Abstract
Context
Cordyceps militaris and Isaria tenuipes (Cordycipitaceae) are high-value fungi that are used for health-promoting food supplements. Since laboratory cultivation has begun for these fungi, increased output has been achieved.
Objective
This study compared the chemical profiles, antioxidant, anti-tyrosinase, and skin extracellular matrix degradation inhibition between mycelium and fruiting body of C. militaris and I. tenuipes.
Materials and methods
The antioxidative potential of 10% v/v aqueous infused extract from each fungus was separately investigated using 2,2-azinobis(3-ethylbenzo-thiazoline-6-sulphonic acid) (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), ferric reducing antioxidant ability, and ferric thiocyanate methods. The inhibition against MMP-1, elastase, and hyaluronidase were determined to reveal their anti-wrinkle potential. Anti-tyrosinase activities were determined.
Results
C. militaris and I. tenuipes extracts were found to contain a wide range of bioactive compounds, including phenolics, flavonoids, and adenosine. A correlation was discovered between the chemical compositions and their biological activities. The extract from I. tenuipes fruiting body (IF) was highlighted as an extraordinary elastase inhibitor (IC50 = 0.006 ± 0.004 mg/mL), hyaluronidase inhibitor (IC50: 30.3 ± 3.2 mg/mL), and antioxidant via radical scavenging (ABTS IC50: 0.22 ± 0.02 mg/mL; DPPH IC50: 0.05 ± 0.02 mg/mL), thereby reducing ability (EC1: 95.3 ± 4.8 mM FeSO4/g extract) and lipid peroxidation prevention (IC50: 0.40 ± 0.11 mg/mL). IF had a three-times higher EC1 value than ascorbic acid and significantly higher elastase inhibition than epigallocatechin gallate.
Discussion and conclusions
IF is proposed as a powerful natural extract with antioxidant and anti-wrinkle properties; therefore, it is suggested for further use in pharmaceutical, cosmeceutical, and nutraceutical industries.
Introduction
Entomopathogenic fungi, a type of fungal pathogen that infects a wide range of insect species, are divided into five categories: Chytridiomycota (Chytrids), the Zygomycota (conjugated fungi), the Ascomycota (sac fungi), the Basidiomycota (club fungi), and the recently described phylum Glomeromycota (Litwin et al. Citation2020). Among Cordycipitaceae, Cordyceps militaris (L.) Fr. Link (Dong-Chung-Ha-Cho) and Isaria tenuipes Peck (Snowflake Dong-Chung-Ha-Cho) are the authentic fungal supplies for food ingredients, herbal products, and dietary supplements in various Asian countries (Moon et al. Citation2018). C. militaris has been reported to grow in many geographic locations and can be artificially cultivated in the laboratory (Nxumalo et al. Citation2020). C. militaris is one of the well-known Cordyceps that have been used in traditional Chinese medicine for over a century as health-promoting supplements (Ng and Wang Citation2005). C. militaris has been shown to have a variety of biological functions, including antioxidant, immunomodulatory, hypolipidemic, and antitumor properties (Ng and Wang Citation2005; Marsup et al. Citation2020).
Apart from C. militaris, I. tenuipes is also an important edible and medicinal fungal species due to its various beneficial pharmacological activities. Nowadays, I. tenuipes can also be cultivated in controlled laboratories and is available as a health food and traditional medicine for anti-ageing, antioxidation, antibacterial use, antidepression, antitumor, lowering blood glucose and blood fat, etc. (Seo et al. Citation2013; Zhang et al. Citation2019). In addition, I. tenuipes has been used as folk medicine for strengthening the immune system and regaining energy in Japan, China, and South Korea, whereas it has been used as a tonic for recovery from tuberculosis and for speedy recovery after childbirth in the Himalayan region (Chhetri et al. Citation2020).
Cordycepin, produced by secondary metabolism in C. militaris and I. tenuipes, has a structure similar to adenosine () (Suparmin et al. Citation2017; Litwin et al. Citation2020). With age, the extracellular levels of adenosine decline (Tescarollo et al. Citation2020). The increase of adenosine content in human peripheral blood mononuclear cells after nicotinamide riboside supplementation has resulted in anti-ageing effects and health benefits (Jacobson and Reitman Citation2020). Since the chemical structure of cordycepin is similar to that of adenosine, it might inhibit adenosine-dependent processes (Litwin et al. Citation2020). Therefore, both C. militaris and I. tenuipes could have anti-ageing potential.
According to the chemical compositions and various biological activities of C. militaris and I. tenuipes, and accompanied with the ability to be cultivated in the laboratory, it is interesting to apply these fungi as active ingredients for anti-ageing. However, there are very few studies investigating the anti-skin ageing and anti-wrinkle activities of C. militaris and I. tenuipes extracts, this is the first study to investigate the anti-skin wrinkle activities of C. militaris and I. tenuipes extracts.
Materials and methods
Chemical materials
Analytical grade cordycepin (purity ≥98.0%), adenosine (purity ≥99.0%), l-ascorbic acid (purity ≥99.0%), epigallocatechin gallate (EGCG; purity ≥95.0%), oleanolic acid (purity ≥97.0%), sodium chloride (NaCl), sodium phosphate (Na3PO4), sodium dihydrogen phosphate (NaH2PO4), disodium phosphate (Na2HPO4), sodium carbonate (Na2CO3), tricine, tris base, elastase from porcine pancreas lyophilised powder (E–E.C.3.4.21.36), N-succinyl-Ala-Ala-Ala-p-nitroanilide (AAAPVN), metalloproteinase-1 (MMP-1) from Clostridium histolyticum (Weinberg and Seguin 1916) (ChC–EC.3.4.23.3), N-[3-(2-furyl)acryloyl]-Leu-Gly-Pro-Ala (FALGPA; purity ≥99.0%), hyaluronidase from bovine testes (E.C.3.2.1.3.5), hyaluronic acid, bovine serum albumin (BSA), and sodium lauryl sulphate (SLS) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Analytical grade ethanol, hexane, ethyl acetate, acetic acid, and dimethyl sulfoxide (DMSO) were purchased from Labscan (Dublin, Ireland). HPLC-grade methanol was purchased from Labscan (Dublin, Ireland).
