Abstract
Context
Gambogic amide (GA-amide) is a non-peptide molecule that has high affinity for tropomyosin receptor kinase A (TrkA) and possesses robust neurotrophic activity, but its effect on angiogenesis is unclear.
Objective
The study investigates the antiangiogenic effect of GA-amide on endothelial cells (ECs).
Materials and methods
The viability of endothelial cells (ECs) treated with 0.1, 0.15, 0.2, 0.3, 0.4, and 0.5 μM GA-amide for 48 h was detected by MTS assay. Wound healing and angiogenesis assays were performed on cells treated with 0.2 μM GA-amide. Chicken eggs at day 7 post-fertilization were divided into the dimethyl sulfoxide (DMSO), bevacizumab (40 μg), and GA-amide (18.8 and 62.8 ng) groups to assess the antiangiogenic effect for 3 days. mRNA and protein expression in cells treated with 0.1, 0.2, 0.4, 0.8, and 1.2 μM GA-amide for 6 h was detected by qRT-PCR and Western blots, respectively.
Results
GA-amide inhibited HUVEC (IC50 = 0.1269 μM) and NhEC (IC50 = 0.1740 μM) proliferation, induced cell apoptosis, and inhibited the migration and angiogenesis at a relatively safe dose (0.2 μM) in vitro. GA-amide reduced the number of capillaries from 56 ± 14.67 (DMSO) to 20.3 ± 5.12 (62.8 ng) in chick chorioallantoic membrane (CAM) assay. However, inactivation of TrkA couldn’t reverse the antiangiogenic effect of GA-amide. Moreover, GA-amide suppressed the expression of VEGF and VEGFR2, and decreased activation of the AKT/mTOR and PLCγ/Erk1/2 pathways.
Conclusions
Considering the antiangiogenic effect of GA-amide, it might be developed as a useful agent for use in clinical combination therapies.
Introduction
Garcinia hanburyi Hook f. (Gittifcrae) is a tropical plant found in southeastern Asia, and bioactive compounds extracted from its’ resin named gamboge have biological activities, such as antitumor, antibacterial, and anti-inflammatory activities (Jia et al. Citation2015; Hatami et al. Citation2020). Gambogic amide (GA-amide) is a derivative of gambogic acid (GA), the main active compound of gamboge. Previous studies reported that GA-amide is a non-peptide molecule with selective high affinity for the tropomyosin receptor kinase A (TrkA) that upregulates the expression and tyrosine phosphorylation of TrkA (Obianyo and Ye Citation2013; Shen and Yu Citation2015). GA-amide can prevent glutamate-induced apoptosis and induce neurite outgrowth in PC12 cells (Jang et al. Citation2007) and is tolerated in vivo. Additionally, GA-amide can promote osteoblastic differentiation, improve fracture healing in mice, and ameliorate leukaemia progression in K562 cell-inoculated nude mice (Chan et al. Citation2009).
TrkA is a tyrosine kinase receptor with a primary function in neurodevelopment (Singer et al. Citation1999). Nerve growth factor (NGF) can bind to TrkA, which activates cAMP response element-binding protein (CREB) to regulate the regeneration, survival, and proliferation of neurons (Lu et al. Citation2010; Hirose et al. Citation2016). Recent studies have shown that TrkA is associated with angiogenesis in a variety of tumours, such as neuroblastoma, ovarian cancer, and breast cancer (Eggert et al. Citation2000; Lagadec et al. Citation2009; Vera et al. Citation2014). Therefore, TrkA may play important roles in angiogenesis in the brain, ovary, and other organs.
PI3K/AKT, Ras/MAPK, and PLCγ are three important signalling pathways downstream of TrkA. Many researchers have reported that pathways stimulated by TrkA activation can mediate cell proliferation, invasion, angiogenesis, and death (Kruttgen et al. Citation2006; Molloy et al. Citation2011; Wang et al. Citation2016). Upon activation by NGF, the Erk1/2 and AKT pathways can promote human choroidal endothelial cell migration and proliferation (Steinle and Granger Citation2003), and TrkA activation can promote cell proliferation and angiogenesis through the oncogenes c-MYC and VEGF in epithelial ovarian cancer (Garrido et al. Citation2020). However, the overexpression of TrkA in SY5Y NB cells inhibited angiogenesis and tumour growth by downregulating angiogenic factors (Eggert et al. Citation2000, Citation2002).
Vascular disorders are found in many kinds of diseases, such as type 2 diabetes mellitus (D2M) (Hassanpour et al. Citation2017), glioma (Chow et al. Citation2016; Ameratunga et al. Citation2018), non-small cell lung cancer (Tian et al. Citation2020) and diabetic retinopathy (Cheung et al. Citation2010), so it is important to find drugs which have antiangiogenic effect.
The effects of TrkA on angiogenesis are not clear, and limited studies have indicated a possible role for GA-amide in angiogenesis. As the formation of vascular is based on the differentiation, proliferation, and migration of endothelial cells (ECs) (Teleanu et al. Citation2019), ECs is an important mode used in the study of antiangiogenic therapies. In this study, we used human umbilical vein endothelial cells (HUVECs), a well-known ECs model (Hassanpour et al. Citation2018; Rezabakhsh et al. Citation2018; Rezabakhsh et al. Citation2019), and normal human brain microvascular endothelial cells (NhECs) as in vitro models and the chick chorioallantoic membrane (CAM) assay as an in vivo model to explore the effect of GA-amide on angiogenesis, and to explore the relationship between the effects of GA-amide and TrkA and the pathways potentially affected by GA-amide.
Materials and methods
Cell lines and cell culture
HUVECs were obtained from the National Infrastructure of Cell Line Resource (Beijing, China). NhECs were purchased from Cell Systems (Seattle, WA, USA). ECs were maintained in EBM-2 medium (Lonza, Switzerland) containing 2% foetal bovine serum (FBS), 0.1% hydrocortisone, 0.1% R3IGF, 0.4% hFGF-b, 0.1% VEGF, 0.1% ascorbic acid, 0.1% GA-1000, 0.1% h EGF, 0.1% heparin, 100 U/mL penicillin, and 100 mg/mL streptomycin (Lonza, Switzerland).
