).
Results
Effect of standardized myrtol on inflammatory cell counts in BALF of LPS-induced ALI mice
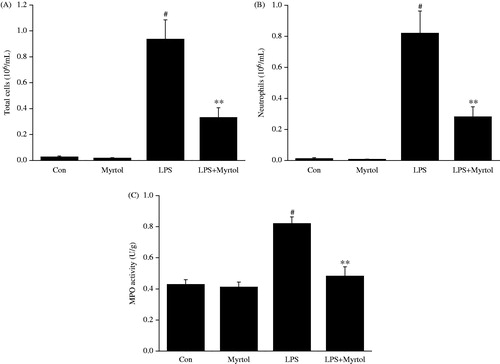
Recruitment of neutrophils into the pulmonary compartment is a key event of acute lung injury. As shown in , administration of saline was associated with few cell numbers in BALF, while LPS challenge caused a brisk and strong influx of total cells and neutrophils into BALF. Treatment of 1200 mg/kg standardized myrtol significantly inhibited LPS-induced cell infiltration. MPO activity in tissues is an indicator of neutrophils infiltration. In parallel with the number of PMNs, the level of MPO activity was markedly inhibited by standardized myrtol.
Figure 1. Effect of Standardized myrtol on inflammatory cell counts in BALF of LPS-induced ALI mice. Standardized myrtol at doses of 1200 mg/kg were administrated 1.5 h before LPS challenge. Mice were killed 6 h after LPS challenge and bronchoalveolar lavage was performed. The numbers of total cells (A) and neutrophils (B) in BALF were examined; (C) Mice were sacrificed 6 h after LPS challenge and lung tissues were homogenized in PBS. The homogenate was used for MPO assay. MPO activities were expressed as units per gram of protein. All values are mean ± SEM (n = 6). #p < 0.05, significant compared with vehicle-treated control; *p < 0.05, significant compared with LPS alone; **p < 0.01, significant compared with LPS alone.
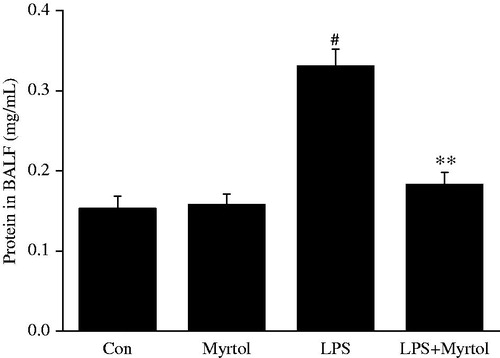
Effect of standardized myrtol on total protein concentration in BALF of LPS-induced ALI mice
Vascular leakage is a hallmark of ALI, thus we examined the total protein concentration in BALF. As shown in , the challenge of LPS resulted in a significantly increase of protein level in BALF, from 0.153 ± 0.016 to 0.331 ± 0.021 mg/mL. Treatment with 1200 mg/kg standardized myrtol did not affect the basal protein level, while dramatically inhibited LPS-induced increase of protein concentration to 0.183 ± 0.015 mg/mL.
Figure 2. Effect of standardized myrtol on total protein concentration in BALF of LPS-induced ALI mice. Standardized myrtol at doses of 1200 mg/kg were administrated 1.5 h before LPS challenge. Mice were killed 6 h after LPS challenge and bronchoalveolar lavage was performed. Total protein concentration in BALF was examined. All values are mean ± SEM (n = 6). #p < 0.05, significant compared with vehicle-treated control; *p < 0.05, significant compared with LPS alone; **p < 0.01, significant compared with LPS alone.
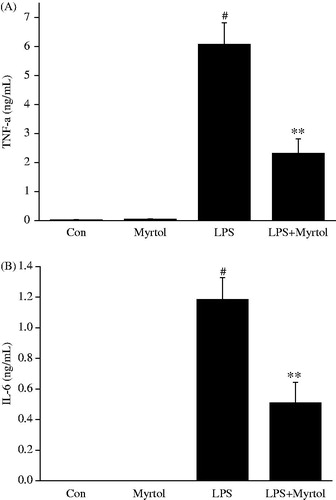
Effect of standardized myrtol on TNF-α and IL-6 levels in BALF of LPS-induced ALI mice
As shown in , LPS challenge causes significant increase levels of TNF-α and IL-6 in BALF, while these elevations were markedly attenuated by administration of 1200 mg/kg standardized myrtol.