Fungi materials
C. militaris and I. tenuipes, obtained fresh from the Mushroom Research and Development Centre (MRDC), Chiang Mai, Thailand, were identified by Prof. Dr. Chaiwat To-anun, an expert in fungal biodiversity at the Department of Entomology and Plant Pathology, Faculty of Agriculture, Chiang Mai University, Chiang Mai, Thailand. The fruiting bodies of C. militaris and I. tenuipes were separated from their mycelium and dried in a hot air oven (Memmert GmbH & Co. KG, Schwabach, Germany) set at 40 °C overnight until dry. The dried fungi were ground into fine powder using a Moulinex DB81 blender (Moulinex, Paris, France) and kept in a well-closed container until further use.
Extraction of C. militaris and I. tenuipes
C. militaris and I. tenuipes were extracted by infusion in boiling DI water. Briefly, 5 mL of boiling DI water was added to 1 g of dried fungi material and mixed for 5 min using a vortex mixer Genie 2 (Scientific Industries, NY, USA). The resulting mixture was then centrifuged at 6,000 rpm for 10 min at ambient temperature using a Thermo Fisher Scientific centrifuge (Sorvall ST16R, Waltham, MA, USA). Subsequently, the supernatant was collected and filtered through a 0.45 μm nylon syringe filter (Whatman Puradisc 25, USA). Four extracts were obtained, including C. militaris fruiting body extract (CF), C. militaris mycelium extract (CM), I. tenuipes fruiting body extract (IF), and I. tenuipes mycelium extract (IM). The colour of each extract was determined using the free online program from https://html-colour-codes.info/colors-from-image/. The extract was kept in the refrigerator until the further experiment.
Determination of chemical compositions of C. militaris and I. tenuipes extracts
Total phenolics content determination by the Folin–Ciocalteu method
The Folin–Ciocalteu method was used to assess the total phenolics content of C. militaris and I. tenuipes extracts, as previously described by Chaiyana et al. (Citation2017). Gallic acid was used as a standard compound and the total phenolic contents were expressed as milligrams per gram of gallic acid equivalents (GAE). Three independent experiments, repeated in triplicate, were performed.
Total flavonoid content determination by the aluminium chloride method
The aluminium chloride colorimetric method was used to assess the total flavonoid content of C. militaris and I. tenuipes extracts (Chaiyana et al. Citation2020). Quercetin was used as a standard compound and the total flavonoid content was expressed as quercetin equivalent (QE). Three independent experiments, repeated in triplicate, were performed.
Cordycepin and adenosine content determination by high performance liquid chromatography (HPLC)
HPLC (Hewlett Packard, Palo Alto, CA, USA) with C-18 reverse phase column (Zorbac Eclipse XDB-C18, 4.6150 mm, 0.5 m) and guard column (Zorbac Eclipse XDB-C18, 4.650 mm, 0.5 m) was used to evaluate the cordycepin and adenosine content of C. militaris and I. tenuipes extracts. To elute the sample, a mobile phase consisting of 5% methanol and 95% of DI water containing 0.05% w/v formic acid was used at a flow rate of 1 mL/min. The sample with an injection volume of 20 μL was detected by the UV detector set at a wavelength of 260 nm. The cordycepin and adenosine standard curves were plotted using a concentration of standard compounds varying from 1 to 150 µg/mL. The tests were carried out twice. Cordycepin content was calculated using the equation: x = (y + 2.6553)/6.3672 (R2 = 0.9998), where x is the cordycepin content and y is the area under the curve (AUC) of cordycepin peak detected around 7.4 min in the HPLC chromatogram. On the other hand, adenosine content was calculated using the equation: x = (y − 6.309)/5.9824 (R2 = 0.9976), where x is the adenosine content and y is the area under the curve (AUC) of adenosine peak detected around 5.9 min in the HPLC chromatogram.
Antioxidant activities determination
2,2′-Azinobis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay
ABTS assay was used to determine the ABTS•+ radical scavenging activity of C. militaris and I. tenuipes sample solutions (Chaiyana et al. Citation2019). The ABTS•+ scavenging activity was calculated using the equation: % ABTS•+ scavenging activity = [1 – (A/B)] × 100, where A is the UV absorbance of the mixture containing sample solution and B is the UV absorbance of the sample solution-free mixture. IC50 values were then calculated from the graph plotted inhibition percentage against the concentration of sample solution using the Graphpad/Prism program version 2.01 (Graphpad Software Inc., La Jolla, CA, USA). Ascorbic acid was used as a positive control. Three independent experiments, repeated in triplicate, were performed.
1,1-Diphenyl-2-picrylhydrazyl radical scavenging (DPPH) assay
DPPH assay was used to determine the DPPH• radical scavenging activity of C. militaris and I. tenuipes sample solutions (Chaiyana et al. Citation2019). The DPPH• scavenging activity was calculated using the equation: % DPPH• scavenging activity = [1 – (A/B)] × 100, where A is the UV absorbance of the mixture containing sample solution and B is the UV absorbance of the sample solution-free mixture. IC50 values were then calculated from the graph plotted inhibition percentage against the concentration of sample solution using the Graphpad/Prism program version 2.01 (Graphpad Software Inc., La Jolla, CA, USA). Ascorbic acid was used as a positive control. Three independent experiments, repeated in triplicate, were performed.
Ferric reducing antioxidant power (FRAP) assay
FRAP assay was used to determine the ferric reducing antioxidant power of C. militaris and I. tenuipes sample solutions (Chaiyana et al. Citation2017). FeSO4 was used as a standard compound and the ferric reducing antioxidant power was expressed as equivalent concentration (EC1) representing the concentration of sample solution with a reducing effect equivalent to 1 mM FeSO4. Three independent experiments, repeated in triplicate, were performed.
