?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context: Bergenin, isolated from the herb of Bergenia purpurascens (Hook. f. et Thoms.) Engl., has anti-inflammatory, antitussive, and wound healing activities. However, whether bergenin affects the activity of human liver cytochrome P450 (CYP) enzymes remains unclear.
Materials and methods: In this study, the inhibitory effects of bergenin (100 μM) on the eight human liver CYP isoforms (i.e., 1A2, 3A4, 2A6, 2E1, 2D6, 2C9, 2C19 and 2C8) were investigated, enzyme kinetics and time-dependent inhibition studies were also performed in vitro using human liver microsomes (HLMs).
Results: The results showed that bergenin inhibited the activity of CYP3A4, 2E1 and 2C9, with IC50 values of 14.39, 22.83 and 15.11 μM, respectively, but other CYP isoforms were not affected. Enzyme kinetic studies showed that bergenin was not only a non-competitive inhibitor of CYP3A4, but also a competitive inhibitor of CYP2E1 and 2C9, with Ki values of 7.71, 11.39 and 8.89 μM, respectively. In addition, bergenin is a time-dependent inhibitor for CYP3A4 with Kinact/KI value of 0.025/3.50 μM−1 min−1.
Discussion and conclusions: The in vitro studies of bergenin with CYP isoforms indicate that bergenin has the potential to cause pharmacokinetic drug interactions with other co-administered drugs metabolized by CYP3A4, 2E1 and 2C9. Further clinical studies are needed to evaluate the significance of this interaction.
Keywords:
Introduction
Bergenia purpurascens (Hook. f. et Thoms.) Engl., a traditional Chinese medicine, possesses anti-inflammation and antidiarrhoeal abilities and is used clinically for the treatment of diarrhoea, dysentery and other gut-associated diseases (Shi et al. Citation2014; Pandey et al. Citation2017; Liu et al. Citation2018). Bergenin is the major bioactive ingredient in the herb–drug (Li et al. Citation2013; Pandey et al. Citation2017). Therapeutically, it is used as an antiarrhythmic, antifungal, anticancer, antidiabetic and antioxidant agent (Bessong et al. Citation2005; Ahmed and Urooj Citation2012; Bajracharya Citation2015; Aggarwal et al. Citation2016).
Cytochrome P450 (CYP450) enzymes are important phase I enzymes in the biotransformation of xenobiotics, and CYP450 enzymes can be inhibited or induced by a variety of drugs and chemicals that can give rise to toxicity or treatment failure (Wrighton and Stevens Citation1992; Li Citation2001; Yan and Caldwell Citation2001; Peng et al. Citation2015). Many adverse herb–drug interactions may be attributed to the inhibition of CYP450 enzymes by co-administrated drugs or herbs (Zhang et al. Citation2007; Nowack Citation2008; Jeong et al. Citation2013; Lee et al. Citation2013; Qi et al. Citation2013; Meng and Liu Citation2014). Therefore, the effects of bergenin on the activity of CYP enzymes should be investigated. To the best of our knowledge, few studies have investigated the effects of bergenin on CYP enzymes, particularly the inhibitory effects, which will increase the risk of therapeutic applications of bergenin and its medical preparations.
The purpose of this study was to investigate the effects of bergenin on eight major CYP isoforms in human liver microsomes (HLMs). In vitro, phenacetin (CYP1A2), testosterone (CYP3A4), coumarin (CYP2A6), chlorzoxazone (CYP2E1), dextromethorphan (CYP2D6), diclofenac (CYP2C9), S-mephenytoin (CYP2C19) and paclitaxel (CYP2C8) were used as probe substrates to determine the effects of bergenin on eight CYP enzymes. In addition, enzyme kinetic studies were conducted to determine the inhibition mode of bergenin on CYP enzymes.
