Abstract
Background. We sought to determine whether descending thoracic aortic occlusion (DTAOC) induced ischemia results in adrenal dysfunction. Methods. Eight pigs underwent DTAOC for 45 min. Six control pigs underwent a sham procedure. Serum cortisol and adrenocorticotropic hormone (ACTH) were measured at baseline, at the end of DTAOC, 30 and 60 min after restoration of flow, and 24 hours later. Statistical analysis was performed using repeated measures ANOVA and t-test. Results. In the study group, cortisol levels decreased during DTAOC (p=0.048) and 30 min after flow restoration (p=0.004). In the control group there was no change in serum cortisol levels. In the study group the drop in serum cortisol was associated with an increase in ACTH levels during DTAOC (p=0.040) and 30 minutes after flow restoration (p=0.070). The increase in ACTH was also significant when compared to the controls during DTAOC (p=0.030) and 30 min after blood flow restoration (p=0.040). Conclusions. There is a transient period of adrenal dysfunction associated with DTAOC that results in stimulation of the pituitary-adrenal axis.
Temporary aortic occlusion (AOC) in the descending thoracic (DT) aorta is utilized during operations on the thoracoabdominal aorta for both aneurysmal and occlusive disease and less frequently during surgery for trauma. Despite major advances in anesthetic and operative management these procedures carry considerable risk for morbidity and mortality Citation1. Major complications after temporary DT AOC are the result of ischemia of the vital organs including the spinal cord with paraparesis/paraplegia, the kidneys with renal insufficiency/failure, and the viscera with liver failure, ischemic enterocolitis or the development of coagulopathy Citation2.
One of the least studied aspects of DT AOC is its effect on adrenal function. The interruption of blood flow during DT AOC subjects the adrenal glands to a period of warm ischemia. This ischemia results in distal sympathoadrenal activation with release of catecholamines during and after DT AOC Citation3. Less is known about the effect of DT AOC on the adrenal cortex. Prolonged ischemia of the adrenal gland leads to focal infarcts in the zonae glomerulata and fascicularis, but whether these infarcts interfere with normal function of the gland is, to the best of our knowledge, not known Citation4.
The purpose of the present study was to examine the response of the adrenal-pituitary axis to DT AOC.
Materials and methods
Experimental protocol
Fourteen healthy market pigs weighting between 25–35 kg were used in the study. After sedation with intramuscular (IM) injection of ketamine (35 mg/kg IM), ace promazine (1.1 mg/kg IM) and atropine (0.04 mg/kg IM) an intravenous catheter was placed in an ear vein. The animal was then given a 10 mg intravenous dose of diazepam to facilitate endotracheal intubation. General anesthesia was maintained using of 1–2% isofluorane with a 5cm H2O Positive End Expiratory Pressure. A one lead ECG was used to monitor heart rate. All animals were placed on a warming blanket and rectal temperature was maintained at 36.0°C to 37.1°C. A left neck cutdown was performed and a 9 Fr pulmonary artery catheter was inserted through the external jugular vein, to ensure optimal hemodynamic monitoring and guide fluid resuscitation during the experiment. An 11 Fr introducer was inserted in the carotid artery. An arterial line was connected to the introducer to allow continuous proximal blood pressure measurement. A separate cutdown was performed in the groin to identify the femoral artery. An 11 Fr introducer was placed in the femoral artery and was connected to an arterial line for continuous distal blood pressure monitoring. Through the carotid artery sheath an aortic occlusion balloon catheter was placed under fluoroscopic guidance in the distal descending thoracic aorta just above the level of the diaphragm Citation5, Citation6. A second occlusion catheter was introduced through the femoral artery and placed under fluoroscopy at the level of the aortic bifurcation. Lactated Ringers solution was used as maintenance intravenous fluid at a rate of 5–10 ml/kg/min. Fluid boluses were administered as necessary to ensure hemodynamic stability.