Viability assay
MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, is a tetrazolium compound widely used in cell viability assay (Yu et al. Citation2016; Chen et al. Citation2020). The cells were seeded in 96-well plates at a density of 1 × 104 cells per well and incubated overnight in EBM-2 medium. Cells were treated with DMSO and GA-amide (0.1, 0.15, 0.2, 0.3, 0.4, and 0.5 μM) for 48 h. Cell viability was assessed by MTS assays (Cell Titer96; PROMEGA. Madison, WI, USA) according to previously described methods(Flores-Arriaga et al. Citation2017).
Wound-healing assay
Cell motility was measured by the wound-healing assay as reported previously (Zhou et al. Citation2017). Typically, HUVECs (4 × 105 cells/well) or NhECs (3 × 105 cells/well) were seeded in 6-well plates and grown for 24 h to reach 90–100% confluence. Then, a linear wound was scratched across the middle of the well surface using a 200 μL pipette tip. Next, culture media were replaced with fresh culture media containing 0.2 μM GA-amide, and the cells were incubated for 12 h. DMSO was used as a vehicle control. At 0, 6, and 12 h, the wound was photographed with a Nikon inverted microscope at 100× magnification, and relative cell migration was quantified using ImageJ image analysis software (Bethesda, MD, USA).
In vitro angiogenesis assay
HUVECs (2 × 105 cells/well) or NhECs (1.5 × 105 cells/well) were seeded to 6-well plates overnight and treated with DMSO or 0.2 μM GA-amide for 6 h. Then, the cells were trypsinized, counted, and resuspended in basal medium. HUVECs (1 × 104 cells/well; 100 μL) or NhECs (1 × 104 cells/well; 100 μL) were seeded in 96-well plates precoated with 60 μL Matrigel at 37 °C for 2 h. After 6 h of incubation at 37 °C and 5% CO2, tube formation was photographed with a Nikon inverted microscope, and tubular structures were analyzed and quantified by ImageJ image analysis software. Data were collected by measuring the total master segment length (pixel) of capillary tubes in five randomly selected standardized fields (the central field of view and four fields up, down, left, and right) of each well under 100× magnification.
Apoptosis assay
HUVECs (2 × 105 cells/well) or NhECs (1.5 × 105 cells/well) were seeded in 6-well plates overnight and treated with DMSO and GA-amide (0.1, 0.2, 0.4, 0.8 and 1.2 μM) for 6 h. Apoptosis assays were performed using a FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen, CA, USA) as previously described (Hu Y et al. Citation2019). Cells were collected after treatment with DMSO or GA-amide and resuspended in 1× binding buffer. Then, 5 μL FITC Annexin V and 5 μL PI were added to the cell suspension, and the mixture was incubated for 15 min at room temperature in the dark. Cell apoptosis was determined by flow cytometry within 1 h.
Reverse transcription real-time quantitative PCR (qRT–PCR)
HUVECs (2 × 105 cells/well) or NhECs (1.5 × 105 cells/well) were seeded in 6-well plates overnight and treated with DMSO or 0.8 μM GA-amide for 6 h. Total RNA was isolated using TRIzol reagent (Life Technologies, CA, USA) according to the manufacturer’s protocol, and qRT–PCR was performed using SYBR Premix Ex Taq Master Mix and a 2-Step kit (TaKaRa, Dalian, China) as previously described (Hu et al. Citation2017). All primers were synthesized by TSINGKE (Beijing, China), and the following primers were used to amplify target mRNA and the internal control: vWF forward (5′-AGCCTTGTGAAACTGAAGCAT-3′) and reverse (5′-GGCCATCCCAGTCCATCTG-3′); CD31 forward (5′-AACAGTGTTGACATGAAGAGCC-3′) and reverse (5′-TGTAAAACAGCACGTCATCCTT-3′); CD105 forward (5′-TGCACTTGGCCTACAATTCCA-3′) and reverse (5′-AGCTGCCCACTCAAGGATCT-3′); and GAPDH forward (5′-GGTCATCCATGACAACTTTGG-3′) and reverse (5′-GGCCATCACGCCACAG-3′).
Western blot assay
HUVECs (2 × 105 cells/well) or NhECs (1.5 × 105 cells/well) were seeded in 6-well plates overnight and treated with DMSO or GA-amide (0.1, 0.2, 0.4, 0.8, and 1.2 μM) for 6 h. Western blot assays were performed as previously described (Tian et al. Citation2020). Cells were lysed with Tris-NaCl-Triton-EDTA (TNTE), and 8 μg protein was separated by SDS-polyacrylamide gel electrophoresis (SDS–PAGE) according to molecular weight. Then, the separated proteins were transferred to NC membranes, and antibodies were used to detect the expression of the corresponding proteins. The following antibodies were used: anti-CD31 (Ab28364, Abcam), anti-CD105 (Ab169545, Abcam), anti-VEGFR2 (2479S, Cell Signalling Technology [CST], MA, USA), anti-vWF (65707S, CST), anti-VEGF (Ab53465, Abcam), anti-mTOR (2983 T, CST), anti-p-mTOR (2974 T, CST), anti-AKT (2938S, CST), anti-p-AKT (9018S, CST), anti-Erk1/2 (4695 T, CST), anti-p-Erk1/2 (4370S, CST), anti-PLCγ (3872 T, CST), anti-p-PLCγ (3871 T, CST), anti-PARP (9542 L, CST), anti-caspase-3 (9665S, CST), anti-cleaved-caspase-3 (9661 L, CST), anti-p-TrkA (9141S, CST), anti-TrkA (Sc-118, Santa Cruz Biotechnology) and anti-β-actin (A5441, Sigma–Aldrich, St. Louis, MO, USA).
siRNA-mediated gene knockdown assay
Cells were seeded into 6-well plates and transfected with siRNA oligonucleotides for 72 h using INTERFERin® (PolyPlus, NY, USA) according to the manufacturer’s protocol. The siRNA sequence was as follows: siTrkA sense: 5′-CGAGAACCCACAAUACUUCAGUGAU-3′.