Materials and methods
Chemicals
Bergenin (≥98%) and testosterone (≥98%) were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). d-Glucose-6-phosphate, glucose-6-phosphate dehydrogenase, corticosterone (≥98%), NADP+, phenacetin (≥98%), acetaminophen (≥98%), 4-hydroxymephenytoin (≥98%), 7-hydroxycoumarin (≥98%), 4′-hydroxydiclofenac (≥98%), sulphaphenazole (≥98%), quinidine (≥98%), tranylcypromine (≥98%), chlorzoxazone (≥98%), 6-hydroxychlorzoxazone (≥98%), paclitaxel (≥98%), 6β-hydroxytestosterone (≥98%), clomethiazole (≥98%) and furafylline (≥98%) were obtained from Sigma Chemical Co. (St. Louis, MO). Montelukast (≥98%) was obtained from Beijing Aleznova Pharmaceutical (Beijing, China). Coumarin (≥98%), diclofenac (≥98%), dextromethorphan (≥98%) and ketoconazole (≥98%) were purchased from ICN Biomedicals (Aurora, OH). Pooled HLMs were purchased from BD Biosciences Discovery Labware (Bedford, MD). All other reagents and solvents were of analytical reagent grade.
Assay with human liver microsomes
As shown in , to investigate the inhibitory effects of bergenin on different CYP isoforms in HLM, the following probe reactions were used, according to a previously described method (Zhang et al. Citation2007; Qi et al. Citation2013): phenacetin O-deethylation for CYP1A2, testosterone 6β-hydroxylation for CYP3A4, coumarin 7-hydroxylation for CYP2A6, chlorzoxazone 6-hydroxylation for CYP2E1, dextromethorphan O-demethylation for CYP2D6, diclofenac 4′-hydroxylation for CYP2C9, S-mephenytoin 4-hydroxylation for CYP2C19 and paclitaxel 6α-hydroxylation for CYP2C8. All incubations were performed in triplicate, and the mean values were utilized. The typical incubation systems contained 100 mM potassium phosphate buffer (pH 7.4), NADPH generating system (1 mM NADP+, 10 mM glucose-6-phosphate, 1 U/mL of glucose-6-phosphate dehydrogenase and 4 mM MgCl2), the appropriate concentration of HLMs, a corresponding probe substrate and bergenin (or positive inhibitor for different probe reactions) in a final volume of 200 μL.
Table 1. Isoforms tested, marker reactions, incubation conditions and Km used in the inhibition study.
The concentration of bergenin was 100 μM, and the positive inhibitor concentrations were as follows: 10 μM furafylline for CYP1A2, 1 μM ketoconazole for CYP3A4, 10 μM tranylcypromine for CYP2A6, 50 μM clomethiazole for CYP2E1, 10 μM quinidine for CYP2D6, 10 μM sulphaphenazole for CYP2C9, 50 μM tranylcypromine for CYP2C19, 5 μM montelukast for CYP2C8. Probe substrates, positive inhibitors (except for dextromethorphan and quinidine which were dissolved in water) and bergenin were dissolved in methanol, with a final concentration of 1% (v/v), and 1% neat methanol was added to the incubations without inhibitor. The final microsomal protein concentration and incubation times for the different probe reactions are shown in . There was a 3 min pre-incubation period (at 37 °C) before the reaction was initiated by adding an NADPH-generating system. The reaction was terminated by adding a 100 μL acetonitrile (10% trichloroacetic acid for CYP2A6) internal standard mix, and the solution was placed on ice. The mixture was centrifuged at 12,000 rpm for 10 min, and an aliquot (50 μL) of the supernatant was transferred for HPLC analysis. The instrument used in this study were Agilent 1260 series instrument (Palo Alto, CA, USA) with DAD and FLD detector, and the quantitative assay for the corresponding metabolites was performed as previously reported (Lang et al. Citation2017; Zhang et al. Citation2017).
Enzyme inhibition and kinetic studies of bergenin
A 100 μM bergenin was used to initially screen for its direct inhibitory effects towards different human CYP isoforms. For the CYP isoforms whose activities were strongly inhibited, secondary studies were performed to obtain the half inhibition concentration (IC50). Ki values were obtained by incubating various concentrations of different probe substrates (20–100 μM testosterone, 25–200 μM chlorzoxazone or 2–20 μM diclofenac) in the presence of 0–50 μM bergenin.