Two experimental groups were used: eight animals underwent occlusion of the descending thoracic aorta by inflating the balloon just above the diaphragm and at the level of the aortic bifurcation for 45 min (DT AOC group) while six animals had placement of the catheters without balloon inflation (sham operation) and were used as controls (control group). The animals were heparinized with 150 units/kg prior to balloon inflation. During balloon inflation blood pressures were obtained from the distal arterial line to ensure complete interruption of blood flow. Blood samples were obtained from the proximal arterial line before balloon inflation, just before balloon deflation, 30 and 60 min after restoration of flow and 24 hours later. After withdrawal of the last blood specimen, animals were sacrificed according to institutional protocol.
The study was approved by the University of Ioannina Care of Experimental Animals Committee and conformed to the “Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Washington: National Academy Press, 1996”.
Blood tests
Samples for cortisol and ACTH were assayed both in DPC Immulite (Diagnostic Products Corporation, Los Angeles, CA, USA). Cortisol was assayed using competitive chemiluminescent immunoassay, while ACTH was assayed using sequential chemiluminescent immunometric assay. Cortisol results are expressed in µg/dl and ACTH in pg/ml. The analytical sensitivity is 0.2 µg/dl for the cortisol measurements and 9 pg/ml for the ACTH measurements.
Statistical analysis
Results are expressed as mean±SD. Analysis of variance (ANOVA) with repeated measures was used to analyze the experimental group and time effects for each parameter. First the simultaneous effect of aortic occlusion (DT AOC vs. control group) and time was tested. If this simultaneous interaction was not significant, then the effects of clamp site (group interaction) and time (time interaction) on the individual parameter were studied separately. When significant group and/or time interactions were observed (p < 0.05), Student's t-tests were used to compare the two groups at each time point. Paired t-testing was used to compare values from each time point to baseline, within each experimental group. Hochberg's method was used to adjust the alpha level for multiple comparisons.
Results
Hemodynamic measurements are shown in . The summary statistics including the number of animals, mean, treatment group and variable in present standard deviation, minimum and maximum values.
Table I. Proximal and distal systolic arterial pressure, rectal temperature and heart rate at baseline, during descending thoracic aortic occlusion and during reperfusion.
Table II. Summary statistics including treatment group, number of animals, as well as cortisol (µg/dl) and ACTH (pg/ml) measurements (mean, standard deviation, minimum and maximum value) at each time point.
Hemodynamic measurements
There were no significant differences between the two groups with regard to systolic arterial pressure (proximal and distal to AOC), rectal temperature and heart rate at baseline and during reperfusion. There were significant differences in systolic arterial pressure (proximal and distal to AOC) and heart rate during DT AOC between the two groups (p < 0.001) as shown in .
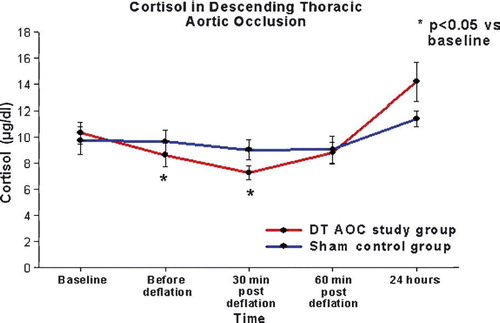
Cortisol levels
There was no significant group/time interactions in the cortisol levels by ANOVA (p = 0.200). However there was significant time effect on the cortisol levels (ANOVA p < 0.001). More specifically in the DT AXC study group the cortisol level decreased from baseline before unclamping (8.6±2.6 µg/dl vs. 10.2±2.6 µg/dl, p = 0.048), although this difference was considered marginal when adjusting the alpha level for multiple comparisons. Cortisol levels in the DT AOC group were lower than baseline 30 min after restoration of flow to the distal circulation (7.3±1.5 µg/dl vs. 10.2±2.6 µg/dl, p = 0.004) and then returned to normal. There was no change in the serum cortisol levels of the control animals throughout the course of the experiment. There was no significant group effect in the serum cortisol levels between the study and the control groups ().