Ferric thiocyanate (FTC) assay
FTC assay was used to determine the inhibitory activity on lipid peroxidation of C. militaris and I. tenuipes sample solutions (Osawa and Namiki Citation1981). The lipid peroxidation inhibition was calculated using the equation: % lipid peroxidation inhibition = [1 – (A/B)] × 100, where A is the UV absorbance of the mixture containing sample solution and B is the UV absorbance of the sample solution-free mixture. IC50 values were then calculated from the graph plotted inhibition percentage against the concentration of sample solution using the Graphpad/Prism program version 2.01 (Graphpad Software Inc., La Jolla, CA, USA). Trolox was used as a positive control. Three independent experiments, repeated in triplicate, were performed.
Anti-skin ageing activities determination
Determination of matrix metalloproteinase-1 (MMP-1) inhibition
The spectrophotometric method was used to investigate the inhibitory activity against MMP-1 of C. militaris and I. tenuipes sample solutions (Thring et al. Citation2009; Chaiyana et al. Citation2019). The MMP-1 inhibition was calculated using the equation: % MMP-1 inhibition = [1 – (A/B)] × 100, where A is the UV absorbance of the mixture containing sample solution and B is the UV absorbance of the sample solution-free mixture. IC50 values were then calculated from the graph plotted inhibition percentage against the concentration of sample solution using the Graphpad/Prism program version 2.01 (Graphpad Software Inc., La Jolla, CA, USA). Oleanolic acid was used as a positive control. Three independent experiments, repeated in triplicate, were performed.
Elastase enzyme inhibition
The spectrophotometric method was used to investigate the inhibitory activity against elastase of C. militaris and I. tenuipes sample solutions (Thring et al. Citation2009; Chaiyana et al. Citation2019). The elastase inhibition was calculated using the following equation: % Elastase inhibition = [1 – (A/B)] × 100, where A is the UV absorbance of the mixture containing sample solution and B is the UV absorbance of the sample solution-free mixture. IC50 values were then calculated from the graph plotted inhibition percentage against the concentration of sample solution using the Graphpad/Prism program version 2.01 (Graphpad Software Inc., La Jolla, CA, USA). Epigallocatechin gallate (EGCG) was used as a positive control. Three independent experiments, repeated in triplicate, were performed.
Hyaluronidase inhibition
The spectrophotometric method was used to investigate the inhibitory activity against hyaluronidase of C. militaris and I. tenuipes sample solutions (Nema et al. Citation2011). The hyaluronidase inhibition was calculated using the equation: % Hyaluronidase inhibition = [1 – (A/B)] × 100, where A is the UV absorbance of the mixture containing sample solution and B is the UV absorbance of the sample solution-free mixture. Oleanolic acid was used as a positive control. Three independent experiments, repeated in triplicate, were performed.
Antityrosinasse activities determination
The spectrophotometric method was used to investigate the inhibitory activity against tyrosinase activity of C. militaris and I. tenuipes sample solutions (Saeio et al. Citation2011). The tyrosinase inhibition was calculated using the equation: % Tyrosinase inhibition = [1 – (A/B)] × 100, where A is the UV absorbance of the mixture containing sample solution and B is the UV absorbance of the sample solution-free mixture. Kojic acid was used as a positive control. Three independent experiments, repeated in triplicate, were performed.
Statistical analysis
The results from each triplicate experiment were expressed as mean ± standard deviation (S.D.) and their comparisons were analysed by using analysis of variance (ANOVA), followed by a Tukey post hoc test. Pearson correlation analysis was applied to determine the relationship between the chemical constituents of the fungi and their antioxidant activities. A p-value of less than 0.05 indicates statistical significance. The rule of thumb for interpreting the size of a correlation coefficient used was: correlation 0.90 to 1.00 (−0.90 to −1.00) as very high positive (negative) correlation; 0.70 to 0.90 (−0.70 to −0.90) as high positive (negative) correlation; 0.50 to 0.70 (−0.50 to −0.70) as moderate positive (negative) correlation; 0.30 to 0.50 (−0.30 to −0.50) as low positive (negative) correlation; and 0.00 to 0.30 (−0.00 to −0.30) as negligible correlation (Mishra et al. Citation2018).
Results and discussion
C. Militaris and I. tenuipes extracts
Both fruiting body and mycelium of C. militaris and I. tenuipes could be extracted using boiling DI water. The external appearance of each extract is shown in . The orange-brown colour of the extracts from the fruiting body and mycelium of C. militaris is similar. This colour was known to be generated by photoinduced carotenogenesis, which resulted in the production of a variety of carotenoid pigments, including lutein, zeaxanthin, cordyxanthins, and cordycepene (Shrestha et al. Citation2006; Dong et al. Citation2013). These pigments have been identified as primary compounds responsible for the orange-brown colour of both the fruiting body and the mycelium of C. militaris (Shrestha et al. Citation2006). On the other hand, the extract from I. tenuipes had a darker colour. Moreover, the extract from the fruiting body of I. tenuipes was noticeably darker than the mycelium part as shown in ; the reasons might be due to the production of pink, red, and reddish-brown pigments from Isaria spp. (Velmurugan et al. Citation2010). The results are in obvious accordance with the shades of water-soluble pigment generated by Isaria spp. previously reported by Velmurugan et al. (Citation2010).
Figure 2. The external appearance of C. militaris fruiting body extract (CF), C. militaris mycelium extract (CM), I. tenuipes fruiting body extract (IF), and I. tenuipes mycelium extract (IM).

Table 1. Hex colour code, RGB colour code model, and CMYK colour code model of extracts from C. militaris and I. tenuipes.