Time-dependent inhibition study of bergenin
To determine whether bergenin could inhibit the activity of CYP3A4, 2E1 and 2C9 in a time-dependent manner, bergenin (20 μM) was pre-incubated with HLMs (1 mg/mL) in the presence of an NADPH-generating system for 30 min at 37 °C. After incubation, an aliquot (20 μL) was transferred to another incubation tube (final volume 200 μL) containing an NADPH-generating system and probe substrates whose final concentrations were approximate to Km. Then, further incubations were performed to measure the residual activity. After being incubated for different time (30 min for CYP2E1 and CYP2C9, 10 min for CYP3A4), the reactions were terminated by adding a 100 μL acetonitrile internal standard mix and then placed on ice; the corresponding metabolites were determined by HPLC.
To determine the KI and Kinact values for the inactivation of CYP3A4, the incubations were conducted using higher probe substrate concentrations (approximately four-fold Km values) and various concentrations of bergenin (0–50 μM) after different pre-incubation times (0–30 min), with a two-step incubation scheme, as described above.
Statistical analysis
The enzyme kinetic parameters for the probe reaction were estimated from the best fit line using least-squares linear regression of the inverse substrate concentration versus the inverse velocity (Lineweaver–Burk plots), and the mean values were used to calculate Vmax and Km. Inhibition data from the experiments that were conducted using multiple compound concentrations were represented by Dixon plots, and inhibition constant (Ki) values were calculated using non-linear regression according to the following equation:
where I is the concentration of the compound, Ki is the inhibition constant, S is the concentration of the substrate and Km is the substrate concentration at half the maximum velocity (Vmax) of the reaction. The mechanism of the inhibition was inspected using the Lineweaver–Burk plots and the enzyme inhibition models. The data comparison was performed using the Student’s t-test and performed using IBM SPSS statistics 20 (SPSS Inc., Chicago, IL).
Results
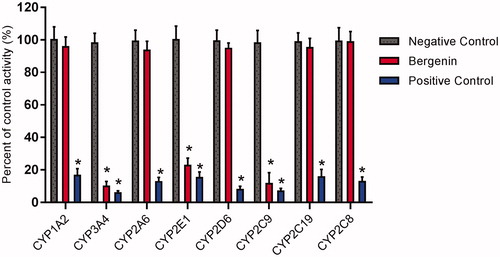
To investigate whether the bergenin affects the catalytic activity of CYP enzymes, the probe reaction assays were conducted with varying concentrations of bergenin (). Specific inhibitors of CYP1A2, 3A4, 2A6, 2E1, 2D6, 2C9, 2C19 and 2C8 were used as positive controls. As shown in , bergenin did not inhibit the activities of CYP1A2, 2A6, 2D6, 2C19 and 2C8 at a concentration of 100 μM. In contrast, the activities of CYP1A2, 3A4 and 2E1 were inhibited to 25.8, 11.0 and 12.2% of their control activities, respectively.
Figure 2. Effects of bergenin (100 μM) on the activity of CYP450 enzymes in pooled HLMs. All data represent mean ± S.D. of the triplicate incubations. *p < 0.05, significantly different from the negative control. Negative control: incubation systems without bergenin; bergenin: incubation systems with bergenin; positive control: incubation systems with their corresponding positive inhibitors.
The enzyme-inhibition study showed that inhibition of CYP3A4, 2E1 and 2C9 by bergenin was concentration-dependent, with IC50 values of 14.39, 22.83 and 15.11 μM, respectively.
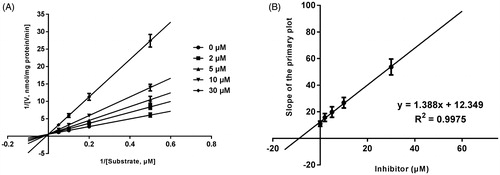
Lineweaver–Burk plots of inhibitory kinetic data suggested that the inhibition of the inhibition of CYP3A4 by bergenin was best fit in a non-competitive manner (), whereas CYP2E1 () and 2C9 () by bergenin was the best fit in a competitive manner. The Ki values of bergenin on CYP3A4 (), 2E1 () and 2C9 () were obtained from the secondary Lineweaver–Burk plot for Ki, with values of 7.71, 11.39 and 8.89 μM, respectively.