Figure 3. Effect of standardized myrtol on the levels of TNF-α and IL-6 in BALF of LPS-induced ALI mice. Standardized myrtol at doses of 1200 mg/kg were administrated 1.5 h before LPS challenge. Mice were killed 6 h after LPS challenge and bronchoalveolar lavage was performed. The levels of TNF-α (A) and IL-6 (B) in BALF were examined. All values are mean ± SEM (n = 6). #p < 0.05, significant compared with vehicle-treated control; *p < 0.05, significant compared with LPS alone; **p < 0.01, significant compared with LPS alone.
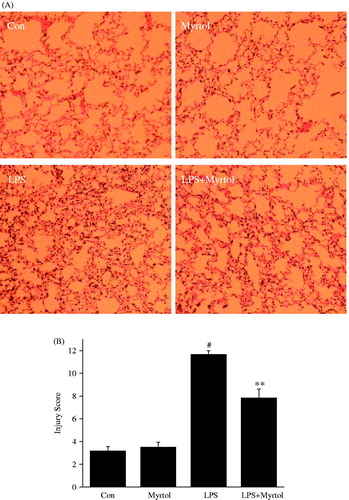
Standardized myrtol attenuated lung histopathology in LPS-induced ALI mice
As shown in , no evident histological alteration was observed in lung specimens of normal mice. However, the inhalation of LPS resulted in significant lung injury, evidenced by the presence of alveolar haemorrhage, a marked swelling of the alveolar walls and remarkable recruitment of neutrophils into the alveolar spaces. These pathological changes were improved by administration of 1200 mg/kg standardized myrtol.
Figure 4. Standardized myrtol ameliorated LPS-induced histological changes in lung tissues. Representative lung sections are shown. 6 h after LPS challenge lungs were fixed, embedded in paraffin and cut into 5 μm slices. After H&E staining, histological examination was performed by light microscopy. Lung sections were obtained from vehicle-treated mice, Standardized myrtol (1200 mg/kg) treated mice, LPS-treated mice or LPS + Standardized myrtol (1200 mg/kg) treated mice (A), and lung injury was scored (B). All values are mean ± SEM (n = 6). #p < 0.05, significant compared with vehicle-treated control; *p < 0.05, significant compared with LPS alone; **p < 0.01, significant compared with LPS alone.
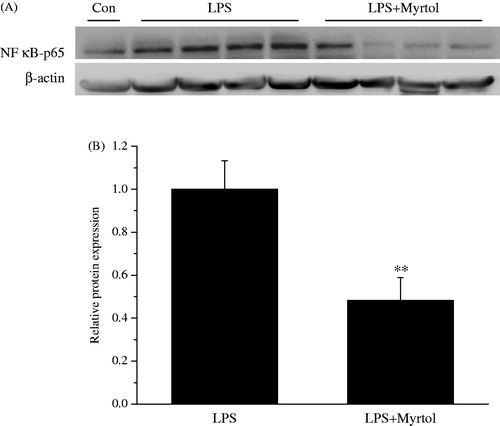
Standardized myrtol inhibited NF-κB activation in LPS-induced ALI mice
We evaluated nuclear NF-κB p65 level by Western blot analysis to investigate the molecular mechanism whereby treatment with standardized myrtol attenuated the development of acute lung injury. As shown in , treatment with of 1200 mg/kg standardized myrtol markedly inhibited the LPS-induced NF-κB-p65 subunits translocation in the lung tissue (Standardized myrtol did not inhibit LPS-induced inflammatory cell counts, MPO activity, pro-inflammatory cytokine releases and protein leakage at the dose of 300 mg/kg, data not shown).
Figure 5. Standardized myrtol inhibited LPS-induced NF-κB activation in lung tissues. Standardized myrtol at doses of 1200 mg/kg were administrated 1.5 h before LPS challenge. Mice were killed 3 h after LPS inhalation and the lungs were removed. The extraction of nuclear and cytoplasmic proteins from lung tissues was performed. The expression of nuclear NF-κB p65 was detected by Western blotting. All values are mean ± SEM (n = 4). **p < 0.01, significant compared with LPS alone.