Chick chorioallantoic membrane (CAM) assay
The CAM assay was performed as described in a previous study (Varinská et al. Citation2018). Chicken eggs at seven days post-fertilization (Boehringer Ingelheim, Beijing) were cleaned with 70% ethanol, and a window of ∼1.5–2.0 cm2 was gently opened on the blunt end of the egg without damaging the embryo. A sterilized silicone ring (inner diameter, 6 mm) was positioned on the CAM surface avoiding major blood vessels. Then, DMSO, 18.8 ng GA-amide, 62.8 ng GA-amide, or 40 μg Bevacizumab (positive control) was placed within the ring. The eggs were incubated in a forced-draft incubator at 38 ± 0.2 °C, with ∼60–65% humidity. After 72 h of treatment, the large vessels, small vessels, and capillaries of the CAM were counted. The angiogenesis index was calculated as the difference between the number of vessels after 72 h and the number of vessels before each treatment. Photographs of CAM blood vessels forming inside the rings were obtained using a Nikon stereomicroscope (Tokyo, Japan).
Statistical analysis
All experiments were repeated at least three times and representative results are presented. All experimental data were processed by GraphPad Prism 9.0 software. The data are presented as the mean ± standard deviation (S.D.). Significant differences between individual groups were determined by one-way ANOVA or two-way RM ANOVA. A t-test was used for statistical analyses between two independent groups. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 indicated statistical significance.
Results
GA-amide inhibited the proliferation and induced the apoptosis of HUVECs and NhECs
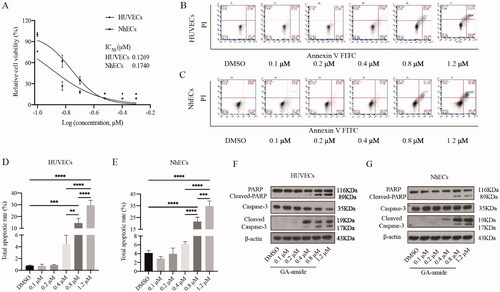
To find a safe dose for use in the angiogenesis assays that does not affect the proliferation of HUVECs and NhECs, we first analyzed the inhibitory effects of GA-amide on EC proliferation by MTS assays. As shown in , GA-amide inhibited the proliferation of HUVECs and NhECs after treatment for 48 h in the range of 0.1–0.5 μM, with IC50 values of 0.1269 and 0.1740 μM against HUVECs and NhECs, respectively. As shown in , GA-amide induced the apoptosis of HUVECs and NhECs in a dose-dependent manner in the range of 0.4–1.2 μM after treatment for 6 h. After treatment with 0.4, 0.8, and 1.2 μM GA-amide for 6 h, ECs showed enhanced expression of apoptosis-related proteins, such as cleaved PARP and cleaved caspase-3 (). From the above experiments, we also observed that treatment with 0.2 μM GA-amide for 6 h did not induce EC death, so the treatment conditions for the angiogenesis assay were set as 0.2 μM GA-amide for 6 h.
Figure 1. GA-amide inhibited the proliferation and induced the apoptosis of HUVECs and NhECs. (A) GA-amide was evaluated for its effects on the cell viability of HUVECs and NhECs using the MTS assay. ECs were treated with GA-amide for 48 h, and the IC50 values were calculated by GraphPad Prism 9.0. (B,C) Flow cytometry-based quantification of HUVEC (B) and NhEC (C) apoptosis after 6 h of exposure to different concentrations of GA-amide by annexin V/PI staining. (D,E) Quantitative analysis of (B) and (C). Data are presented as the mean ± S.D., and samples were assayed in triplicate. Significant differences between individual groups were determined by one-way ANOVA. **p < 0.01; ***p < 0.001; ****p < 0.0001. (F,G) Western blotting was used to analyze the cleavage of cell apoptosis proteins (PARP and Caspase-3) in HUVECs and NhECs after treatment with GA-amide for 6 h.
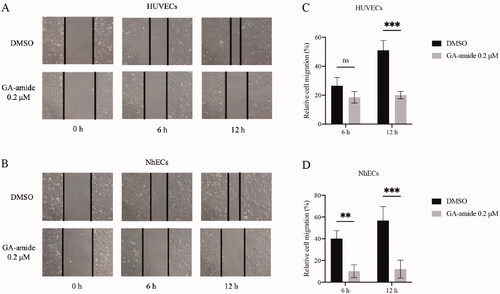
GA-amide suppressed HUVECs and NhECs migration in vitro
EC migration is essential for angiogenesis; thus, we assessed the antimigration effects of GA-amide on HUVECs and NhECs using a relatively safe dose of 0.2 μM in vitro. As shown in , ECs actively migrated into the wounded area in the DMSO, and 0.2 μM GA-amide treatment slowed EC migration. The statistical analysis revealed no significant difference in relative cell migration between HUVECs treated with GA-amide or DMSO for 6 h, but 0.2 μM GA-amide significantly inhibited the migration of HUVECs after 12 h of treatment (). Moreover, 0.2 μM GA-amide significantly inhibited the migration of NhECs after 6 and 12 h ().
Figure 2. GA-amide inhibited the migration of HUVECs and NhECs. (A,B) Representative images showing scratch width after ECs were treated with 0.2 μM GA-amide for 0, 6, and 12 h. Images were taken under a phase-contrast microscope at 100× magnitude. Scale bar, 200 μm. (C,D) Quantitative analysis of (A) and (B). Data are presented as the mean ± S.D., and samples were assayed in triplicate. Significant differences between individual groups were determined using two-way RM ANOVA with Sidak’s multiple comparisons test. **p < 0.01; ***p < 0.001.