Figure 3. Lineweaver–Burk plots (A) and the secondary plot for Ki (B) of effects of bergenin on CYP3A4 catalyzed reactions (testosterone 6β-hydroxylation) in pooled HLM. Data were obtained from 30 min incubation with testosterone (20–100 μM) in the absence or presence of bergenin (0–30 μM). All data represent mean ± S.D. of the triplicate incubations.
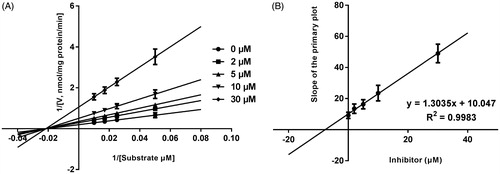
Figure 4. Lineweaver–Burk plots (A) and the secondary plot for Ki (B) of effects of bergenin on CYP2E1 catalyzed reactions (chlorzoxazone 6-hydroxylation) in pooled HLM. Data were obtained from 30 min incubation with diclofenac (25–250 μM) in the absence or presence of bergenin (0–50 μM). All data represent mean ± S.D. of the triplicate incubations.
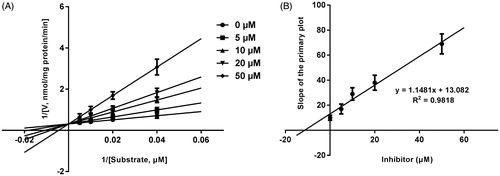
Figure 5. Lineweaver–Burk plots (A) and the secondary plot for Ki (B) of effects of bergenin on CYP2C9 catalyzed reactions (diclofenac 4′-hydroxylation) in pooled HLM. Data were obtained from 30 min incubation with phenacetin (2–20 μM) in the absence or presence of bergenin (0–30 μM). All data represent mean ± S.D. of the triplicate incubations.
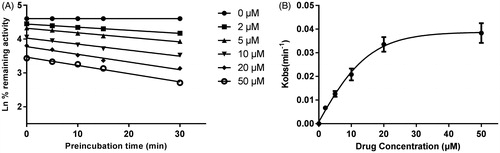
As shown in , after pre-incubation of bergenin with HLM for 30 min, the activity of CYP3A4 decreased with the incubation time, however, the activity of CYP2E1 and 2C9 was not affected. To characterize the time-dependent inhibition of CYP3A4 by bergenin, inactivation parameters of KI and Kinact values were calculated using non-linear regression analysis in HLM. As calculated from the inactivation plot of , the Kinact/KI value for CYP3A4 was 0.025/3.50 μM−1 min−1. The Kinact values imply that approximately 2.5% of CYP3A4 is inactivated each minute when a saturating concentration of bergenin is incubated with HLM.
Figure 6. Time-dependent inhibition investigations of CYP3A4, 2E1 and 2C9 catalyzed reactions by bergenin (20 μM). All data represent mean ± S.D. of the triplicate incubations.
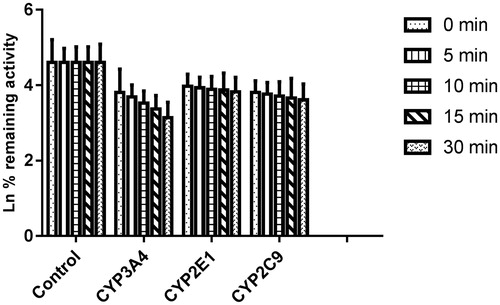
Figure 7. Time and concentration-inactivation of microsomal CYP3A4 activity by bergenin in the presence of NADPH. The initial rate constant of inactivation of CYP3A4 by each concentration (Kobs) was determined through linear regression analysis of the natural logarithm of the percentage of remaining activity versus pre-incubation time (A). The KI and Kinact values were determined through non-linear analysis of the Kobs versus the bergenin concentration (B).