Chemical compositions of C. militaris and I. tenuipes extracts
HPLC chromatograms as shown in reveals the presence of cordycepin (peak detected around 7.4 min) and adenosine (peak detected around 5.9 min) in C. militaris and I. tenuipes extracts. The chemical compositions of the extracts are shown in . The fruiting body of both C. militaris and I. tenuipes contained significantly higher phenolic compounds than their mycelium part (p < 0.05). In contrast, the total flavonoids and cordycepin content were found to be identical in the fruiting body and mycelium. Adenosine content was also similar in the fruiting body and mycelium of I. tenuipes, but significantly higher in the C. militaris fruiting body compared to its mycelium part.
Figure 3. HPLC chromatograms of C. militaris fruiting body extract (CF) (a), C. militaris mycelium extract (CM) (b), I. tenuipes fruiting body extract (IF) (c), I. tenuipes mycelium extract (IM) (d).
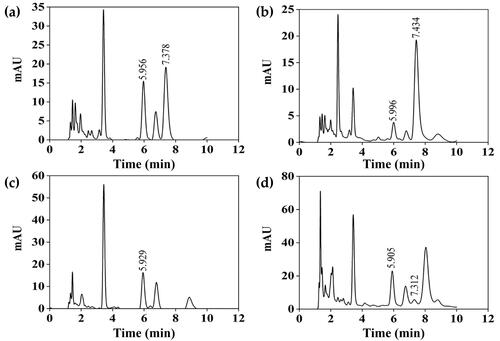
Table 2. Total phenolics, total flavonoids, cordycepin, and adenosine content of C. militaris and I. tenuipes extracts.
Despite the fact that no prior studies had compared the phenolic content of the fruiting body and mycelium parts of C. militaris and I. tenuipes, the results were consistent with the study of Ganoderma lucidum (FR.) Karst (Ganodermataceae) (Chandrasekaran et al. Citation1978). Heleno et al. (Citation2012) observed that the fruiting body of G. lucidum contained the highest phenolics content of 28.64 mg GAE/g extract, which was significantly higher than that found in mycelium grown in any culture medium, including solid Melin–Norkans medium (14.22 ± 0.29 mg GAE/g extract), liquid Melin–Norkans medium (6.03 ± 0.98 mg GAE/g extract), and Potato Dextrose Agar medium (15.19 ± 0.59 mg GAE/g extract). Mishra et al. (Citation2018) also reported that the fruiting body of G. lucidum had the highest phenolics content of 4.13 μg GAE/g extract compared to its mycelium part. In contrast, the mycelium part of Coprinus comatus (O.F. Müll.) Pers. (Agaricaceae) and Coprinellus truncorum (Scop.) Redhead, Vilgalys & Monclavo (Psathyrellaceae) contained significantly higher phenolic content compared to the fruiting body (Tešanović et al. Citation2017). Therefore, the different distribution of phenolic compounds in each part of the fungus depended on the varying bioactive compound synthesis mechanism of each fungus species (Park et al. Citation2013).
In contrast to the phenolics content, flavonoid content was not significantly different in the fruiting body and the mycelium part of both C. militaris and I. tenuipes (). Findings were consistent with those of Carvajal et al. (Citation2012), who observed no significant differences in flavonoid content in the fruiting body (1.8 ± 0.16 mg catechin/g extract), young mycelia (2.1 ± 0.20 mg catechin/g extract), and old mycelia (2.3 ± 0.20 mg catechin/g extract) of Agaricus brasiliensis Wasser. (Agaricaceae) On the other hand, a significant flavonoid content has been reported in several edible mushrooms (Barros et al. Citation2007). Flavonoids were found to be significantly more abundant than phenolics in all extracts in the present study (p < 0.05). The findings were consistent with those of Zhu et al. (Citation2013), who found that the flavonoid content of both fresh and dry C. militaris fruiting bodies (25.74 ± 1.14 and 16.34 ± 1.23 mg rutin/g extract) was significantly higher than that of phenolics (1.93 ± 0.08 and 1.85 ± 0.07 mg gallic acid/g extract).
Similar to the flavonoid content, cordycepin was present in a comparable amount in both the fruiting body and the mycelium part of C. militaris (). However, the cordycepin distribution in the fruiting body or mycelium of C. militaris was variable. In some cases, cordycepin was higher in the fruiting body (2.654 ± 0.02 mg/g) compared to the mycelium (0.9040 ± 0.02 mg/g) (Huang et al. Citation2009), but in another case, mycelial biomass of C. militaris had higher cordycepin content (0.182 ± 0.08% w/w) than that in the fruiting body (0.110 ± 0.03% w/w) (Chan et al. Citation2015). On the other hand, no cordycepin was detected in I. tenuipes in the present study. This was consistent with the previous report noting that cordycepin was not detected in I. tenuipes but was considered a bioactive component of Cordyceps spp. and some Isaria spp., such as Isaria cicadae Miquel (Zhang et al. Citation2019).
Adenosine, a crucial parameter for Cordyceps quality control, was found to be higher in the fruiting body than the mycelium part (). The results were in accordance with the study of Huang et al. (Citation2009) reported that the fruiting body of C. militaris contained higher adenosine (2.45 ± 0.03 mg/g) than the mycelium (1.592 ± 0.03 mg/g) (Zhu et al. Citation2013). On the other hand, comparable adenosine was detected in the fruiting body and mycelium of I. tenuipes (). Although there have been few studies comparing the adenosine content of I. tenuipes fruiting body and mycelium, the fruiting body has been previously reported to contain adenosine (Kang et al. Citation2010; Ji et al. Citation2011; Pham et al. Citation2020).
Antioxidant activities of C. militaris and I. tenuipes extracts
The extracts of C. militaris and I. tenuipes inhibited ABTS•+, DPPH•, and lipid peroxidation in a dose-dependent manner () and the dose-response curve was used to quantify the IC50 value as presented in . IF was an extract with extraordinary antioxidant activities via radical scavenging activity, reducing ability, and prevention of lipid peroxidation. The most distinguished antioxidant activity of IF was via reducing ability since the EC1 value of IF was about three times higher than that of ascorbic acid, a well-known natural antioxidant.