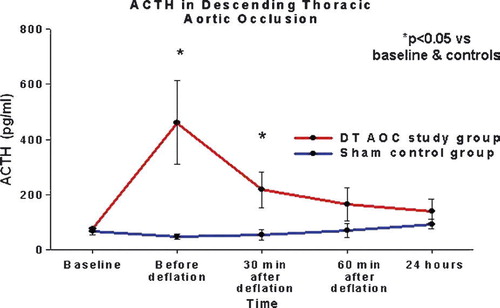
ACTH levels
There was a significant group/time interaction in the ACTH levels by ANOVA (p = 0.006). Additional analyses were run in order to compare ACTH between groups and time points. First, Student's t-tests were run comparing the study and control groups at each time point. Results are presented in . The mean ACTH was considerably higher in the study group when compared to the sham control animals just before balloon deflation and 30 min after restoration of flow, although these differences were considered marginal when adjusting the alpha level for multiple comparisons. Next, we run pair t-tests in both experimental groups to compare ACTH levels between the different time points. In the control group there was no difference in serum cortisol levels throughout the experiment. Serum ACTH levels increased in the study group from 83±41 pg/ml at baseline to 462±424 pg/ml during aortic occlusion (p = 0.040) and 218±186 pg/ml 30 min after flow restoration (p = 0.070). These differences were considered marginal when adjusting for alpha level ().
Table III. T-test Results for ACTH control vs. study group.
Discussion
DT AOC is necessary during operations on the thoracoabdominal aorta Citation1. The interruption of blood flow during cross-clamping, without the use of shunts or distal bypass, results in underperfusion of the distal vascular beds. It is this temporary ischemia that has been invoked in the generation of a number of vital organ ischemic complications such as renal insufficiency, spinal cord ischemia and bowel ischemia Citation2–4, Citation7, Citation8. The adrenal glands derive their vascular supply from the pararenal aorta. DT AOC temporarily interrupts the blood supply to the adrenals and subjects the glands to a period of warm ischemia. The physiologic effect of this ischemia on adrenal function is one of the least studied aspects of thoracoabdominal aneurysm repair.
We know that plasma catecholamines rise during and after cross-clamping of the descending thoracic aorta Citation9. The causes of this catecholamine release are not clear. Direct ischemic excitation of the adrenal medulla does not appear to be the main mechanism; elevation of plasma catecholamines has been observed also after elective abdominal aortic aneurysm repair and various general surgery procedures Citation10–12. Less is known about the response of the adrenal cortex to ischemia. The effects of temporary warm adrenal ischemia on the histologic structure of the adrenal gland depend on the ischemic time. The adrenals tolerate up to 30 min of warm ischemia without any consistent histologic changes. The adrenal cortex parenchyma appears to be quite resistant to ischemia; however extension of the ischemic time beyond 30 min induces focal infarcts in the zonae fasciculata and reticularis. Deprivation of the blood supply for 2 hours leads to massive necrosis of the cortex Citation13.
