Abstract
Context: Virgin coconut oil (VCO) has been reported to possess antioxidative, anti-inflammatory and anti-stress properties.
Objective: Capitalizing on these therapeutic effects, this study investigated for the first time the potential of VCO on memory improvement in vivo.
Materials and methods: Thirty male Wistar rats (7–8 weeks old) were randomly assigned to five groups (n = six per group). Treatment groups were administered with 1, 5 and 10 g/kg VCO for 31 days by oral gavages. The cognitive function of treated-rats were assessed using the Morris Water Maze Test. Brains were removed, homogenized and subjected to biochemical analyses of acetylcholine (ACh) and acetylcholinesterase (AChE), antioxidants [superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), glutathione peroxidase (GPx) and glutathione reductase (GRx)], lipid peroxidase [malondialdehyde (MDA)] as well as nitric oxide (NO). α-Tocopherol (αT; 150 mg/kg) was also included for comparison purposes.
Results: VCO-fed Wistar rats exhibited significant (p < 0.05) improvement of cognitive functions [reduced escape latency (≥ 1.8 s), reduced escape distance (≥ 0.3 m) and increased total time spent on platform (≥ 1 s)]. The findings were accompanied by elevation of ACh (15%), SOD (8%), CAT (≥ 54%), GSH (≥ 20%) and GPx (≥ 12%) and reduction of AChE (≥17%), MDA (> 33%) and NO (≥ 34%). Overall, memory improvement by VCO was comparable to αT.
Discussion and conclusion: VCO has the potential to be used as a memory enhancer, the effect of which was mediated, at least in part, through enhanced cholinergic activity, increased antioxidants level and reduced oxidative stress.
Introduction
Virgin coconut oil (VCO) is increasingly known for its usefulness as functional food oil. This is evident by the increased availability of VCO in the South East Asian countries (Marina et al. Citation2009b). In general, VCO is predominantly made up of triglycerides (i.e., short- and medium-chain saturated fatty acids). Interestingly, 60% of the medium-chain triglycerides (MCT) in the VCO are almost similar to those in mother’s milk, an ideal natural food formula that protects infants from infections and other illnesses (Isaacs & Thormar Citation1990). Lauric acid, in particular, is an MCT that encompasses the majority of VCO nutritional content (Bawalan & Chapman Citation2006). Its amount in VCO is comparable to that in the coconut oil (Marina et al. Citation2009b). Lauric acid is commonly used as nutritional supplements for infants or patients suffering from malabsorption (Nik Norulaini et al. Citation2009). Besides, VCO is also rich in active polyphenol compounds, which are strong inhibitors of lipid peroxidation (Dosumu et al. Citation2010). Polyphenols are known for their neuroprotective actions, especially in preventing the neurotoxic effects of β-amyloid (Menard et al. Citation2013).
The beneficial effects of VCO have been widely investigated. In fact, VCO has been reported for its excellent antioxidative, anti-inflammatory and antistress properties (Yeap et al. Citation2015). Capitalizing on these therapeutic effects, the present study assessed the memory-enhancing effect of VCO in normal adult Wistar rats. It is now clear that some aspects of age-related cognitive decline begin in healthy educated adults when they are in their 20s and 30s (Salthouse Citation2009). Prevention or delay of the progression of age-related cognitive decline would definitely help to reduce the risk of functional impairment. Given that cognitive impairment is correlated with increased vulnerability of various biological components (e.g., lipids, proteins, nucleic acids and cholinergic neurotransmitters) to damaging effects of oxidative stress (Papandreou et al. Citation2009), VCO appeared to be an ideal nutraceutical-based memory-enhancing agent.
This study also sought to understand the underlying mechanisms of VCO-induced memory-enhancing effects, by which vital insights into the effective use of VCO in the prevention of neurodegeneration would be obtained. The present study focused on the association of VCO-induced memory-enhancing effects with cholinergic activity and oxidative stress process. To the best of our knowledge, this is the first mechanistic study on VCO-induced memory improvement in vivo. α-Tocopherol (α-T), a monophenolic lipid-soluble vitamin E, was included in this study for comparison purpose.
Materials and methods
Ethics approval
The present animal study was approved by the Committee on Animal Research and Ethics of the Universiti Teknologi MARA (UiTM) [Reference No: 600-FF (PT. 5/2); dated 8 March 2013]. The laboratory animals were handled and managed in accordance to the Guide for the Care and Use of Laboratory Animal (National Research Council Citation1996).
Animals
Adult male Wistar rats (7–8 weeks old) weighing between 250–300 g were obtained from the Laboratory Animal Facility and Management (LAFAM) of the Faculty of Pharmacy, UiTM Puncak Alam Campus. The animals were housed under standard laboratory conditions and maintained on a 12 h light/dark cycle with access to food and water ad libitum. The rodents were acclimatized to laboratory conditions before the study.