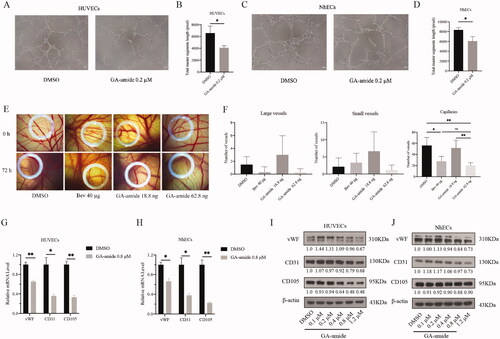
GA-amide inhibited angiogenesis both in vitro and in vivo and suppressed the expression of EC markers at both the mRNA and protein level
Since angiogenesis is characterized by the formation of tubular structures by capillary ECs, we conducted capillary tube formation assays to assess the effects of GA-amide on tube formation. As shown in , when grown freely on Matrigel, HUVECs and NhECs became aligned into cords on the Matrigel, and highly organized capillary tubes formed. Treatment of HUVECs and NhECs with 0.2 μM GA-amide effectively suppressed tube formation compared with the control (). We also evaluated the in vivo antiangiogenic effects of GA-amide by using the CAM assay. We counted the number of large vessels, small vessels, and capillaries in CAM models treated with DMSO, bevacizumab, and GA-amide. As shown in , GA-amide had the same antiangiogenic effects as bevacizumab (positive control), and 62.8 ng GA-amide suppressed the formation of capillaries but not of large or small vessels. To further confirm the antiangiogenic effects of GA-amide, we detected the expression of EC markers, such as vWF, CD31, and CD105; the results showed that GA-amide downregulated the expression of vWF, CD31, and CD105 at both the mRNA and protein levels ().
Figure 3. GA-amide inhibited angiogenesis both in vitro and in vivo and suppressed the expression of EC markers at both the mRNA and protein levels. (A) Representative images showing the tube formation by HUVECs treated with 0.2 μM GA-amide for 6 h. (B) Quantitative analysis of A after images were analyzed by ImageJ software. Scale bar, 200 μm. (C) Representative images showing tube formation by NhECs treated with 0.2 μM GA-amide for 6 h. (D) Quantitative analysis of C after images were analyzed by the ImageJ software. Scale bar, 200 μm. (E) Representative images showing the antiangiogenic effects of GA-amide (18.8 and 62.8 ng) and bevacizumab (Bev, 40 μg) on vessel sprouting and branching. (F) The graphs summarize the data for large vessels, small vessels, and capillaries from the CAM assay. Bev: Positive control. (G,H) The mRNA expression levels of EC markers in HUVECs (G) and NhECs (H) treated with GA-amide were evaluated by qRT–PCR. Data are presented as the mean ± S.D. of three independent determinations. *p < 0.05 and **p < 0.01 compared with DMSO by two-tailed Student’s t-test. (I,J) The protein expression levels of EC markers in HUVECs (I) and NhECs (J) treated with GA-amide were evaluated by Western blot.
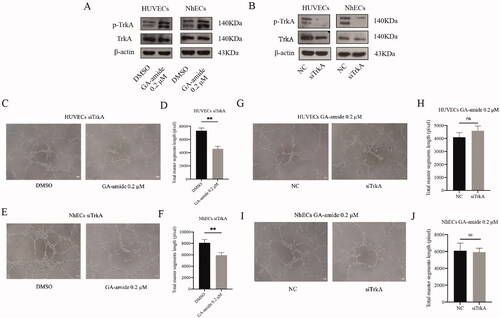
TrkA knockdown did not reverse the antiangiogenic effects of GA-amide on HUVECs and NhECs
GA-amide can activate TrkA, so we wanted to determine whether the antiangiogenic effects of GA-amide occur through TrkA. First, we found that 0.2 μM GA-amide enhanced the tyrosine phosphorylation of TrkA in HUVECs and NhECs (). Then, we used siRNA to knockdown the expression of TrkA in ECs (). As shown in , GA-amide reduced tube formation by TrkA-knockdown ECs. Meanwhile, the tube formation capability was not significantly different between normal ECs and TrkA-knockdown ECs treated with GA-amide ().
Figure 4. TrkA knockdown did not reverse the antiangiogenic effects of GA-amide on HUVECs and NhECs. (A) p-TrkA levels in HUVECs and NhECs after treatment with 0.2 μM GA-amide for 6 h were evaluated by Western blot. (B) TrkA and p-TrkA expression levels in HUVECs and NhECs after treatment with siTrkA were evaluated by Western blot. (C) Representative images showing the antiangiogenic effects of GA-amide on TrkA knockdown HUVECs. (D) Quantitative analysis of C. (E) Representative images showing the antiangiogenic effects of GA-amide on TrkA-knockdown NhECs. (F) Quantitative analysis of (E). (G) Representative images showing the antiangiogenic effects of GA-amide on normal and TrkA-knockdown HUVEC. (H) Quantitative analysis of (G). (I) Representative images showing the antiangiogenic effects of GA-amide on normal and TrkA knockdown NhECs. (J) Quantitative analysis of (I). Images were taken under a phase-contrast microscope at 100× magnitude. Images were analyzed by the ImageJ software, and the numbers of capillary-like structures in five separated fields of one well was calculated. Scale bar, 200 μm. Data are presented as the mean ± S.D. of three independent determinations. **p < 0.01 compared with the DMSO by two-tailed Student’s t-test.