The normal response of the adrenal cortex to surgical trauma has been characterized. Serum and urine cortisol levels increased from baseline in patients that underwent urgent operations for thoracic and/or abdominal traumatic injuries and returned to the preoperative levels 24–48 h later Citation14. Similarly, patients who underwent surgical procedures lasting longer than one hour under general anesthesia and patients undergoing coronary revascularisation and valve replacement experienced elevation of serum cortisol that peaked during surgery and then returned to the preoperative levels Citation15, Citation16. In our experimental model serum cortisol levels did not increase. In fact there was a decrease in serum cortisol levels during the period of DT AOC that persisted up to 30 min after flow restoration. The decrease in serum cortisol levels was associated with an increase in the levels of the ACTH. The cause of this temporary adrenal dysfunction is not clear. It is interesting to note that in a previous study serum cortisol increased with the severity of the injury until the injury was very severe, at which point the serum cortisol levels decreased. In this same group of severely injured patients (like in our study group animals) the serum ACTH levels were elevated compared to noninjured controls. The authors postulated that shock-induced adrenal gland hypoperfusion was responsible for the decrease in serum cortisol levels Citation17. It is generally believed that ACTH is the principal stimulus of the adrenal cortex after trauma. It appears that after trauma and temporary DT AOC there is impairment of the adrenal cortical response to ACTH. This response is different from the response of the adrenal medulla to trauma and severe hypoperfusion: the cortex “shuts down” while the medulla (with the help of the sympathetic system) oversecretes serum catecholamines.
The great variability of the ACTH values during and after DT AOC is not surprising. Similar variability has been observed in normal subjects and in patients after trauma Citation17, Citation18. Even during periods of maximal stress ACTH is released in bursts, which are irregular and non-predictable. Furthermore, one could ask whether the decrease in the cortisol levels we documented in the study group is a mere reflection of a normal diurnal cycle of cortisol secretion. Does the drop in serum cortisol through the first day of the experiment represent the expected decrease of adrenal cortical secretion past the early morning surge, and thus it has nothing to do with the DT AOC? It is known that surgery, burns and trauma disrupt the diurnal cycle of cortisol secretion, and this disruption persists for several days Citation17. Moreover, a decrease in serum cortisol levels was not observed in control animals.
Supraceliac aortic cross-clamping leads also to transient cessation of renal and intestinal blood flow. Ischemia-reperfusion renal injury results in impaired postoperative renal function in up to 50% of the cases and this has been also confirmed in a porcine model of suprarenal aortic cross-clamping Citation19, Citation20. The development of postoperative renal impairment is a major cause of perioperative morbidity and mortality after thoracic aortic surgery. Studies in patients undergoing supraceliac aortic cross-clamping show that the decrease in renal blood flow does not correlate with any decrease in cardiac output or change in mean arterial pressure. The mechanism underlines this phenomenon may, in part, be a result of humoral alterations such as increased release of renin, which may also affect adrenal function Citation20. In addition, intestinal ischemia-reperfusion injury can trigger injuries in remote tissues Citation21, Citation22. Reperfusion of the intestine releases inflammatory mediators, which promote neutrophil priming. Primed neutrophils adhere endothelial cells on the reperfused and remote vascular beds and elicit damaging substances such as reactive oxygen species and neutrophil elastase, which has been regarded as a key mediator because it degrades the basement membrane and other important intercellular matrixes to cause severe tissue damage Citation22. Adrenal reperfusion injury may be also related to intestinal reperfusion injury.
Moreover, studies on human abdominal aortic procedures with infrarenal aortic cross-clamping have shown adrenal dysfunction as measured by cortisol. Salartash et al. found that serum cortisol levels were elevated over the 24-hour study period in both conventional open surgery group and endovascular group Citation23. However, 6 hours after the initiation of the operative procedure, the increase in cortisol levels was statistically significant in conventional open surgery group Citation23. In our experimental porcine model we used balloon occlusion of the thoracic aorta making the surgical procedures simple, quickly accomplished and less invasive than aortic cross-clamping through a thoracotomy. Therefore, our experimental model was closer to the endovascular group indicating that we were not able to study any increase in cortisol levels related to open surgical procedure. In our study we evaluated the variation in cortisol levels and ACTH levels which represented either the direct effect of adrenal ischemia-reperfusion injury or the remote reperfusion injury of the adrenal glands caused by other affected organs such as the kidneys or the intestine.