Treatment groups
The rats (n = 6) were randomly assigned into the following groups: Control (saline only), VCO of 1, 5 and 10 g/kg, respectively (commercially available; Dr Azimuth Formula, Kelantan, Malaysia) as well as α-T [150 mg/kg (Sigma, St Louis, MO)] (Yamada et al. Citation1999). The VCO, which was prepared via fermentation process, comprised of lauric acid (47.03%), myristic acid (18.71%), caprylic acid (7.93%), palmitic acid (8.86%), capric acid (5.84%), oleic acid (5.52%), stearic acid (3.27%), linoleic acid (0.87%) and caproic acid (1.88%). VCO or αT were administered to the animals p.o. for 31 days ().
Morris Water Maze (MWM) Test
The MWM task is a widely accepted method to assess spatial learning and memory of rodents (Morris Citation1984). Briefly, each rat was exposed to three tracks a day for three days (Day 26–28) before the actual task (). For each training trial, each rat was placed at one of the three randomly determined locations and allowed to find the hidden platform. The animal was allowed to remain at the platform for 20 s before being placed in a holding cage for 30 s until the start of the next trial. After training, the actual task was conducted for three consecutive days (Day 29 to Day 31; ). During the actual task, each rat was placed into the pool at a fixed starting position whereby it was allowed to swim and escape latency, escape distance and swimming speed recorded. The cut off time was 60 s. If the animal crossed the cut off time, it would be guided gently to the platform by hand. Time taken from initial point to platform (escape latency), travelled distance and swimming speed were monitored and analyzed using a video tracking system (ANY-Maze, San Diego Instrument, San Diego, CA). In order to assess memory consolidation, a probe test (day 32) was conducted after the last acquisition session (). During this test, platform was removed. Time spent in the platform quadrant was recorded.
Biochemical assays
Further to behavioural test on day 32, the animals were sacrificed by cervical dislocation (). Blood was collected through cardiac puncture. The whole brain was carefully removed from the skull and transferred into phosphate-buffered saline (PBS) before being subjected to homogenization using a glass Wise Stir Homogenizer (Daihan Scientific, Korea). The brain homogenates were centrifuged (2000 g at 4 °C for 10 min) and resultant supernatant was collected and stored at −80 °C until biochemical assays were conducted.
Acetylcholinesterase (AChE) assay
AChE was measured using the QuantiChromTM Acetylcholinesterase Assay Kit (BioAssay System, CA) in accordance with the manufacturer’s instruction (Anonymous Citation2007). Briefly, 10 μL supernatant from brain homogenate of each sample was added into 190 μL working reagent in a 96-well plate. The intensity of the colour change was measured at 412 nm of the 2nd and 10th min using the microplate reader (Tecan, Mannedorf, Switzerland). The activity of this enzyme was determined by calculating the difference of absorbance between the 2nd and the 10th min in comparison to the standard.
Acetylcholine (ACh) abundance assay
ACh was measured using the EnzyChromTM Acethylcholine Assay Kit (BioAssay System, CA) in accordance to the manufacturer’s instruction (Anonymous Citation2013). Briefly, 20 μL of supernatant from brain tissue lysates was added onto wells containing 80 μL of working reagent. The colour intensity was measured at 570 nm.
Superoxide dismutase (SOD) assay
Measurement of SOD activity was performed using the Cayman’s Superoxide Dismutase Assay Kit (Cayman Chemical, Ann Arbor, MI) in accordance with the manufacturer’s instructions (Anonymous Citation2014e). Briefly, 10 μL of the sample was added into 200 μL radical detector. The reaction was initiated by adding 20 μL xanthine oxidase and the resultant mixture was incubated at room temperature for 20 min on a shaker. The absorbance was measured at 440 nm.
Catalase (CAT) assay
Measurement of CAT activity was performed using the Cayman’s Catalase Assay Kit (Cayman Chemical, Ann Arbor, MI) in accordance with the manufacturer’s instructions (Anonymous Citation2014a). Briefly, 20 μL of the sample was added into 30 μL of methanol and 100 μL of Assay Buffer. The reaction was initiated by adding 20 μL of H2O2. The mixture was incubated at room temperature for 20 min. To terminate the reaction, 30 μL of potassium hydroxide and purpald chromagen were added and the mixture was incubated at room temperature for 10 min. Finally, 10 μL of potassium periodate was added and incubated at room temperature for 5 min in a shaker. The solution was then measured at 540 nm.