Figure 4. Dose-response curve on the inhibition of ABTS•+ (a), DPPH• (b), and lipid peroxidation (c) of ascorbic acid (AS), Trolox (TX), C. militaris fruiting body extract (CF), C. militaris mycelium extract (CM), I. tenuipes fruiting body extract (IF), and I. tenuipes mycelium extract (IM).
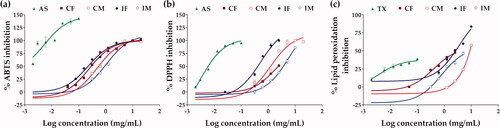
Table 3. Antioxidant activities of C. militaris and I. tenuipes extracts.
In contrast to I. tenuipes, the differences in antioxidant activity between the fruiting body and the mycelium of C. militaris were observed in the present study. This is well related to previous research reporting that the fruiting body and mycelium of C. militaris had their own role and worked in different ways. The mycelium of C. militaris exhibited stronger chelating ability, reducing power, and total antioxidant capacity than the fruiting body, which have been shown to have higher DPPH• radical scavenging activity (Dong et al. Citation2014).
The relationship between the chemical constituents and antioxidant activities of the extracts as shown in indicated adenosine content as a factor strongly affecting the lipid peroxidation inhibitory activity (p < 0.0006). The higher adenosine content resulted in a lower IC50 value, indicating more potent inhibitory activity since a lower concentration was needed for 50% inhibitory activity. The results were related to those of a previous study, which found that adenosine could interact with adenosine receptors (A1 and A3AR), causing the antioxidant enzymes to be activated and resulting in a reduction in lipid peroxidation in cells (Maggirwar et al. Citation1994; Ford et al. Citation1997). Since an increase in lipid peroxidation end products, such as lipid alkoxyl (LO•) and lipid peroxyl (LOO•) radicals, is the most widely cited evidence for free radical involvement in human disease, the extracts which possessed strong lipid peroxidation inhibition would be beneficial for human’s health (Ayala et al. Citation2014).
Table 4. The relationship between the chemical constituents and antioxidant activities of C. militaris and I. tenuipes extracts.
On the other hand, the findings from this study indicated phenolic compounds as the major chemical constituents responsible for the radical scavenging activities of the extracts since the total phenolic content possessed a high correlation with the ABTS•+ inhibition (r = −0.8613) and moderate correlation with the DPPH• inhibition (r = −0.6558) (). The negative correlations revealed that higher phenolics content was associated with a lower IC50 value, suggesting more potent radical scavenging activity because a lower concentration was required for the 50% inhibition. The findings were in good agreement with previous research indicating that phenolic compounds are essential chemical constituents that facilitate free radical scavenging through their hydroxyl groups (Soobrattee et al. Citation2005).
Furthermore, flavonoids were found to be responsible for the reducing abilities with a moderate correlation (r = 0.8842) in the present study (); related to a previous report that found flavonoids to be capable of reducing Fe3+ to Fe2+ (Mira et al. Citation2002). Since the presence of high-reducing-capacity reductants is highly correlated with potential antioxidant activity (Olugbami et al. Citation2014), IF with the highest EC1 value would be a potent antioxidant.
Anti-wrinkle activities of C. militaris and I. tenuipes extracts
Skin ageing naturally occurs from a decline in the extracellular matrix (ECM) in the dermis, including collagen fibres, elastin fibres, and hyaluronan by matrix metalloproteinase-1 (MMP-1), elastase, and hyaluronidase, respectively (Maity et al. Citation2011; Kim et al. Citation2019). Therefore, the inhibition of these enzymes can slow ECM deterioration and delay the appearance of wrinkles on the skin. depicts the inhibitory activity of C. militaris and I. tenuipes extracts on MMP-1, elastase, and hyaluronidase. The extracts of C. militaris and I. tenuipes inhibited MMP-1 and elastase in a dose-dependent manner and their IC50 value is shown in . CF was found to be the most effective inhibitor of MMP-1, while IF was found to be the most effective inhibitor of elastase (p < 0.05). Although the bioassay results indicated CF as the most effective inhibitor to MMP-1 in all extracts, its inhibitory activity was not very potent when compared with the positive control (oleanolic acid). The MMP-1 inhibitory effect of oleanolic acid was about 1,000 times more potent than CF. On the other hand, the elastase inhibitory effect of IF was close to the positive control (EGCG). Only a small amount of IF was required to inhibit the activity of elastase as its IC50 was very low (0.006 ± 0.004 mg/mL). Moreover, the inhibitory effect on elastase of EGCG was only six times more potent than IF, which was considered to be not significantly different (p > 0.05). Therefore, IF was worth using as an elastase inhibitor, whereas, CF was not worth using as an MMP-1 inhibitor since it required a large amount of high concentration of formulation. The present study is the first to reveal anti-elastase of C. militaris and I. tenuipes extracts. Apart from the extreme effectiveness of IF on elastase inhibition, equalling the effectiveness of EGCG, a natural elastase inhibitor (Andrade et al. Citation2021), IF was the strongest hyaluronidase inhibitor compared to the others (p < 0.05). Therefore, IF was suggested as a potent natural extract, which exerted anti-wrinkle activities.
Figure 5. Dose-response curve on the inhibition of collagenase (a) and elastase (b), and hyaluronidase inhibition (c) f oleanolic acid (OA), epigallocatechin gallate (EGCG), C. militaris fruiting body extract (CF), C. militaris mycelium extract (CM), I. tenuipes fruiting body extract (IF), and I. tenuipes mycelium extract (IM). The letter a, b, c, and d denote significant differences among samples at p < 0.05.
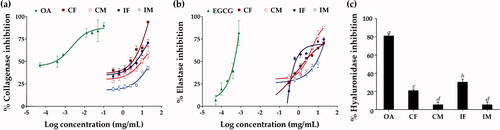
Table 5. Anti-wrinkle activities of C. militaris and I. tenuipes extracts.