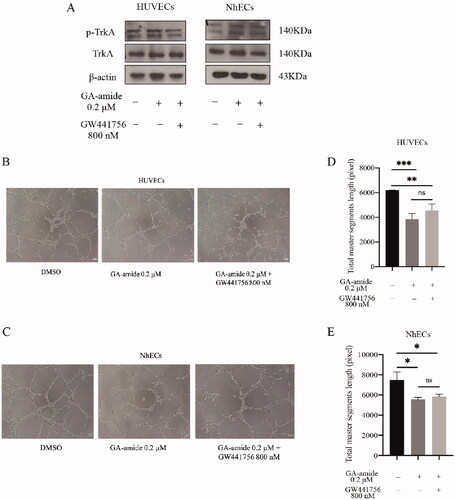
The inhibition of TrkA could not reverse the antiangiogenic effects of GA-amide on HUVECs and NhECs
To confirm that the antiangiogenic effects of GA-amide were unrelated to TrkA, we treated ECs with a selective TrkA inhibitor, GW441756, and evaluated the antiangiogenic effects of GA-amide. As shown in , we verified that GW441756 inhibited the phosphorylation of TrkA in ECs, and this treatment did not affect the antiangiogenic activity of GA-amide in ECs (). Additionally, there was no significant differences in tube formation by ECs upon treatment with GA-amide in the presence or absence of GW441756 ().
Figure 5. The inhibition of TrkA could not reverse the antiangiogenic effects of GA-amide on HUVECs and NhECs. (A) The inhibitory effects of GW441756 (TrkA inhibitor) on TrkA in HUVECs and NhECs were evaluated by Western blot. (B,C) Representative images showing the antiangiogenic effects of GA-amide with or without GW441756 on HUVECs (B) and NhECs (C). (D,E) Quantitative analysis of (B) and (C). Images were analyzed by ImageJ software, and the numbers of capillary-like structures in five separated fields of one well was calculated. Scale bar, 200 μm. Data are presented as the means ± S.D. of three independent determinations. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with DMSO by two-tailed Student’s t-test.
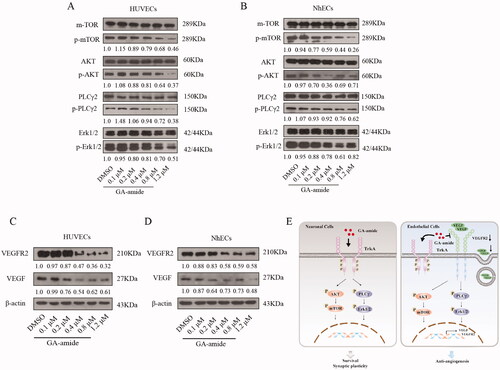
GA-amide suppressed the expression of VEGF/VEGFR2 and downregulated the activation of the AKT/mTOR and PLCγ/Erk1/2 pathways
AKT/mTOR and PLCγ/Erk1/2 pathways are the two main pathways related to angiogenesis (Ahir et al. Citation2020), so we investigated the influence of GA-amide on the activation of these two pathways. As shown in , GA-amide downregulated the activation of the AKT/mTOR and PLCγ/Erk1/2 pathways in cells treated for 6 h. The expression of VEGF and VEGFR2 can be regulated by the AKT/mTOR and PLCγ/Erk1/2 pathways (Nicolas et al. Citation2019; Wang et al. Citation2019; Zhong et al. Citation2020), and our results showed that GA-amide suppressed the expression of VEGF and VEGFR2 in cells treated for 6 h (). According to previous studies, GA-amide might act as an NGF analogue to activate TrkA and its downstream pathways to help neuronal cell to survive (Roux and Barker Citation2002; Szegezdi et al. Citation2008; Greenberg et al. Citation2009). In our study, we found that GA-amide suppressed the expression of VEGF and VEGFR2 and decreased AKT/mTOR and PLCγ/Erk1/2 pathway activation in ECs; this may reduce VEGF secretion into the extracellular space and the binding of VEGF to VEGFR2, resulting in the observed antiangiogenic effects of GA-amide ().
Figure 6. GA-amide suppressed the expression of VEGF and VEGFR2 and downregulated the activation of the AKT/mTOR and PLCγ/Erk1/2 pathways. (A,B) The expression levels of proteins involved in the AKT/mTOR and PLCγ/Erk1/2 pathways in GA-amide- or DMSO-treated HUVECs (A) and NhECs (B) were evaluated by Western blot. Quantitative analysis of phosphorylated protein levels normalized to non-phosphorylated protein levels in the corresponding samples. (C,D) The expression levels of VEGF and VEGFR2 in GA-amide- or DMSO-treated HUVECs (C) and NhECs (D) were evaluated by Western blot. (E) A model of the mechanism by which GA-amide inhibits angiogenesis by ECs.
Discussion
GA-amide is a derivative of GA, and most studies on GA-amide, have focussed on its’ function as a selective TrkA agonist (Jang et al. Citation2007; Obianyo and Ye Citation2013; Shen and Yu Citation2015). In our present study, we showed for the first time that GA-amide has an antiangiogenic effect in vitro and in vivo, and this effect does not involve TrkA.
Angiogenesis, the formation of new blood vessels from pre-existing vessels by the proliferation, migration, and differentiation of vascular endothelial cells (ECs), is an important process in building the mature vascular network essential for the delivery of oxygen and nutrients in normal tissues. Researchers have found that angiogenesis is overactivated in tumours and can promote tumour growth, progression, and metastasis (Mazzone et al. Citation2009; Nagy et al. Citation2010; Carmeliet and Jain Citation2011; De Bock et al. Citation2011; Farzaneh Behelgardi et al. Citation2018). After Judah Folkman established the antiangiogenic concept in cancer therapy (Folkman Citation1971), an increasing number of preclinical and clinical studies have focussed on this topic. Currently, angiogenesis has been validated as a target in several tumour types through randomized trials, and vascular endothelial growth factor (VEGF) pathway inhibitors have been incorporated into the therapeutic armoury (Kerbel and Kamen Citation2004; Kim et al. Citation2006).