Glutathione (GSH) assay
Measurement of GSH concentration was performed using the Cayman’s GSH Assay (Cayman Chemical, Ann Arbor, MI) in accordance with the manufacturer’s instructions (Anonymous Citation2014b). Briefly, 150 μL freshly prepared assay cocktail containing MES buffer, cofactor mixture, enzyme mixture, water and DTNB was added into 50 μL samples in a 96-well plate. The absorbance was measured at 405 nm and 5 min interval for 30 min.
Glutathione peroxidase (GPx) assay
Measurement of GPx was performed using Cayman’s GPx Assay Kit (Cayman Chemical, Ann Arbor, MI) in accordance with the manufacturer’s instructions (Anonymous Citation2014c). Briefly, 20 μL of the sample was mixed with 100 μL of assay buffer and 50 μL of co-substrate mixture. The reaction was initiated by adding 20 μL cumene hydroperoxide and the absorbance was read every minute (at least 5 time points) at 340 nm.
Glutathione reductase (GR) assay
Measurement of GR was performed using the Cayman’s Glutathione Reductase Assay Kit (Cayman Chemical, Ann Arbor, MI) in accordance with the manufacturer’s instructions (Anonymous Citation2014d). Briefly, 20 μL sample was added into 20 μL GSSG and 100 μL assay buffer. The reactions of the enzyme were initiated by adding 50 μL NADPH to the wells. Absorbance was read at 340 nm every minute (at least 5 time points).
Thiobarbituric acid reactive substances (TBARS) assay
The measurement of TBARS, which was essential for screening and monitoring lipid peroxidation, was performed using the Cayman’s TBARS Assay Kit (Cayman Chemical, Ann Arbor, MI) in accordance to the manufacturer’s instructions (Anonymous Citation2014f). Briefly, 100 μL of the sample was mixed with SDS (sodium dodecyl sulphate) solution and 4 mL colour reagent containing TBA, acetic acid and sodium hydroxide. The solution was then boiled vigorously for 1 h, and immediately cooled down on ice for 10 min to stop the reaction. The solution was centrifuged (2 000 g and 4 °C for 10 min). Supernatant was collected and the absorbance was measured at 530 nm.
Nitric oxide (NO) assay
Measurement of nitric oxide (NO), a pro-inflammatory mediator, was performed using the QuantiChromTM Nitric Oxide Assay Kit (BioAssay System, CA) in accordance with the manufacturer’s instructions (Anonymous Citation2010). Briefly, 100 μL of deproteinated sample was added to 200 μL working reagent containing three mixture of reagent provided by manufacturer. The solution was then incubated at 60 °C for 10 min. After centrifugation (2000 g and 4 °C for 10 min), 250 μL supernatant was transferred into 96-well plates and the absorbance was read at 540 nm.
Statistical analyses
Statistical significance was analyzed using GraphPad Prism version 6.01 (GraphPad software Inc., La Jolla, CA). Mean differences was evaluated using the One-Way Analysis of Variance (ANOVA) (with post hoc Befforoni’s Test). p Values <0.05 were considered as statistically significant.
Results
VCO-enhanced memory of Wistar rats but not their locomotor
Cognitive functions of VCO-treated rats were examined in terms of their escape latency (time to reach platform), escape distance (searching distance) and time spent in platform quadrant (probe test) (). In addition, the present study also assessed the motor function of treated animals by taking into account the average swimming speed (). In general, VCO (1–10 g/kg) significantly (p < 0.05) reduced escape latency (Day 1–3) when compared to control (). On average, the escape latency of 10 g/kg VCO-treated rats (p < 0.001) was reduced by 2.3 s as opposed to that of control (Day 1–3).
Figure 2. VCO-enhanced memory in Wistar rats. VCO treated rats were subjected to behavioural study using the Morris Water Maze Test. The rodents were assessed as follows: (A) Escape latency, (B) escape distance, (C) time spent by the rat in the escape platform zone (probe test) and (D) average speed taken by the rat to reach escape platform. αT was also included for comparison purposes. Each bar represents mean ± SEM of n = 6. *p < 0.05, **p < 0.01, ***p < 0.001 when compared to control group.
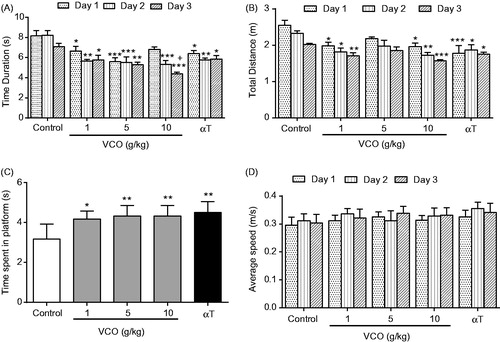
The escape distance, on the other hand, was significantly (p < 0.05) reduced only in VCO-treated rats at 1 g/kg (by 0.6 m, 0.5 m and 0.3 m; Day 1–3) and 10 g/kg (by 0.6 m, 0.6 m and 0.5 m; Day 1–3) when compared to control (). Remarkably, the attenuation of escape latency and escape distance observed among the VCO-treated rodents was comparable to that of αT at day 3 (1.2 s and 0.3 m, respectively). It is noteworthy that 10 g/kg VCO-treated rats exhibited significantly (p < 0.05) better escape latency than αT rats at Day 3.