Previous studies on the MMP-1 inhibitory activity of C. militaris and I. tenuipes extracts indicated cordycepin and adenosine as main components that inhibited the activator protein-1 and suppressed MMP-1 expression (Noh et al. Citation2009, Citation2010). Cordycepin has a poor correlation with MMP-1 inhibition, while adenosine has a negligible correlation (). Phenolic compounds were found to be strongly correlated with the inhibitory activities of not only MMP-1 but also elastase and hyaluronidase.
Table 6. The relationship between the chemical constituents and anti-ageing activities of C. militaris and I. tenuipes extracts.
On the other hand, flavonoids have been found to be strongly affected by the hyaluronidase inhibitory activity; due to changes in the microenvironment and hyaluronidase conformation caused by the presence of flavonoids (Smiderle et al. Citation2014). It has been proposed that placing hyaluronan in topical skincare products or using it as a temporary dermal filling agent could slow down hyaluronan degradation and increase hyaluronan levels in the skin layer, resulting in plumping and youthful skin being achieved (Yusuf et al. Citation2021). IF, a hyaluronidase inhibitor, is another natural extract that can help to slow the skin ageing process.
Antityrosinasse activities of C. militaris and I. tenuipes extracts
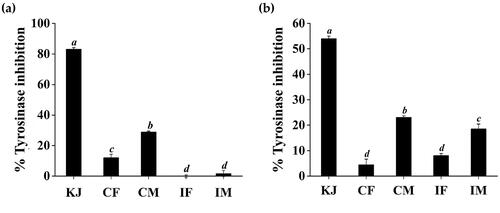
Tyrosinase, an enzyme involved in the mechanism of melanogenesis, is found in mammals, plants, and microorganisms. Tyrosinase is also a primary rate-limiting enzyme that catalyses enzyme browning and melanin synthesis. Tyrosinase also exhibits monophenolase and diphenolase activities, which catalyse the hydroxylation of l-tyrosine to L-DOPA and the oxidation of L-DOPA to dopaquinone, which can then be polymerised in a nonenzymatic way to produce dark pigments (Cui et al. Citation2018). Tyrosinase inhibitors can be used for skin whitening and have been widely used in cosmetics. Kojic acid is a metabolite of fungal that exhibits inhibitory activity towards tyrosinase. The inhibitory effects of kojic acid in this context have been attributed to its ability to chelate copper at the active site of this enzyme. Thus, kojic acid is often used as a positive control to tyrosinase inhibitors. Anti-tyrosinase activities of C. militaris and I. tenuipes extracts were analysed using l-tyrosine and L-DOPA as a substrate with kojic acid as a reference compound (). It was remarked that CM possessed significantly higher tyrosinase inhibitory activity than CF, IF, and IM (p < 0.05). The tyrosinase inhibitory activity of CM was 28.9 ± 0.6% and 23.1 ± 0.6% when a substrate was l-tyrosine and L-DOPA, respectively. However, the inhibitory effects of all extracts were less than that with kojic acid.
Figure 6. Inhibitory activities against tyrosinase when the substrates were l-tyrosine (a) and L-DOPA (b) of kojic acid (KJ), C. militaris fruiting body extract (CF), C. militaris mycelium extract (CM), I. tenuipes fruiting body extract (IF), and I. tenuipes mycelium extract (IM). The letter a, b, c, and d denote significant differences among samples at p < 0.05.
Conclusions
Extracts from C. militaris and I. tenuipes contained a wide range of biologically active compounds, including phenolics, flavonoids, and adenosine, whereas, cordycepin was rarely detected in I. tenuipes. Although the chemical profiles of C. militaris have previously been reported, the present study focussed on the comparison between different parts, i.e., mycelium and fruiting body of C. militaris, as well as the comparison between different fungi, i.e., C. militaris and I. tenuipes. The findings highlighted that the chemicals varied in the fruiting body and mycelium part. The phenolics and flavonoids in the fruiting body were considerably higher than in the mycelium. Phenolic compounds were found to be moderately correlated with the radical scavenging activities and strongly correlated with the inhibitory activities of MMP-1, elastase, and hyaluronidase. Flavonoids were shown to have a moderate correlation with reducing ability and had a significant impact on hyaluronidase inhibitory function. Adenosine was strongly affected by the lipid peroxidation inhibitory activity (p < 0.0006). Apart from the compounds investigated in the present study, other hydrophilic compounds in the extracts may also be responsible for the bioactivities. Therefore, the fractionation of crude extracts to identify key compounds is suggested for further study. IF (I. tenuipes) was highlighted as an extract with extraordinary elastase and hyaluronidase inhibitory activities. Furthermore, IF was a strong antioxidant with radical scavenging activity, reducing ability, and lipid peroxidation prevention. Surprisingly, IF had a three-times higher EC1 value than ascorbic acid and significantly higher elastase inhibition than EGCG. Therefore, IF is proposed as a powerful natural extract with antioxidant and anti-wrinkle properties.
Acknowledgments
The authors gratefully acknowledge C. militaris support materials received from the Mushroom Research and Development Center (MRDC), Chiang Mai, Thailand. This research was funded by Research and Researchers for Industries (RRi), grant number MSD62I0068. The APC was funded by the Innovation Center for Holistic Health, Nutraceuticals, and Cosmeceuticals, Faculty of Pharmacy, Chiang Mai University, Thailand and the Research Center of Pharmaceutical Nanotechnology, Research Center of Pharmaceutical Nanotechnology, Chiang Mai University, Thailand. Pachabadee Marsup is grateful to the Graduate School at Chiang Mai University for the Teaching Assistance/Research Assistance (TA/RA) Scholarship for the academic year 2018. Suwannee Sriyab is grateful to the CMU Presidential Scholarship 2020 for a Post-Doctoral researcher. Adchara Prommaban is grateful to the CMU Presidential Scholarship 2021 for a Post-Doctoral researcher.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Andrade JM, Domínguez-Martín EM, Nicolai M, Faustino C, Rodrigues LM, Rijo P. 2021. Screening the dermatological potential of Plectranthus species components: antioxidant and inhibitory capacities over elastase, collagenase and tyrosinase. J Enzyme Inhib Med Chem. 36(1):257–269.