The clinical implications of the temporary adrenal dysfunction following DT AOC are not clear. It is known, that experimentally induced acute adrenal insufficiency in canine and feline animal models leads to severe hypotension and severe impairment of the cardiac function Citation24. The plasma cortisol levels, however, in these adrenalectomized animals decline to virtually undetectable, after the adrenalectomy. This reduction is very different from the 20–25% serum cortisol reduction observed in our study group. The clinical significance of this temporary reduction of adrenal cortical secretion definitively deserves further study. Postoperative adrenal insufficiency is a rare complication. The clinical manifestations of the disease are non-specific. If not recognized, the condition can progress to refractory hypotension, circulatory collapse, multiple organ failure and death Citation25–29. There are reports in the literature suggesting that postoperative acute adrenal insufficiency in ICU patients may be more common than previously thought Citation30. The true incidence of postoperative adrenal insufficiency after operations on the thoracoabdominal aorta is not known.
Conclusion
DT AOC leads to adrenal dysfunction, which is manifested by decrease in the serum cortisol levels, in a porcine model, despite an increase in ACTH production. This adrenal dysfunction is temporary, as sustained adrenal injury cannot be detected 24 hours later. The etiology as well as the role of this transient adrenal dysfunction in morbidity and mortality, following operations on the thoracoabdominal aorta, needs to be studied in the human.
Acknowledgements
To Athanasoulas Dimitrios, a nursing staff of the Cardiac Surgery Department at the University of Ioannina, Greece for his help and cooperation.
References
- Cambria RP. Thoracoabdominal aortic aneurysm repair: How I do it. Cardiovasc Surg. 1999; 7: 597–606
- Nypaver TJ, Shepard AD, Reddy DJ, Elliott JP, Jr, Ernst CB. Supraceliac aortic cross-clamping: Determinants of outcome in elective abdominal aortic reconstruction. J Vasc Surg. 1993; 17: 868–75
- Jean-Claude JM, Reilly LM, Stoney RJ, Messina LM. Pararenal aortic aneurysms: The future of open aortic aneurysm repair. J Vasc Surg. 1999; 29: 902–12
- Hines GL, Chorost M. Supraceliac aortic occlusion: A safe approach to pararenal aortic aneurysms. Ann Vasc Surg. 1998; 12: 335–40
- Papakostas JC, Matsagas MI, Toumpoulis IK, Malamou-Mitsi VD, Pappa LS, Gkrepi C, et al. Evolution of spinal cord injury in a porcine model of prolonged aortic occlusion. J Surg Res. 2006; 133: 159–66
- Toumpoulis IK, Papakostas JC, Matsagas MI, Malamou-Mitsi VD, Pappa LS, Drossos GE, et al. Superiority of early relative to late ischemic preconditioning in spinal cord protection after descending thoracic aortic occlusion. J Thorac Cardiovasc Surg. 2004; 128: 724–30
- Green RM, Ricotta JJ, Ouriel K, DeWeese JA. Results of supraceliac aortic clamping in the difficult elective resection of infrarenal abdominal aortic aneurysm. J Vasc Surg. 1989; 9: 124–34
- Schneider JR, Gottner RJ, Golan JF. Supraceliac versus infrarenal aortic cross-clamp for repair of non-ruptured infrarenal and juxtarenal abdominal aortic aneurysm. Cardiovasc Surg. 1997; 5: 279–85
- Normann NA, Taylor AA, Crawford ES, DeBakey ME, Saleh SA. Catecholamine release during and after cross clamping of descending thoracic aorta. J Surg Res. 1983; 34: 97–103