Probe test reflects memory consolidation. VCO (1, 5 and 10 g/kg) and αT (150 mg/kg) were able to significantly (p < 0.05) retain the rodents in the platform quadrant for a relatively longer time when compared to control ().
In terms of swimming speed, there was no significant difference between the groups. The average swimming speed over three days was ∼0.32 ± 0.01 m/s (). This strongly suggest that VCO and αT enhanced only the memory of Wistar rats but not their locomotor activity.
VCO-improved cholinergic activity in rat brain
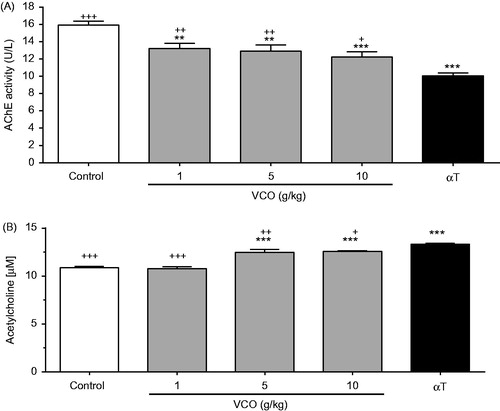
illustrates the effects of VCO on AChE activity in rat brain. VCO (1, 5 and 10 g/kg) significantly inhibited AChE activity in a dose-dependent manner when compared to control [17.1% (p < 0.01), 19.1% (p < 0.01) and 23.2% (p < 0.001), respectively]. αT, on the other hand, significantly inhibited AChE by 37.0% (p < 0.001) when compared to control. As such, the AChE inhibitory effect of αT was more superior to the highest dose of VCO (10 g/kg). , on the other hand, shows the effects of VCO on ACh concentration in rat brain. Whilst 1 g/kg showed no effect, 5 and 10 g/kg VCO significantly up-regulated ACh by 14.8% (p < 0.001) and 15.4% (p < 0.001), respectively, when compared to control. αT, on the other hand, significantly up-regulated ACh by 22.3% (p < 0.001) when compared to control. As such, αT was more superior in up-regulating ACh when compared to VCO.
VCO-enhanced antioxidant activity in rat brain
depicts the effects of VCO on antioxidant activity in rat brain. In general, treatment with VCO-enhanced antioxidant level in rat brain. For SOD (), while 1 and 5 g/kg VCO showed no effect, 10 g/kg VCO significantly increased its activity by 8.3% (p < 0.05) when compared to control. αT, on the other hand, enhanced SOD by 9.7% (p < 0.01) in relative to control. For CAT (), whilst 1 g/kg VCO showed no effect, 5 and 10 g/kg VCO significantly up-regulated its activity in a dose-dependent manner [54.0%; (p < 0.01) and 67.2% (p < 0.001), respectively] when compared to control. αT exerted greater effect, increasing CAT concentration by 90.0% (p < 0.001) as opposed to control.
Figure 4. Effects of VCO on antioxidant activity in rat brain. The concentration of antioxidants in rat brain was measured: (A) SOD, (B) CAT, (C) GSH, (D) GPx and (E) GR. Each bar represents mean ± SEM of n = 6. *p < 0.05, **p < 0.01, ***p < 0.001 when compared to control; +p < 0.05, ++p < 0.01, +++p < 0.001 when compared to αT.
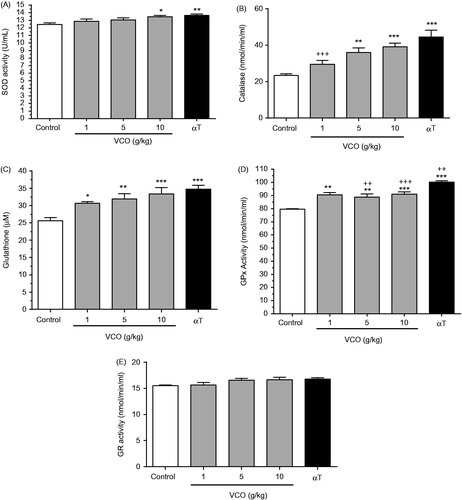
For GSH (), 1, 5 and 10 g/kg VCO increased its level by 19.7% (p < 0.05), 24.8% (p < 0.01) and 30.5% (p < 0.001), respectively when compared to control. αT enhanced GSH by 35.9% (p < 0.001) than control. For GPx (), 1, 5 and 10 g/kg VCO increased its level by 13.7%, (p < 0.01), 11.7% (p < 0.01), 14.3% (p < 0.001), respectively, when compared to control. αT significantly increased GPx activity by 26.1% (p < 0.001; almost twofold of VCO) in rat brain as opposed to control. For GR (), there was no significant difference between treatments.