- Ayala A, Muñoz MF, Argüelles S. 2014. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014:360438.
- Barros L, Calhelha RC, Vaz JA, Ferreira IC, Baptista P, Estevinho LM. 2007. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur Food Res Technol. 225(2):151–156.
- Carvajal AES, Koehnlein EA, Soares AA, Eler GJ, Nakashima AT, Bracht A, Peralta RM. 2012. Bioactives of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. LWT- Food Sci Technol. 46(2):493–499.
- Chaiyana W, Anuchapreeda S, Punyoyai C, Neimkhum W, Lee K-H, Lin W-C, Lue S-C, Viernstein H, Mueller M. 2019. Ocimum sanctum Linn. As a natural source of skin anti-ageing compounds. Ind Crops Prod. 127:217–224.
- Chaiyana W, Chansakaow S, Intasai N, Kiattisin K, Lee KH, Lin W, Lue SC, Leelapornpisid P. 2020. Chemical constituents, antioxidant, anti-MMPs, and anti-hyaluronidase activities of Thunbergia laurifolia Lindl. leaf extracts for skin aging and skin damage prevention. Molecules. 25(8):1923.
- Chaiyana W, Punyoyai C, Somwongin S, Leelapornpisid P, Ingkaninan K, Waranuch N, Srivilai J, Thitipramote N, Wisuitiprot W, Schuster R, et al. 2017. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex Vaucher extract as functional food and nutraceuticals ingredients. Nutrients. 9(10):1105.
- Chan JSL, Barseghyan GS, Asatiani MD, Wasser SP. 2015. Chemical composition and medicinal value of fruiting bodies and submerged cultured mycelia of caterpillar medicinal fungus Cordyceps militaris CBS-132098 (Ascomycetes). Int J Med Mushrooms. 17(7):649–659.
- Chandrasekaran SK, Benson H, Urquhart J. 1978. Methods to achieve controlled drug delivery: the biomedical engineering approach. In: Robinson JR, ed. Sustained and Controlled Release Drug Delivery Systems. New York: Marcel Dekker. p. 557–593.
- Chhetri DR, Chhetri A, Shahi N, Tiwari S, Karna SKL, Lama D, Pokharel YR. 2020. Isaria tenuipes Peck, an entomopathogenic fungus from Darjeeling Himalaya: evaluation of in-vitro antiproliferative and antioxidant potential of its mycelium extract. BMC Complement Med Ther. 20(1):14.
- Cui H, Duan F, Jia S, Cheng F, Yuan K. 2018. Antioxidant and tyrosinase inhibitory activities of seed oils from Torreya grandis Fort. ex Lindl. Biomed Res Int. 2018:5314320.
- Dong C, Yang T, Lian T. 2014. A comparative study of the antimicrobial, antioxidant, and cytotoxic activities of methanol extracts from fruit bodies and fermented mycelia of caterpillar medicinal mushroom Cordyceps militaris (Ascomycetes). Int J Med Mushrooms. 16(5):485–495.
- Dong JZ, Wang SH, Ai XR, Yao L, Sun ZW, Lei C, Wang Y, Wang Q. 2013. Composition and characterization of cordyxanthins from Cordyceps militaris fruit bodies. J Funct Foods. 5(3):1450–1455.
- Ford MS, Nie Z, Whitworth C, Rybak LP, Ramkumar V. 1997. Up-regulation of adenosine receptors in the cochlea by cisplatin. Hear Res. 111(1–2):143–152.
- Heleno SA, Barros L, Martins A, Queiroz MJR, Santos-Buelga C, Ferreira IC. 2012. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: a comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res Int. 46(1):135–140.
- Huang L, Li Q, Chen Y, Wang X, Zhou X. 2009. Determination and analysis of cordycepin and adenosine in the products of Cordyceps spp. Afr J Microbiol Res. 3:57–961.
- Jacobson KA, Reitman ML. 2020. Adenosine-related mechanisms in non-adenosine receptor drugs. Cells. 9(4):956.
- Ji SD, Sung GB, Kang PD, Kim KY, Choi YS, Kim NS, Woo SO, Han SM, Hong IP, Ha NG. 2011. Synnemata production using silkworm variety, female Yangwonjam by Isaria tenuipes. Mycobiology. 39(3):158–163.
- Kang PD, Sung GB, Kim KY, Kim MJ, Hong IP, Ha NG. 2010. Breeding of a silkworm variety for synnemata production of Isaria tenuipes. Mycobiology. 38(3):180–183.
- Kim DJ, Chang SS, Lee J. 2019. Anti-aging potential of substance P-based hydrogel for human skin longevity. IJMS. 20(18):4453.
- Litwin A, Nowak M, Różalska S. 2020. Entomopathogenic fungi: unconventional applications. Rev Environ Sci Biotechnol. 19(1):23–42.
- Maggirwar SB, Dhanraj DN, Somani SM, Ramkumar V. 1994. Adenosine acts as an endogenous activator of the cellular antioxidant defense system. Biochem Biophys Res Commun. 201(2):508–515.
- Maity N, Nema NK, Abedy MK, Sarkar BK, Mukherjee PK. 2011. Exploring Tagetes erecta Linn flower for the elastase, hyaluronidase and MMP-1 inhibitory activity. J Ethnopharmacol. 137(3):1300–1305.
- Marsup P, Yeerong K, Neimkhum W, Sirithunyalug J, Anuchapreeda S, To-Anun C, Chaiyana W. 2020. Enhancement of chemical stability and dermal delivery of Cordyceps militaris extracts by nanoemulsion. Nanomaterials. 10(8):1565.