In the past decade, first-generation antivascular drugs have undergone unprecedented development and have shown very promising therapeutic effects in the treatment of various tumours, such as prolonging progression-free survival and overall survival. However, antivascular drugs have side effects, and tumours can develop intrinsic and acquired resistance, which leads to therapeutic failure (Ellis and Hicklin Citation2008). Bevacizumab is an antivascular treatment for glioblastoma, but because of its low blood–brain barrier permeability, this agent is administered at a high dose, which can cause serious systemic side effects, including intestinal perforation and pulmonary embolism (Riina et al. Citation2009). GA-amide possesses robust neurotrophic activity, indicating that it can pass through the blood–brain barrier and enter brain tissue. Our research showed that GA-amide can inhibit EC angiogenesis, indicating that it has potential for the treatment of brain tumours in the future.
The AKT/mTOR and PLCγ/Erk1/2 pathways have been reported to be overactivated in many kinds of cancers, and these phenomena are related to cancer metastasis. The PI3K/AKT and Erk1/2 pathways can promote the proliferation and migration of ECs and thus promote the formation of tumour blood vessels (Ahir et al. Citation2020). Our research found that GA-amide could downregulate the activation of these two pathways and inhibit EC proliferation, migration, and angiogenesis.
Previous studies have demonstrated the important roles of VEGF and VEGFR2 in angiogenesis, and VEGFR2 is the main mediator of VEGF-triggered angiogenesis (Gho et al. Citation2019). When VEGF binds to VEGFR2, the receptor autophosphorylates its tyrosine residues, increasing its tyrosine kinase activity and the activation of downstream intracellular signalling molecules, including AKT/mTOR and PLCγ/Erk1/2 (Zhang et al. Citation2016; Assareh et al. Citation2019). Our research found that GA-amide could downregulate the expression of VEGF and VEGFR2. Considering the VEGF and VEGFR2 can be regulated by the AKT/mTOR and PLCγ/Erk1/2 pathways (Nicolas et al. Citation2019; Wang et al. Citation2019; Zhong et al. Citation2020), we hypothesized that GA-amide can suppress the expression of VEGF and VEGFR2 by downregulating the activation of the AKT/mTOR and PLCγ/Erk1/2 pathways, thereby inhibiting angiogenesis by ECs.
Many phytochemicals can inhibit angiogenesis by inhibiting the expression of VEGF/VEGFR2 and the activation of downstream pathways. Gambogic acid can inhibit angiogenesis by suppressing the activation of VEGFR2 and its downstream protein kinases c-Src, FAK, and AKT in prostate cancer (Yi et al. Citation2008; Wang and Chen Citation2012). Polydatin can significantly decrease the VEGF-induced phosphorylation of AKT, eNOS, and Erk (Hu WH et al. Citation2019). Curcumin can inhibit the activation of FAK, AKT, and Erk in HUVECs and inhibit tumour growth in a VEGF-overexpressing tumour model (Fu et al. Citation2015).
Overall, we found that GA-amide, a phytochemical that has antiangiogenic effects, has potential in angiogenesis-related diseases, such as cancer and diabetic retinopathy. However, our current research mainly focussed on the effect of GA-amide on angiogenesis by normal ECs. To further clarify the role GA-amide in tumours and fundus vascular diseases, we need more in vivo and in vitro research models, such as tumour-derived endothelial cells, tumour cells, and diabetic retinopathy animal models. By exploring the effects of agent in these models, we can further evaluate the therapeutic effects of GA-amide on diseases and promote its translation into clinical applications.
Conclusions
Our research demonstrated the antiangiogenic property of GA-amide in vitro and in vivo via the suppression of VEGF/VEGFR2 and the downstream AKT/mTOR and PLCγ/Erk1/2 pathways in a TrkA-independent manner. These findings provide pharmacological support for GA-amide as a candidate agent in anticancer therapy.
Author contributions
Tongtong Sui designed and performed research, analyzed data, and wrote the paper. Bojun Qiu, Jiaorong Qu, Yuxin Wang, and Kunnian Ran collected data and performed the statistical analysis. Wei Han and Xiaozhong Peng directed the experimental design and data analysis.
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Ahir BK, Engelhard HH, Lakka SS. 2020. Tumor development and angiogenesis in adult brain tumor: Glioblastoma. Mol Neurobiol. 57(5):2461–2478.
- Ameratunga M, Pavlakis N, Wheeler H, Grant R, Simes J, Khasraw M. 2018. Anti-angiogenic therapy for high-grade glioma. Cochrane Database Syst Rev. 11:CD008218.
- Assareh E, Mehrnejad F, Mansouri K, Esmaeili Rastaghi AR, Naderi-Manesh H, Asghari SM. 2019. A cyclic peptide reproducing the α1 helix of VEGF-B binds to VEGFR-1 and VEGFR-2 and inhibits angiogenesis and tumor growth. Biochem J. 476(4):645–663.
- Carmeliet P, Jain RK. 2011. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 10(6):417–427.
- Chan CB, Liu X, Jang SW, Hsu SI, Williams I, Kang S, Chen J, Ye K. 2009. NGF inhibits human leukemia proliferation by downregulating cyclin A1 expression through promoting acinus/CtBP2 association. Oncogene. 28(43):3825–3836.
- Chen Z, Gao Y, Gao S, Song D, Feng Y. 2020. MiR-135b-5p promotes viability, proliferation, migration and invasion of gastric cancer cells by targeting Krüppel-like factor 4 (KLF4). Arch Med Sci. 16(1):167–176.
- Cheung N, Mitchell P, Wong TY. 2010. Diabetic retinopathy. Lancet. 376(9735):124–136.
- Chow DS, Horenstein CI, Canoll P, Lignelli A, Hillman EM, Filippi CG, Grinband J. 2016. Glioblastoma induces vascular dysregulation in nonenhancing peritumoral regions in humans. Am J Roentgenol. 206(5):1073–1081.
- De Bock K, Cauwenberghs S, Carmeliet P. 2011. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev. 21(1):73–79.