Discussion
In clinical practice, many patients undergo multiple-drug therapy. Multiple-drug therapy possesses several advantages, such as simultaneously treatment of diseases, or multi-drug therapy for the treatment of complex chronical disorders, resulting in a better treatment outcome compared with monotherapy. However, many herb–drug interactions resulting from the concurrent use of herbal drugs with prescription and over-the-counter drugs may cause adverse reactions such as toxicity and treatment failure (Zhou et al. Citation2004). The most common causes of herb–drug interactions are a modification of the enzyme activity of cytochrome P450 enzymes, specifically through inhibitory effects. Inhibition of CYP enzymes in vivo may result in unexpected elevations in the plasma concentrations of concomitant drugs, leading to adverse effects (Hu et al. Citation2015; Liu et al. Citation2015). Therefore, regulatory authorities require preclinical (in vitro) and clinical (in vivo) interaction studies in the drug development.
As bergenin possesses numerous pharmacological activities and has the potential of becoming a lead compound of anticancer drugs, it is essential to investigate the inhibitory effects of bergenin on the major CYP enzymes. To the best of our knowledge, this study is the first to investigate the effects of bergenin on the metabolism of probe substrates of several CYP isoforms, including CYP1A2, 3A4, 2A6, 2E1, 2D6, 2C9, 2C19 and 2C8.
The CYP3A subfamily is one of the dominant CYP enzymes in the liver and extra-hepatic tissues, such as the intestines, and it plays an important role in the oxidation of xenobiotics and contributes to the biotransformation of approximately 60% of currently used therapeutic drugs (Pandit et al. Citation2011). Human CYP3A4 is one of the most abundant drug-metabolizing CYP isoforms in human liver microsomes, accounting for approximately 40% of the total CYP enzymes (Zhou Citation2008). In fact, characterization of the CYP3A4 isoform responsible for the metabolism of drugs and herbal constituents is important for identifying potential drug–drug or herb–drug interactions in humans. The present study showed that bergenin had inhibitory effects in vitro on CYP3A4 isoform, with Ki and IC50 values of 7.71 μM and 14.39 μM, respectively. The results suggested that bergenin was also a weak CYP3A4 inhibitor, and the potential of herb–drug interaction with CYP3A4 would also be low. However, the results also indicated that bergenin is a time-dependent inhibitor for CYP3A4 with Kinact/KI value of 0.025/3.50 μM−1 min−1, which revealed that bergenin would inhibit the activity of CYP3A4 with an increase of pre-incubation time of concentration. Therefore, to avoid adverse drug interactions, bergenin should not be used with other drugs metabolized by CYP3A4.
CYP2E1 also play an important role in the metabolism of many drugs (Sun et al. Citation2014; Bedada and Neerati Citation2018). Our study showed that bergenin competitively inhibited human liver microsomal CYP2E1 activity. Therefore, bergenin should also be used carefully with drugs metabolized by CYP2E1 to avoid possible drug interactions even though bergenin was an inhibitor for these two CYP isoforms.
Our study showed that bergenin competitively inhibited human liver microsomal CYP2C9 activity. Therefore, bergenin should also be used carefully with drugs metabolized by CYP2C9 to avoid possible drug interactions even though bergenin was an inhibitor for these two CYP isoforms.
As we know, in vitro data are essential for understanding a potential enzyme inhibition and DDI in vivo. However, an observed in vitro inhibition of a CYP enzyme does not mean that the drug will cause clinically relevant interactions. Many other factors might influence drug interactions mediated by CYP inhibition, including the contribution of the hepatic clearance to the total clearance of the affected drug, the fraction of the hepatic clearance which is subject to metabolic inhibition, and the ratio of the inhibition constant (Ki) over the in vivo concentration of the inhibitor (Ito et al. Citation1998; Ericsson et al. Citation2014). Therefore, further in vivo system studies are needed to identify the interactions of bergenin with CYP isoform in humans.