- Mira L, Tereza Fernandez M, Santos M, Rocha R, Helena Florêncio M, Jennings KR. 2002. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 36(11):1199–1208.
- Mishra J, Joshi A, Rajput R, Singh K, Bansal A, Misra K. 2018. Phenolic rich fractions from mycelium and fruiting body of Ganoderma lucidum inhibit bacterial pathogens mediated by generation of reactive oxygen species and protein leakage and modulate hypoxic stress in HEK 293 cell line. Adv Pharmacol Sci. 20182018. :6285615.
- Moon BC, Kim WJ, Park I, Sung GH, Noh P. 2018. Establishment of a PCR assay for the detection and discrimination of authentic Cordyceps and adulterant species in food and herbal medicines. Molecules. 23(8):1932.
- Nema NK, Maity N, Sarkar B, Mukherjee PK. 2011. Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Arch Dermatol Res. 303(4):247–252.
- Ng TB, Wang HX. 2005. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol. 57(12):1509–1519.
- Noh E-M, Youn HJ, Jung SH, Han J-H, Jeong Y-J, Chung E-Y, Jung J-Y, Kim B-S, Lee S-H, Lee Y-R, et al. 2010. Cordycepin inhibits TPA-induced matrix metalloproteinase-9 expression by suppressing the MAPK/AP-1 pathway in MCF-7 human breast cancer cells. Int J Mol Med. 25(2):255–260.
- Noh E-M, Kim J-S, Hur H, Park B-H, Song E-K, Han M-K, Kwon K-B, Yoo W-H, Shim I-K, Lee SJ, et al. 2009. Cordycepin inhibits IL-1beta-induced MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts. Rheumatology. 48(1):45–48.
- Nxumalo W, Elateeq AA, Sun Y. 2020. Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis?–a review. J Ethnopharmacol. 257:112879.
- Olugbami JO, Gbadegesin MA, Odunola OA. 2014. In vitro evaluation of the antioxidant potential, phenolic and flavonoid contents of the stem bark ethanol extract of Anogeissus leiocarpus. Afr. J Med Health Sci. 43(Suppl 1):101–109.
- Osawa T, Namiki M. 1981. A novel type of antioxidant isolated from leaf wax of Eucalyptus leaves. Agric Biol Chem. 45(3):735–739.
- Park SJ, Hyun SH, Suh HW, Lee SY, Sung GH, Kim SH, Choi HK. 2013. Biochemical characterization of cultivated Cordyceps bassiana mycelia and fruiting bodies by 1H nuclear magnetic resonance spectroscopy. Metabolomics. 9(1):236–246.
- Pham TMN, Ha TN, Nguyen VKD, Nguyen TH, Nguyen C, Dinh MH, Ngo KS. 2020. The cytotoxic activity of extracts from the biomass and fruit bodies of Isaria tenuipes VHI-2 fungus on MCF-7 cancer cell line. Technologies. 16:907–916.
- Saeio K, Chaiyana W, Okonogi S. 2011. Antityrosinase and antioxidant activities of essential oils of edible Thai plants. Drug Discov Ther. 5(3):144–149.
- Seo DS, Kang JK, Jeong MH, Kwon M, Park CB. 2013. Anti-diabetic effects of Isaria tenuipes in OLETF rats as an animal model of diabetes mellitus type II. J Food Hyg Saf. 28(2):152–157.
- Shrestha B, Lee WH, Han SK, Sung JM. 2006. Observations on some of the mycelial growth and pigmentation characteristics of Cordyceps militaris isolates. Mycobiology. 34(2):83–91.
- Smiderle FR, Baggio CH, Borato DG, Santana-Filho AP, Sassaki GL, Iacomini M, Van Griensven LJ. 2014. Anti-inflammatory properties of the medicinal mushroom Cordyceps militaris might be related to its linear (1→3)-β-D-glucan. PLOS One. 9(10):e110266.
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. 2005. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res. 579(1-2):200–213.
- Suparmin A, Kato T, Dohra H, Park EY. 2017. Insight into cordycepin biosynthesis of Cordyceps militaris: comparison between a liquid surface culture and a submerged culture through transcriptomic analysis. PLOS One. 12(11):e0187052.
- Tešanović K, Pejin B, Šibul F, Matavulj M, Rašeta M, Janjušević L, Karaman MA. 2017. A comparative overview of antioxidative properties and phenolic profiles of different fungal origins: fruiting bodies and submerged cultures of Coprinus comatus and Coprinellus truncorum. J Food Sci Technol. 54(2):430–438.
- Tescarollo FC, Rombo DM, DeLiberto LK, Fedele DE, Alharfoush E, Tomé ÂR, Cunha RA, Sebastião AM, Boison D. 2020. Role of adenosine in epilepsy and seizures. J Caffeine Adenosine Res. 10(2):45–60.
- Thring TS, Hili P, Naughton DP. 2009. Anti-collagenase, anti-elastase, and anti-oxidant activities of extracts from 21 plants. BMC Complement Med Ther. 9:27.
- Velmurugan P, Kamala-Kannan S, Balachandar V, Lakshmanaperumalsamy P, Chae JC, Oh BT. 2010. Natural pigment extraction from five filamentous fungi for industrial applications and dyeing of leather. Carbohydr Polym. 79(2):262–268.
- Yusuf L, Girsang E, Nasution AN, Elvira C, Wibowo SHB, Widowati W. 2021. H2O2-scavenging activity and hyaluronidase inhibition scutellarein and apigenin in Ocimum basilicum extract. JKB. 31(3):133–138.
- Zhang X, Hu Q, Weng Q. 2019. Secondary metabolites (SMs) of Isaria cicadae and Isaria tenuipes. RSC Adv. 9(1):172–184.
- Zhu SJ, Pan J, Zhao B, Liang J, Ze-Yu W, Yang JJ. 2013. Comparisons on enhancing the immunity of fresh and dry Cordyceps militaris in vivo and in vitro. J Ethnopharmacol. 149(3):713–719.