Overall, VCO-enhanced antioxidant activity in rat brain was almost comparable to that of αT. Significant differences between VCO and αT were only observed in catalase [1 g/kg VCO only] and GPx [1–5 g/kg VCO only].
VCO-reduced oxidative stress level in rat brain
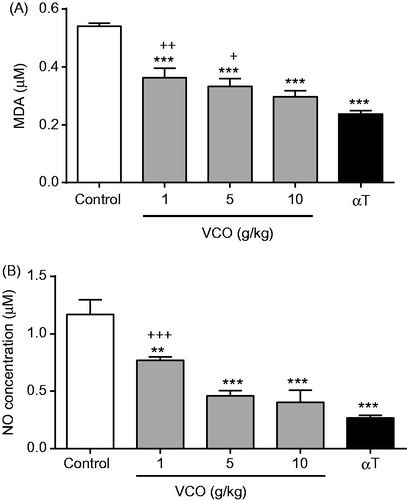
highlights the effect of VCO on oxidative stress level in rat brain. In general, treatment with VCO-reduced oxidative level in rats. For MDA (), 1, 5 and 10 g/kg VCO significantly (p < 0.001) inhibited its level in a dose-dependent manner (by 32.8, 38.3 and 45.1%, respectively) when compared to control. αT, on the other hand, significantly inhibited MDA level by 56.3% (p < 0.001) as opposed to control. Significant differences were observed between the lower doses of VCO (1 and 5 g/kg) and αT but not at 10 g/kg VCO. For NO (), 1, 5 and 10 g/kg VCO significantly inhibited its level in a dose-dependent manner [by 34.4% (p < 0.01), 60.6% (p < 0.001) and 65.4% (p < 0.001), respectively] when compared to control. αT, on the other hand, significantly inhibited NO level by 77.1% (p < 0.001). No significant differences were observed between VCO (5 and 10 g/kg) and αT.
Discussion
The present study investigated the potential use of VCO as a natural memory enhancer in normal adult Wistar rats. The MWM was first performed to evaluate the spatial memory function of VCO-treated rats. The behavioural test requires subjects to search for a hidden platform in opaque water and memorize the location of the platform (Li et al. Citation2011). The present study demonstrated for the first time that treatment with VCO enhanced and consolidated memory of tested rodents. The VCO-induced memory-enhancing effect could be due to the presence of ketones. VCO, a natural source of medium-chain triglyceride (MCT), is readily oxidized to ketones. Ketone is known to supply a rapid alternative source of energy to the neurons (Sabitha & Vasudeva Citation2010; Gandotra et al. Citation2014). It is a short-chain fatty acid that can readily cross the blood–brain barrier to be absorbed by neuron and oxidized in the mitochondria to generate ATP (energy) (Page et al. Citation2009; Fernando et al. Citation2015). Interestingly, the memory-enhancing effect of VCO was comparable to that of αT. αT is a monophenolic lipid-soluble Vitamin E that protects cell membranes against lipid peroxidation (Bharrhan et al. Citation2010). Its in vivo memory-enhancing effect appeared to vary among reports. Morris et al. (Citation2015) reported that αT acetate significantly improved learning in normal adult Wistar rat by reducing escape latency and exhibiting higher target quadrant preference in the probe test. Shichiri et al. (Citation2011), on the contrary, found αT to show no significant cognitive improvement in normal adult Wistar rat model. Nevertheless, in a recent clinical study, αT was reported to slow down disease progression in AD patients. Sano et al. (Citation1997) found that treatment with αT (2000 IU a day) reduced neuronal damage and slowed disease progression of patients with moderately severe impairment from AD.
The memory-enhancing effect of VCO was found to be accompanied by increased cholinergic activity in rat brain. The present study found that VCO inhibited AChE activity and at the same time enhanced ACh in treated rats. ACh is a cholinergic neurotransmitter that plays important role in regulating the cognitive functions of brain. Increased AChE activity, however, hydrolyzes ACh into acetate and choline, resulting in the termination of synaptic transmission (Zugno et al. Citation2014). Anwar et al. (Citation2012) reported that phenolic compounds decreased AChE activity in the cerebral cortex and striatum of adult Wistar rats. As such, the improved cholinergic activity in this study might also be attributed to phenolic compounds in VCO (Marina et al. Citation2009a). The VCO-enhanced cholinergic activity was also consistent with that of αT. Nevertheless, the effect of αT on cholinergic activity could be dose-dependent. Previous report found that lower concentration of 50 mg/kg αT did not alter AChE activity in normal Wistar rat brain (Gutierres et al. Citation2012).