Discussion
In the beginning of ALI, the earliest immune cell recruited to the site of injury is neutrophil, which is vital for the host defense. However, an excess of activated neutrophils leads to tissue damage by release of various inflammatory cytokines, proteinases and cationic polypeptides (Grommes & Soehnlein Citation2011). MPO, an enzyme located mainly in the primary granules of neutrophils, reflects the adhesion and margination of neutrophils in the lung. Proofs from clinical data and animal experience have been shown that both decreased number of PMNs and lowering of MPO activity are associated with favourable prognosis of ALI (Steinberg et al. Citation1994; Abraham et al. Citation2000). In the present study, the rapid neutrophils influx induced by LPS was markedly inhibited by standardized myrtol. These observations were further confirmed by the results that standardized myrtol suppressed MPO activity. Because protein extravasation is considered as a pointer of vascular leakage, we examined total protein contents in BALF and detected lower protein levels in standardized myrtol treated mice. These results suggested that standardized myrtol attenuated neutrophils influx and vascular leakage in LPS-challenged mice, which thereby ameliorating the lung pathological changes.
Numerous studies suggest that a network of pro-inflammatory cytokines, such as TNF-α and IL-6, plays a vital role in lung injury (Bhatia & Moochhala Citation2004; Tunceroglu et al. Citation2013). These cytokines not only cause inflammatory cascade injury, but also recruited neutrophils into lung tissues (Bauer et al. Citation2000). Standardized myrtol has been reported to decrease the release of TNF-α, IL-8 and GM-SCF in cultured alveolar macrophages from COPD patients (Rantzsch et al. Citation2009). In the present study, greatly increased levels of TNF-α and IL-6 in BALF were induced by inhalation of LPS, which was suppressed by pretreatment with standardized myrtol. The inflammatory cytokine levels have been confirmed to reflect the extent of pulmonary inflammation in ALI (Bhatia & Moochhala Citation2004). Data obtained from our study demonstrated the potently anti-inflammatory activity of standardized myrtol in vivo.
NF-κB is a transcription factor regulating various genes involved in inflammatory and immune response (Schuliga Citation2015). Clinical research has reported that the degree of NF-κB activation was increased in patients with sepsis or acute lung injury (Schwartz et al. Citation1996). Additionally, animal studies showed that inactivation of NF-κB pathway by pharmacological inhibition or gene modification decreased the expression of pro-inflammatory mediators and diminished the severity of endotoxemia-induced acute lung injury (Wang et al. Citation2011; Lopez et al. Citation2015). In the present study, the nuclear proteins from lung tissues were extracted. LPS inhalation significantly increased the translocation of NF-κB subunits from cytoplasm into nucleus, which was consistent with those of previous studies (Shi et al. Citation2014; Zhang et al. Citation2014). However, the events induced by LPS were reversed by pretreatment with standardized myrtol. Thus, it is suggested that NF-κB pathway plays an important role in the process of antagonizing acute lung injury by standardized myrtol.
Dispensatory reports that standardized myrtol is well tolerated by human and host animal, this phytomedicine induces few and mild adverse reactions even treated with the maximal tolerance dose (about 1500 mg/kg for human; above 2000 mg/kg for rats). In the present study, oral administration of standardized myrtol did not provoke behavioural effects in mice, and no acute toxicity and mortality occurred at the dose of 1200 mg/kg. Additionally, sham groups (mice treated 1200 mg/kg standardized myrtol alone) showed no difference on neutrophils influx, protein exudation and pro-inflammatory cytokine levels in BALF, when compared with control group. Thus, the doses of standardized myrtol used in this study are considered to be safe.
In conclusion, we have demonstrated the protective effects of standardized myrtol on lung injury induced by LPS, which is evidenced by improvement of the pathologic changes in the lung, inhibition of NF-κB activation, and decrease of neutrophils infiltration, vascular leakage and pro-inflammatory cytokine release. Our study suggests that standardized myrtol might be useful in the therapy of lung injury.
Disclosure statement
The authors report no conflicts of interest.
References
- Abraham E, Carmody A, Shenkar R, Arcaroli J. 2000. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 279:L1137–L1145.
- Bauer TT, Montón C, Torres A, Cabello H, Fillela X, Maldonado A, Nicolás JM, Zavala E. 2000. Comparison of systemic cytokine levels in patients with acute respiratory distress syndrome, severe pneumonia, and controls. Thorax. 55:46–52.
- Begrow F, Böckenholt C, Ehmen M, Wittig T, Verspohl EJ. 2012. Effect of Myrtol standardized and other substances on the respiratory tract: ciliary beat frequency and mucociliary clearance as parameters. Adv Ther. 29:350–358.