- Brismar B, Hedenstierna G, Lundh R, Tokics L. Oxygen uptake, plasma catecholamines and cardiac output during neurolept-nitrous oxide and halothane anaesthesias. Acta Anaesthesiol Scand. 1982; 26: 541–9
- Brown FF, III, Owens WD, Felts JA, Spitznagel EL, Jr, Cryer PE. Plasma epinephrine and norepinephrine levels during anesthesia: Enflurane-N2O-O2 compared with fentanyl-N2O-O2. Anesth Analg. 1982; 61: 366–70
- Riles TS, Fisher FS, Schaefer S, Pasternack PF, Baumann FG. Plasma catecholamine concentrations during abdominal aortic aneurysm surgery: The link to perioperative myocardial ischemia. Ann Vasc Surg. 1993; 7: 213–9
- Horvath E, Kovacs K. Effect of temporary ischemia on the fine structure of the rat adrenal cortex. Pathol Eur. 1973; 8: 43–59
- Harris MJ, Baker RT, McRoberts JW, Mohler JL. The adrenal response to trauma, operation and cosyntropin stimulation. Surg Gynecol Obstet. 1990; 170: 513–6
- Mohler JL, Michael KA, Freedman AM, Griffen WO, Jr, McRoberts JW. The serum and urinary cortisol response to operative trauma. Surg Gynecol Obstet. 1985; 161: 445–9
- Oka Y, Wakayama S, Oyama T, Orkin LR, Becker RM, Blaufox MD, et al. Cortisol and antidiuretic hormone responses to stress in cardiac surgical patients. Can Anaesth Soc J. 1981; 28: 334–8
- Barton RN, Stoner HB, Watson SM. Relationships among plasma cortisol, adrenocorticotrophin, and severity of injury in recently injured patients. J Trauma. 1987; 27: 384–92
- Krieger DT, Allen W. Relationship of bioassayable and immunoassayable plasma ACTH and cortisol concentrations in normal subjects and in patients with Cushing's disease. J Clin Endocrinol Metab. 1975; 40: 675–87
- Hauser B, Froba G, Bracht H, Strater J, Chkhouta AB, Vassilev D, et al. Effects of intrarenal administration of the cox-2 inhibitor parecoxib during porcine suprarenal aortic cross-clamping. Shock. 2005; 24: 476–81
- Sear JW. Kidney dysfunction in the postoperative period. Br J Anaesth. 2005; 95: 20–32
- Juel IS, Solligard E, Lyng O, Stromholm T, Tvedt KE, Johnsen H, et al. Intestinal injury after thoracic aortic cross-clamping in the pig. J Surg Res. 2004; 117: 283–95
- Kotake Y, Yamamoto M, Matsumoto M, Morisaki H, Takeda J. Sivelestat, a neutrophil elastase inhibitor, attenuates neutrophil priming after hepatoenteric ischemia in rabbits. Shock. 2005; 23: 156–60
- Salartash K, Sternbergh WC, III, York JW, Money SR. Comparison of open transabdominal AAA repair with endovascular AAA repair in reduction of postoperative stress response. Ann Vasc Surg. 2001; 15: 53–9
- Lefer AM, Verrier RL, Carson WW. Cardiac performance in experimental adrenal insufficiency in cats. Circ Res. 1968; 22: 817–27
- Barquist E, Kirton O. Adrenal insufficiency in the surgical intensive care unit patient. J Trauma. 1997; 42: 27–31
- Claussen MS, Landercasper J, Cogbill TH. Acute adrenal insufficiency presenting as shock after trauma and surgery: Three cases and review of the literature. J Trauma. 1992; 32: 94–100
- Hubay CA, Weckesser EC, Levy RP. Occult adrenal insufficiency in surgical patients. Ann Surg. 1975; 181: 325–32
- Messiant F, Duverger D, Verheyde I, Declerck N, Pruvot FR, Scherpereel P. Postoperative acute adrenal insufficiency. Ann Fr Anesth Reanim. 1993; 12: 594–7
- Steer M, Fromm D. Recognition of adrenal insufficiency in the postoperative patient. Am J Surg. 1980; 139: 443–6
- Merry WH, Caplan RH, Wickus GG, Reynertson RH, Kisken WA, Cogbill TH, et al. Postoperative acute adrenal failure caused by transient corticotropin deficiency. Surgery. 1994; 116: 1095–100