VCO-derived biologically active compounds such as polyphenols, tocopherols, sterols and squalene are known for their potent antioxidative activity (Abujazia et al. Citation2012). In parallel to this, the present findings indicated increased antioxidants and decreased oxidative stress in rat brains. This strongly suggested that VCO-enhanced memory was closely associated with changes of antioxidant status in brain. Oxidative stress is postulated to be a major causal factor of senescence. Accumulation of oxidative stress such as ROS in tissues during aging process modifies macromolecules including protein, DNA and lipid (Sasaki Citation2010). Antioxidants like SOD, CAT, GSH, GPx and GR are mutually supportive team of defence against oxidative assault by ROS and lipid peroxidation (Priya et al. Citation2011; Adhikari & Arora Citation2014). Catechin is a polyphenolic compound that is found in VCO (Marina et al. Citation2009a). It has been reported to elevate the activity of SOD and CAT in mice striatum (Mandel et al. Citation2008). Elsewhere, polyphenol fraction extracted from VCO enhanced antioxidant status and reduced lipid peroxide content in hearts, livers and kidneys of Sprague-Dawley rats (Nevin & Rajamohan Citation2006). In addition, it was reported that the presence of cerebral ketones was associated with reduction of apoptosis, inflammation and oxidative stress (Krikorian et al. Citation2012). The antioxidative capacity of ketone (Veech et al. Citation2001; Ziegler et al. Citation2003; Krikorian et al. Citation2012) may have also contributed to the attentuation of ROS by VCO as observed in this study. Similar antioxidative effect was also observed amongst αT-treated rats. Previous study found αT to reduce hepatic MDA level and restored CAT and GPx activity in aged Wistar rat (Helmy Citation2012). αT is able to detoxify H2O2 by converting it to O2 and H2O under physiological conditions (Feng & Wang Citation2012). Inhibition of free radicals formation generated from lipid peroxidation by αT prevented complete oxidation (Dumont & Beal Citation2011).
Conclusions
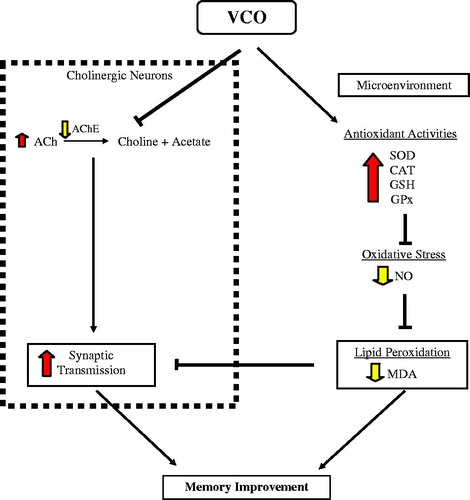
Altogether, findings from the present animal behavioural study indicated that both VCO and αT enhanced cognitive function of normal adult Wistar rats. Nevertheless, the observed effect was not accompanied by apparent changes in locomotor function. The memory-enhancing effect of VCO appeared to be more apparent at 10 g/kg and above. The enhanced memory under the influence of VCO is likely mediated through two biological components in the brain (): cholinergic neurons and the microenvironment. For cholinergic neurons, VCO reduced AChE action which in turn increased ACh concentration in the brain. The increased ACh plays important role in effective synaptic transmission during acquiring new information and consolidation of memory. Within the microenvironment, on the other hand, VCO increased production of antioxidants in brain, namely SOD, CAT, GSH and GPx. The up-regulated antioxidants served as protection against lipid peroxidation (MDA) by oxidative stress (NO). The collective effects of these two components would eventually prevent or delay neurodegeneration. The promising outcomes of this study strongly imply the possible use of VCO, not only as neuroprotective agents for those suffering from neurodegenerative diseases, but also as brain food (supplements for the health populations).
Figure 6. Proposed mechanisms underlying VCO-induced memory improvement in normal adult Wistar rats. The enhanced memory under the influence of VCO is likely mediated through two biological components in the brain: cholinergic neurons and the microenvironment. For cholinergic neurons, VCO reduced AChE action which in turn increased ACh concentration in the brain. The increased ACh plays important role in effective synaptic transmission during acquiring new information and consolidation of memory. Within the microenvironment, on the other hand, VCO increased production of antioxidants in brain, namely SOD, CAT, GSH and GPx. The up-regulated antioxidants served as protection against lipid peroxidation (MDA) by oxidative stress (NO). The collective effects of these two components would eventually prevent or delay neurodegeneration.