- Eggert A, Grotzer MA, Ikegaki N, Liu XG, Evans AE, Brodeur GM. 2000. Expression of neurotrophin receptor TrkA inhibits angiogenesis in neuroblastoma. Med Pediatr Oncol. 35(6):569–572.
- Eggert A, Grotzer MA, Ikegaki N, Liu XG, Evans AE, Brodeur GM. 2002. Expression of the neurotrophin receptor TrkA down-regulates expression and function of angiogenic stimulators in SH-SY5Y neuroblastoma cells. Cancer Res. 62(6):1802–1808.
- Ellis LM, Hicklin DJ. 2008. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 14(20):6371–6375.
- Farzaneh Behelgardi M, Zahri S, Mashayekhi F, Mansouri K, Asghari SM. 2018. A peptide mimicking the binding sites of VEGF-A and VEGF-B inhibits VEGFR-1/-2 driven angiogenesis, tumor growth and metastasis. Sci Rep. 8(1):17924–17936.
- Flores-Arriaga JC, de J, Pozos-Guillen A, Escobar-Garcia DM, Grandfils C, Cerda-Cristerna BI. 2017. Cell viability and hemocompatibility evaluation of a starch-based hydrogel loaded with hydroxyapatite or calcium carbonate for maxillofacial bone regeneration. Odontology. 105(4):398–407.
- Folkman J. 1971. Tumor angiogenesis: therapeutic implications. N Engl J Med. 285(21):1182–1186.
- Fu Z, Chen X, Guan S, Yan Y, Lin H, Hua ZC. 2015. Curcumin inhibits angiogenesis and improves defective hematopoiesis induced by tumor-derived VEGF in tumor model through modulating VEGF-VEGFR2 signaling pathway. Oncotarget. 6(23):19469–19482.
- Garrido MP, Torres I, Avila A, Chnaiderman J, Valenzuela-Valderrama M, Aramburo J, Orostica L, Duran-Jara E, Lobos-Gonzalez L, Romero C. 2020. NGF/TRKA decrease miR-145-5p levels in epithelial ovarian cancer cells. IJMS. 21(20):7657–7677.
- Gho Y, Shin SS, Choi YH, Ko K, Kim WJ, Moon SK. 2019. Hydrangenol suppresses VEGF-stimulated angiogenesis by targeting p27KIP1-dependent G1-cell cycle arrest, VEGFR-2-mediated signaling, and MMP-2 expression. Anim Cells Syst. 23(2):72–81.
- Greenberg ME, Xu B, Lu B, Hempstead BL. 2009. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 29(41):12764–12767.
- Hassanpour M, Rezabakhsh A, Pezeshkian M, Rahbarghazi R, Nouri M. 2018. Distinct role of autophagy on angiogenesis: highlights on the effect of autophagy in endothelial lineage and progenitor cells. Stem Cell Res Ther. 9(1):305–320.
- Hassanpour M, Rezabakhsh A, Rahbarghazi R, Nourazarian A, Nouri M, Avci CB, Ghaderi S, Alidadyani N, Bagca BG, Bagheri HS. 2017. Functional convergence of Akt protein with VEGFR-1 in human endothelial progenitor cells exposed to sera from patient with type 2 diabetes mellitus. Microvasc Res. 114:101–113.
- Hatami E, Jaggi M, Chauhan SC, Yallapu MM. 2020. Gambogic acid: a shining natural compound to nanomedicine for cancer therapeutics. Biochim Biophys Acta Rev Cancer. 1874(1):188381–188420.
- Hirose M, Kuroda Y, Murata E. 2016. NGF/TrkA signaling as a therapeutic target for pain. Pain Pract. 16(2):175–182.
- Hu WH, Wang HY, Kong XP, Xiong QP, Poon KK, Xu L, Duan R, Chan GK, Dong TT, Tsim KW. 2019. Polydatin suppresses VEGF-induced angiogenesis through binding with VEGF and inhibiting its receptor signaling. FASEB J. 33(1):532–544.
- Hu PS, Xia QS, Wu F, Li DK, Qi YJ, Hu Y, Wei ZZ, Li SS, Tian NY, Wei QF, et al. 2017. NSPc1 promotes cancer stem cell self-renewal by repressing the synthesis of all-trans retinoic acid via targeting RDH16 in malignant glioma. Oncogene. 36(33):4706–4718.
- Hu Y, Zhang M, Tian N, Li D, Wu F, Hu P, Wang Z, Wang L, Hao W, Kang J, et al. 2019. The antibiotic clofoctol suppresses glioma stem cell proliferation by activating KLF13. J Clin Invest. 129(8):3072–3085.
- Jang SW, Okada M, Sayeed I, Xiao G, Stein D, Jin P, Ye K. 2007. Gambogic amide, a selective agonist for TrkA receptor that possesses robust neurotrophic activity, prevents neuronal cell death. Proc Natl Acad Sci USA. 104(41):16329–16334.
- Jia B, Li S, Hu X, Zhu G, Chen W. 2015. Recent research on bioactive xanthones from natural medicine: Garcinia hanburyi. AAPS PharmSciTech. 16(4):742–758.
- Kerbel RS, Kamen BA. 2004. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 4(6):423–436.
- Kim DW, Huamani J, Fu A, Hallahan DE. 2006. Molecular strategies targeting the host component of cancer to enhance tumor response to radiation therapy. Int J Radiat Oncol Biol Phys. 64(1):38–46.
- Kruttgen A, Schneider I, Weis J. 2006. The dark side of the NGF family: neurotrophins in neoplasias. Brain Pathol. 16(4):304–310.
- Lagadec C, Meignan S, Adriaenssens E, Foveau B, Vanhecke E, Romon R, Toillon RA, Oxombre B, Hondermarck H, Le Bourhis X. 2009. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene. 28(18):1960–1970.