The results of this study indicate that bergenin may influence the in vitro metabolism of drugs that are substrates of CYP3A4, 2E1 and 2C9. Pan et al. (Citation2016) have reported that the plasma Cmax values of bergenin in rats treated with an oral dose (100 mg/kg) were less than 300 ng/mL (0.9 μM). Therefore, the rat plasma concentrations of bergenin were much lower than the Ki and IC50 values of bergenin determined in this study, and severe drug–drug interaction might not occur if bergenin were co-administered with the substrates of the CYP3A4, 2E1 and 2C9. However, due to the pharmacokinetic differences of human and rats, further in vivo system studies are needed to identify the interactions of bergenin with CYP isoform in humans.
In conclusion, the effects of bergenin on the activity of CYP enzymes were systematically investigated. The results showed that bergenin could inhibit the activity of CYP3A4, 2E1 and 2C9, while the activities of other CYP enzymes were not affected. Therefore, to avoid adverse drug interactions, it is recommended that bergenin should not be used with other drugs metabolized by CYP3A4, 2E1 and 2C9.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aggarwal D, Gautam D, Sharma M, Singla SK. 2016. Bergenin attenuates renal injury by reversing mitochondrial dysfunction in ethylene glycol induced hyperoxaluric rat model. Eur J Pharmacol. 791:611–621.
- Ahmed F, Urooj A. 2012. Cardioprotective activity of standardized extract of Ficus racemosa stem bark against doxorubicin-induced toxicity. Pharm Biol. 50:468–473.
- Bajracharya GB. 2015. Diversity, pharmacology and synthesis of bergenin and its derivatives: potential materials for therapeutic usages. Fitoterapia. 101:133–152.
- Bedada SK, Neerati P. 2018. The effect of quercetin on the pharmacokinetics of chlorzoxazone, a CYP2E1 substrate, in healthy subjects. Eur J Clin Pharmacol. 74:91–97.
- Bessong PO, Obi CL, Andreola ML, Rojas LB, Pouysegu L, Igumbor E, Meyer JJ, Quideau S, Litvak S. 2005. Evaluation of selected South African medicinal plants for inhibitory properties against human immunodeficiency virus type 1 reverse transcriptase and integrase. J Ethnopharmacol. 99:83–91.
- Ericsson T, Sundell J, Torkelsson A, Hoffmann KJ, Ashton M. 2014. Effects of artemisinin antimalarials on Cytochrome P450 enzymes in vitro using recombinant enzymes and human liver microsomes: potential implications for combination therapies. Xenobiotica. 44:615–626.
- Hu X, Huang W, Yang Y. 2015. Cytochrome P450 isoenzymes in rat and human liver microsomes associate with the metabolism of total coumarins in Fructus Cnidii. Eur J Drug Metab Pharmacokinet. 40:373–377.
- Ito K, Iwatsubo T, Kanamitsu S, Nakajima Y, Sugiyama Y. 1998. Quantitative prediction of in vivo drug clearance and drug interactions from in vitro data on metabolism, together with binding and transport. Annu Rev Pharmacol Toxicol. 38:461–499.
- Jeong HU, Kong TY, Kwon SS, Hong SW, Yeon SH, Choi JH, Lee JY, Cho YY, Lee HS. 2013. Effect of honokiol on cytochrome P450 and UDP-glucuronosyltransferase enzyme activities in human liver microsomes. Molecules. 18:10681–10693.
- Lang J, Li W, Zhao J, Wang K, Chen D. 2017. Inhibitory effects of curculigoside on human liver cytochrome P450 enzymes. Xenobiotica. 47:849–855.
- Lee SY, Lee JY, Kang W, Kwon KI, Park SK, Oh SJ, Ma JY, Kim SK. 2013. Cytochrome P450-mediated herb-drug interaction potential of Galgeun-tang. Food Chem Toxicol. 51:343–349.
- Li AP. 2001. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov Today. 6:357–366.
- Li BH, Wu JD, Li XL. 2013. LC-MS/MS determination and pharmacokinetic study of bergenin, the main bioactive component of Bergenia purpurascens after oral administration in rats. J Pharm Anal. 3:229–234.