Acknowledgements
We acknowledge receipt of financial grant support from Ministry of Higher Education (MOHE) [Reference no: 600-RMI/ST/ERGS 5/3 (52/2011) and 600-RMI/LRGS 5/3 (3/2012)].
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Abujazia MA, Muhammad N, Shuid AN, Soelaiman IN. 2012. The effects of virgin coconut oil on bone oxidative status in ovariectomised rat. Evid Based Complement Alternat Med. 2012. Available from: http://dx.doi.org/10.1155/2012/525079.
- Adhikari M, Arora R. 2014. The flavonolignan-silymarin protects enzymatic, hematological, and immune system against γ-radiation-induced toxicity. Environ Toxicol. 31:641–654.
- Anonymous. (2007). QuantiChromTM Acetylcholinesterase Assay Kit (DACE-100): rapid colorimetric determination of acetylcholinesterase activity [Internet] [cited 2014 January 20]. Available from: http://www.bioassaysys.com/file_dir/DACE.pdf
- Anonymous. (2010). QuantiChromTM Nitric Oxide Assay Kit (D2NO-100): quantitative colorimetric determination of nitric oxide at 540nm [Internet] [cited 2014 January 20]. Available from: http://www.bioassaysys.com/file_dir/D2NO.pdf
- Anonymous. (2013). EnzyChromTM Acetylcholine Assay Kit (EACL-100): quantitative colorimetric/fluorimetric acetylcholine determination [Internet] [cited 2014 January 20]. Available from: http://www.bioassaysys.com/file_dir/EACL.pdf
- Anonymous. (2014a). Catalase Assay Kit [Internet] [cited 2014 January 20]. Available from: https://www.caymanchem.com/pdfs/707002.pdf
- Anonymous. (2014b). Glutathione Assay Kit [Internet] [cited 2014 January 20]. Available from: https://www.caymanchem.com/pdfs/703002.pdf
- Anonymous. (2014c). Glutathione Peroxidase Assay Kit [Internet] [cited 2014 January 20]. Available from: https://www.caymanchem.com/pdfs/703102.pdf
- Anonymous. (2014d). Glutathione Reductase Assay Kit [Internet] [cited 2014 January 20]. Available from: https://www.caymanchem.com/pdfs/703202.pdf
- Anonymous. (2014e). Superoxide Dismutase Assay Kit [Internet] [cited 2014 January 20]. Available from: https://www.caymanchem.com/pdfs/706002.pdf
- Anonymous. (2014f). TBARS Assay Kit [Internet] [cited 2014 January 20]. Available from: https://www.caymanchem.com/pdfs/10009055.pdf
- Anwar J, Spanevello RM, Thomé G, Stefanello N, Schmatz R, Gutierres JM, Vieira JM, Baldissarelli J, Carvalho FB, da Rosa MM, et al. 2012. Effects of caffeic acid on behavioral parameters and on the activity of acetylcholinesterase in different tissues from adult rats. Pharmacol Biochem Behav. 103:386–394.
- Bawalan DD, Chapman KR. 2006. Virgin coconut oil production manual for micro- and village-scale processing. In: FAO Regional Office for Asia and the Pacific. Thailand: Thammada Press Co. Ltd.
- Bharrhan S, Chopra K, Rishi P. 2010. Vitamin E supplementation modulates endotoxin-induced liver damage in a rat model. Am J Biomed Sci. 2:51–62.
- Dosumu OO, Duru FIO, Osinubi AA, Oremosu AA, Noronha CC. 2010. Influence of virgin coconut oil (VCNO) on oxidative stress, serum testosterone and gonadotropic hormones (FSH, LH) in chronic ethanol ingestion. Agri Biol J North Am. 1.
- Dumont M, Beal MF. 2011. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic Biol Med. 51:1014–1026.
- Feng Y, Wang X. 2012. Antioxidant therapies for Alzheimer's disease. Oxid Med Cell Longev. 2012. Available from: http://dx.doi.org/10.1155/2012/472932.
- Fernando WM, Martins IJ, Goozee KG, Brennan CS, Jayasena V, Martins RN. 2015. The role of dietary coconut for the prevention and treatment of Alzheimer's disease: potential mechanisms of action. Br J Nutr. 114:1–14.
- Gandotra S, Kour J, Van der Waag A. 2014. Efficacy of adjunctive extra virgin coconut oil use in moderate to severe Alzheimer’s disease. Int J Sch Cog Psychol. doi:10.4172/2469-9837.1000108.
- Gutierres JM, Carvalho FB, Rosa MM, Schmatz R, Rodrigues M, Vieira JM, Mazzanti CM, Morsch VM, Rubin MA, Schetinger MRC, et al. 2012. Protective effect of α-tocopherol on memory deficits and Na+,K+-ATPase and acetylcholinesterase activities in rats with diet-induced hypercholesterolemia. Biomed Aging Pathol. 2:73–80.