- Lu J, Wu DM, Hu B, Zheng YL, Zhang ZF, Wang YJ. 2010. NGF-Dependent activation of TrkA pathway: a mechanism for the neuroprotective effect of troxerutin in d-galactose-treated mice. Brain Pathol. 20(5):952–965.
- Mazzone M, Dettori D, de Oliveira RL, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, de Almodovar CR, et al. 2009. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 136(5):839–851.
- Molloy NH, Read DE, Gorman AM. 2011. Nerve growth factor in cancer cell death and survival. Cancers. 3(1):510–530.
- Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. 2010. Heterogeneity of the tumor vasculature. Semin Thromb Hemost. 36(3):321–331.
- Nicolas S, Abdellatef S, Haddad MA, Fakhoury I, El-Sibai M. 2019. Hypoxia and EGF stimulation regulate VEGF expression in human glioblastoma multiforme (GBM) cells by differential regulation of the PI3K/Rho-GTPase and MAPK pathways. Cells. 8(11):1397–1421.
- Obianyo O, Ye K. 2013. Novel small molecule activators of the Trk family of receptor tyrosine kinases. Biochim Biophys Acta. 1834(10):2213–2218.
- Rezabakhsh A, Fathi F, Bagheri HS, Malekinejad H, Montaseri A, Rahbarghazi R, Garjani A. 2018. Silibinin protects human endothelial cells from high glucose-induced injury by enhancing autophagic response. J Cell Biochem. 119(10):8084–8094.
- Rezabakhsh A, Rahbarghazi R, Malekinejad H, Fathi F, Montaseri A, Garjani A. 2019. Quercetin alleviates high glucose-induced damage on human umbilical vein endothelial cells by promoting autophagy. Phytomedicine. 56:183–193.
- Riina HA, Fraser JF, Fralin S, Knopman J, Scheff RJ, Boockvar JA. 2009. Superselective intraarterial cerebral infusion of bevacizumab: a revival of interventional neuro-oncology for malignant glioma. J Exp Ther Oncol. 8(2):145–150.
- Roux PP, Barker PA. 2002. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 67(3):203–233.
- Shen J, Yu Q. 2015. Gambogic amide selectively upregulates TrkA expression and triggers its activation. Pharmacol Rep. 67(2):217–223.
- Singer HS, Hansen B, Martinie D, Karp CL. 1999. Mitogenesis in glioblastoma multiforme cell lines: a role for NGF and its TrkA receptors. J Neurooncol. 45(1):1–8.
- Steinle JJ, Granger HJ. 2003. Nerve growth factor regulates human choroidal, but not retinal, endothelial cell migration and proliferation. Auton Neurosci. 108(1–2):57–62.
- Szegezdi E, Herbert KR, Kavanagh ET, Samali A, Gorman AM. 2008. Nerve growth factor blocks thapsigargin-induced apoptosis at the level of the mitochondrion via regulation of Bim. J Cell Mol Med. 12(6A):2482–2496.
- Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. 2019. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. JCM. 9(1):84–104.
- Tian W, Cao C, Shu L, Wu F. 2020. Anti-angiogenic therapy in the treatment of non-small cell lung cancer. Onco Targets Ther. 13:12113–12129.
- Tian Y, Wang Z, Wang Y, Yin B, Yuan J, Qiang B, Han W, Peng X. 2020. Glioma-derived endothelial cells promote glioma cells migration via extracellular vesicles-mediated transfer of MYO1C. Biochem Biophys Res Commun. 525(1):155–161.
- Varinská L, Fáber L, Kello M, Petrovová E, Balážová Ľ, Solár P, Čoma M, Urdzík P, Mojžiš J, Švajdlenka E, et al. 2018. beta-ESCIN effectively modulates HUVECS proliferation and tube formation. Molecules. 23(1):197–211.
- Vera C, Tapia V, Vega M, Romero C. 2014. Role of nerve growth factor and its TRKA receptor in normal ovarian and epithelial ovarian cancer angiogenesis. J Ovarian Res. 7:82–89.
- Wang J, He C, Zhou T, Huang Z, Zhou L, Liu X. 2016. NGF increases VEGF expression and promotes cell proliferation via ERK1/2 and AKT signaling in Muller cells. Mol Vis. 22:254–263.
- Wang J, Yang L, Liang F, Chen Y, Yang G. 2019. Integrin alpha x stimulates cancer angiogenesis through PI3K/Akt signaling-mediated VEGFR2/VEGF-A overexpression in blood vessel endothelial cells. J Cell Biochem. 120(2):1807–1818.
- Wang X, Chen W. 2012. Gambogic acid is a novel anti-cancer agent that inhibits cell proliferation, angiogenesis and metastasis. Anticancer Agents Med Chem. 12(8):994–1000.
- Yi T, Yi Z, Cho SG, Luo J, Pandey MK, Aggarwal BB, Liu M. 2008. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 68(6):1843–1850.
- Yu P, Zhang Z, Li S, Wen X, Quan W, Tian Q, Chen J, Zhang J, Jiang R. 2016. Progesterone modulates endothelial progenitor cell (EPC) viability through the CXCL12/CXCR4/PI3K/Akt signalling pathway. Cell Prolif. 49(1):48–57.
- Zhang Z, Zhang H, Peng T, Li D, Xu J. 2016. Melittin suppresses cathepsin S-induced invasion and angiogenesis via blocking of the VEGF-A/VEGFR-2/MEK1/ERK1/2 pathway in human hepatocellular carcinoma. Oncol Lett. 11(1):610–618.
- Zhong M, Li N, Qiu X, Ye Y, Chen H, Hua J, Yin P, Zhuang G. 2020. TIPE regulates VEGFR2 expression and promotes angiogenesis in colorectal cancer. Int J Biol Sci. 16(2):272–283.
- Zhou P, Qin J, Li Y, Li G, Wang Y, Zhang N, Chen P, Li C. 2017. Combination therapy of PKCζ and COX-2 inhibitors synergistically suppress melanoma metastasis. J Exp Clin Cancer Res. 36(1):115–126.