- Beuscher N, Kietzmann M, Bien E, Champeroux P. 1998. Interference of Myrtol standardized with inflammatory and allergic mediators. Arzneimittelforschung. 48:985–989.
- Bhatia M, Moochhala S. 2004. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 202:145–156.
- Cao L, Chen Y, Zhao Y, Zhang H, Wang S, Wang Z, Kang J. 2011. Effect of Myrtol standardized on mucus hypersecretion and clearance of Pseudomonas aeruginosa in a rat model of chronic obstructive pulmonary disease. Arzneimittelforschung. 61:685–692.
- Chen H, Bai C, Wang X. 2010. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev Respir Med. 4:773–783.
- Galvin IM, Steel A, Pinto R, Ferguson ND, Davies MW. 2013. Partial liquid ventilation for preventing death and morbidity in adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 7:CD003707.
- Grassmann J, Hippeli S, Dornisch K, Rohnert U, Beuscher N, Elstner EF. 2000. Antioxidant properties of essential oils. Possible explanations for their anti-inflammatory effects. ArzneimittelForschung. 50:135–139.
- Grommes J, Soehnlein O. 2011. Contribution of neutrophils to acute lung injury. Mol Med. 17:293–307.
- Herridge MS, Angus DC. 2005. Acute lung injury-affecting many lives. N Engl J Med. 353:1736–1738.
- Lopez B, Maisonet TM, Londhe VA. 2015. Alveolar NF-κB signaling regulates endotoxin-induced lung inflammation. Exp Lung Res. 41:103–114.
- Matthys H, de Mey C, Carls C, Ryś A, Geib A, Wittig T. 2000. Efficacy and tolerability of Myrtol standardized in acute bronchitis. A multi-centre, randomised, double-blind, placebo-controlled parallel group clinical trial vs. cefuroxime and ambroxol. ArzneimittelForschung. 50:700–711.
- Matute-Bello G, Frevert CW, Martin TR. 2008. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 295:L379–L399.
- Meister R, Wittig T, Beuscher N, de Mey C. 1999. Efficacy and tolerability of Myrtol standardized in long-term treatment of chronic bronchitis. A double-blind, placebo-controlled study. Study Group investigators. ArzneimittelForschung. 49:351–358.
- Rantzsch U, Vacca G, Dück R, Gillissen A. 2009. Anti-inflammatory effects of Myrtol standardized and other essential oils on alveolar macrophages from patients with chronic obstructive pulmonary disease. Eur J Med Res. 14:205–209.
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. 2005. Incidence and outcomes of acute lung injury. N Engl J Med. 353:1685–1693.
- Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. 2010. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 184:5271–5279.
- Schuliga M. 2015. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules. 5:1266–1283.
- Schwartz MD, Moore EE, Moore FA, Shenkar R, Moine P, Haenel JB, Abraham E. 1996. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med. 24:1285–1292.
- Shi D, Zheng M, Wang Y, Liu C, Chen S. 2014. Protective effects and mechanisms of mogroside V on LPS-induced acute lung injury in mice. Pharm Biol. 6:729–734.
- Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. 1994. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med. 150:113–122.
- Takano Y, Mitsuhashi H, Ueno K. 2011. 1α,25-Dihydroxyvitamin D3 inhibits neutrophil recruitment in hamster model of acute lung injury. Steroids. 76:1305–1309.
- Tunceroglu H, Shah A, Porhomayon J, Nader ND. 2013. Biomarkers of lung injury in critical care medicine: past, present, and future. Immunol Invest. 42:247–261.
- Wang M, Liu T, Wang D, Zheng Y, Wang X, He J. 2011. Therapeutic effects of pyrrolidine dithiocarbamate on acute lung injury in rabbits. J Transl Med. 9:61.
- Ying B, Maimaiti AK, Song D, Zhu S. 2015. Myrtol ameliorates cartilage lesions in an osteoarthritis rat model. Int J Clin Exp Pathol. 8:1435–1442.
- Zhang Y, Du Z, Zhou Q, Wang Y, Li J. 2014. Remifentanil attenuates lipopolysaccharide-induced acute lung injury by downregulating the NF-κB signaling pathway. Inflammation. 37:1654–1660.
- Zimmermann T, Seiberling M, Thomann P, Karabelnik D. 1995. The relative bioavailability and pharmacokinetics of standardized Myrtol. ArzneimittelForschung. 45:1198–1201.