- Helmy MM. 2012. Potential hepato-protective effect of α-tocopherol or simvastatin in aged rats. Pharmacol Rep. 64:698–705.
- Isaacs CE, Thormar H. 1990. Human milk lipids inactivate enveloped viruses. Canada: Arts Biomedical Publishers and Distributors; p. 161–174.
- Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. 2012. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 33:425. e419–e427.
- Li Y, Li F, Gong Q, Wu Q, Shi J. 2011. Inhibitory effects of Dendrobium alkaloids on memory impairment induced by lipopolysaccharide in rats. Planta Med. 77:117–121.
- Mandel SA, Amit T, Weinreb O, Reznichenko L, Youdim MBH. 2008. Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci Ther. 14:352–365.
- Marina AM, Man YB, Nazimah SA, Amin I, 2009a. Antioxidant capacity and phenolic acids of virgin coconut oil. Int J Food Sci Nutr. 6:114–123.
- Marina A, Che Man Y, Amin I. 2009b. Virgin coconut oil: emerging functional food oil. Trends Food Sci Technol. 20:481–487.
- Menard C, Bastianetto S, Quirion R. 2013. Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Front Cell Neurosci. 7:281.
- Morris MC, Schneider JA, Li H, Tangney CC, Nag S, Bennett DA, Honer WG, Barnes LL. 2015. Brain tocopherols related to Alzheimer's disease neuropathology in humans. Alzheimers Dement. 11:32–39.
- Morris R. 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 11:47–60.
- National Research Council. 1996. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press.
- Nevin KG, Rajamohan T. 2006. Virgin coconut oil supplemented diet increases the antioxidant status in rats. Food Chem. 99:260–266.
- Nik Norulaini NA, Setianto WB, Zaidul ISM, Nawi AH, Azizi CYM, Omar AK. 2009. Effects of supercritical carbon dioxide extraction parameters on virgin coconut oil yield and medium-chain triglyceride content. Food Chem. 116:193–7.
- Page KA, Williamson A, Yu N, McNay EC, Dzuira J, McCrimmon RJ, Sherwin RS. 2009. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes. 58:1237–1244.
- Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, et al. 2009. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res. 198:352–358.
- Priya DK, Gayathri R, Gunassekaran G, Murugan S, Sakthisekaran D. 2011. Chemopreventive role of sulforaphane by upholding the GSH redox cycle in pre- and post-initiation phases of experimental lung carcinogenesis. Asian Pac J Cancer Prev. 12:103–110.
- Sabitha P, Vasudeva DM, 2010. Lipid profile and antioxidant enzymes in coconut oil consumers. Indian Coconut J. 7:3–6.
- Salthouse TA. 2009. When does age-related cognitive decline begin? Neurobiol Aging. 30:507–14.
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, et al. 1997. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 336:1216–1222.
- Sasaki T. 2010. Analysis of aging-related oxidative stress status in normal aging animals and development of anti-aging interventions. Yakugaku Zasshi. 130:29–42.
- Shichiri M, Yoshida Y, Ishida N, Hagihara Y, Iwahashi H, Tamai H, Niki E. 2011. alpha-Tocopherol suppresses lipid peroxidation and behavioral and cognitive impairments in the Ts65Dn mouse model of Down syndrome. Free Radic Biol Med. 50:1801–1811.
- Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF. 2001. Ketone bodies, potential therapeutic uses. Iubmb Life. 51:241–247.
- Yamada K, Tanaka T, Han D, Senzaki K, Kameyama T, Nabeshima T. 1999. Protective effects of idebenone and alpha-tocopherol on beta-amyloid-(1-42)-induced learning and memory deficits in rats: implication of oxidative stress in beta-amyloid-induced neurotoxicity in vivo. Eur J Neurosci. 11:83–90.
- Yeap SK, Beh BK, Ali NM, Yusof HM, Ho WY, Koh SP, et al. 2015. Antistress and antioxidant effects of virgin coconut oil in vivo. Exp Ther Med. 9:39–42.
- Ziegler DR, Ribeiro LC, Hagenn M, Araújo E, Torres ILS, Gottfried C, Netto CA, Gonçalves CA. 2003. Ketogenic diet increases glutathione peroxidase activity in rat hippocampus. Neurochem Res. 28:1793–1797.
- Zugno AI, Matos MP, Canever L, Fraga DB, De Luca RD, Ghedim FV, Deroza PF, de Oliveira MB, Pacheco FD, Valvassori SS. 2014. Evaluation of acetylcholinesterase activity and behavioural alterations induced by ketamine in an animal model of schizophrenia. Acta Neuropsychiatr. 26:43–50.