- Liu B, Wang M, Wang X. 2018. Phytochemical analysis and antibacterial activity of methanolic extract of Bergenia purpurascens against common respiratory infection causing bacterial species in vitro and in neonatal rats. Microb Pathog. 117:315–319.
- Liu T, Qian G, Wang W, Zhang Y. 2015. Molecular docking to understand the metabolic behavior of GNF-351 by CYP3A4 and its potential drug-drug interaction with ketoconazole. Eur J Drug Metab Pharmacokinet. 40:235–238.
- Meng Q, Liu K. 2014. Pharmacokinetic interactions between herbal medicines and prescribed drugs: focus on drug metabolic enzymes and transporters. Curr Drug Metab. 15:791–807.
- Nowack R. 2008. Cytochrome P450 enzyme, and transport protein mediated herb-drug interactions in renal transplant patients: grapefruit juice, St John's Wort - and beyond! Nephrology. 13:337–347.
- Pan RH, He HM, Dai Y, Xia YF. 2016. Comparative pharmacokinetics of bergenin, a main active constituent of Saxifraga stolonifera Curt., in normal and hepatic injury rats after oral administration. Chin J Nat Med. 14:776–782.
- Pandey R, Kumar B, Meena B, Srivastava M, Mishra T, Tiwari V, Pal M, Nair NK, Upreti DK, Rana TS. 2017. Major bioactive phenolics in Bergenia species from the Indian Himalayan region: method development, validation and quantitative estimation using UHPLC-QqQLIT-MS/MS. PLoS One. 12:e0180950.
- Pandit S, Mukherjee PK, Ponnusankar S, Venkatesh M, Srikanth N. 2011. Metabolism mediated interaction of α-asarone and Acorus calamus with CYP3A4 and CYP2D6. Fitoterapia. 82:369–374.
- Peng Y, Wu H, Zhang X, Zhang F, Qi H, Zhong Y, Wang Y, Sang H, Wang G, Sun J. 2015. A comprehensive assay for nine major cytochrome P450 enzymes activities with 16 probe reactions on human liver microsomes by a single LC/MS/MS run to support reliable in vitro inhibitory drug-drug interaction evaluation. Xenobiotica. 45:961–977.
- Qi XY, Liang SC, Ge GB, Liu Y, Dong PP, Zhang JW, Wang AX, Hou J, Zhu LL, Yang L, et al. 2013. Inhibitory effects of sanguinarine on human liver cytochrome P450 enzymes. Food Chem Toxicol. 56:392–397.
- Shi X, Li X, He J, Han Y, Li S, Zou M. 2014. Study on the antibacterial activity of Bergenia purpurascens extract. Afr J Tradit Complement Altern Med. 11:464–468.
- Sun M, Tang Y, Ding T, Liu M, Wang X. 2014. Inhibitory effects of celastrol on rat liver cytochrome P450 1A2, 2C11, 2D6, 2E1 and 3A2 activity. Fitoterapia. 92:1–8. DOI:10.1016/j.fitote.2013.10.004
- Wrighton SA, Stevens JC. 1992. The human hepatic cytochromes P450 involved in drug metabolism. Critical Rev in Toxicol. 22:1–21.
- Yan Z, Caldwell GW. 2001. Metabolism profiling, and cytochrome P450 inhibition & induction in drug discovery. Curr Top Med Chem. 1:403–425.
- Zhang H, Ya G, Rui H. 2017. Inhibitory effects of triptolide on human liver cytochrome P450 enzymes and P-glycoprotein. Eur J Drug Metab Pharmacokinet. 42:89–98.
- Zhang JW, Liu Y, Cheng J, Li W, Ma H, Liu HT, Sun J, Wang LM, He YQ, Wang Y, et al. 2007. Inhibition of human liver cytochrome P450 by star fruit juice. J Pharm Pharm Sci. 10:496–503.
- Zhou S, Chan E, Li SC, Huang M, Chen X, Li X, Zhang Q, Paxton JW. 2004. Predicting pharmacokinetic herb-drug interactions. Drug Metab Drug Interac. 20:143–158.
- Zhou SF. 2008. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 9:310–322.