Abstract
Objective. We sought to evaluate if patients with proximal critical circumflex (CX) lesions did better with single SV-CABG on the best obtuse marginal (OM), or with sequential SV-CABG on two OM branches. Design. Ninety patients were prospectively randomised to single SV-CABG on the best OM (sSV-CABG-45 patients; Group A) or to sequential SV-CABG on 2 OM (seqSV-CABG 45 patients; Group B). Transit-time flowmetry (TTF), and graft flow reserve were evaluated. Recurrent angina, acute myocardial infarction, readmission for coronary reintervention were defined “treatment failure” during follow-up. Results. SeqSV-CABG showed better intraoperative maximum (119.1±57.5 ml/min vs. sSV-CABG 62.4±29.6; p=0.001), mean (56.3±31.5 ml/min vs. 30.8±12.8; p=0.0001), minimum flow (22.8±9.2 ml/min vs. 11.8±8.9; p=0.001) and P.I. (0.71±0.4 vs.1.46±0.9; p=0.006). Graft flow reserve also proved to be higher (95.4±29.7 ml/min mean flow vs. sSV-CABG 42.3±15.2 ml/min mean flow; p=0.0001; flow reserve 1.72±0.99 vs 1.32±1.09; p=0.001) as well as freedom from treatment failure (97.5±0.5% vs 88.7±0.4%; p=0.05). Conclusions. SeqSV-CABG showed higher TTF flows, with no incremental risk for perioperative morbidity. Higher flows and graft flow reserve may allow lower treatment failure at mid-term follow-up.
Despite the recent enthusiasm for arterial revascularization, having superior long-term patency rate, saphenous vein grafts (SVG) continue to be the backbone of daily coronary revascularization Citation1, Citation2. One of the issues of concern in regard to the patency of the SVGs is whether to use them as individual or sequential conduits Citation3. It can be argued that proximal occlusion of a sequential SVG may jeopardize a large amount of viable myocardium, causing catastrophic acute myocardial infarction Citation4–6. Furthermore, it is still a matter of debate whether an extensive revascularization of all the diseased coronaries is better than grafting the qualitatively best affected vessels, minimizing cross-clamp time Citation7, Citation8. Studies that focus on this subject are scarce, and go back at least a decade, in a pre-transit-time flow method (TTF) era Citation4–6.
The recent availability of TTF to assess coronary blood flow has led to its increased use as a tool in determining perfusion abnormalities. TTF is fast and easy, is less affected by artifacts than other techniques, and is also predictive of graft occlusion at angiographic follow-up Citation9, Citation10. Not only are the absolute flow values valuable, but also the flow curve patterns and the pulsatile index (P.I.) useful in correctly using this technology Citation11. Troponin I (TnI), on the other hand, has been recently shown as a sensitive and specific marker of myocardial damage, able to detect even minor differences of myocardial ischemia and prognostic for cardiovascular events at follow-up Citation12.
Therefore, it was the aim of our study to evaluate the influence of single versus sequential saphenous vein grafting technique on perioperative TTF results, as well as biochemical, echocardiographic, perioperative and follow-up clinical results in patients with a severely diseased circumflex artery (CX) undergoing CABG.
Material and methods
Ninety patients with three-vessels coronary disease with critical proximal CX stenosis, admitted for isolated CABG were enrolled in the study between January 2003 and January 2005. After obtaining Institutional Review Board approval and informed consent, patients were prospectively randomised by card to undergo single SVG (sSV-CABG; Group A) on the best obtuse marginal branch (OM), or sequential SVG on 2 OM vessels (seqSV-CABG; Group B). Exclusion criteria were additional surgical procedures, severe comorbidities (dyalisis, hepatic failure, cancer, autoimmune disease), coronary revascularization with “Y-” or “T-”graft construction. Follow-up data were collected by the database of our outpatient clinic.
End-points
The primary endpoints were: intraoperative TTF results, in-hospital mortality and morbidity [defined as any of following: pneumonia, respiratory failure, need for non-invasive positive-pressure ventilation, acute renal failure, perioperative acute myocardial infarction (AMI – defined as new Q waves greater than 0.04 ms and/or reduction in R waves greater than 25% in at least two leads, new akinetic/dyskinetic segment at echocardiography, and TnI >3.1 µg/l at 12 hours or >3.7 µg/l at any time), postoperative arrhythmias (considered to be a complication only if they were life-threatening or needed medical treatment), and low cardiac output syndrome], and follow-up freedom from treatment failure (defined as any of the following: AMI during follow-up, recurrence of angina, need for PCI or redo CABG).
Perioperative enzymatic leakage (TnI I >3.1 µg/l at 12 hours or >3.7 µg/l at any time Citation12, need for inotropic support, in-hospital and intensive therapy unit (ITU) stay, and echocardiographic recovery of the left ventricular function – either before hospital discharge and at 12 months of follow-up – were the secondary endpoints.
Surgery
A standard anaesthetic protocol was used: this consisted of intravenous anaesthesia with a propofol infusion at 3 mg/kg per hour combined with fentanyl administration at 0.10 mg each 20 min. Neuromuscular blockade was achieved by 4 mg/h pancuronium bromide and the lungs ventilated to normocapnia with air and oxygen (45–50%).
In all patients CABG was performed through a median sternotomy, always on cardiopulmonary bypass (CPB). A standard cardiopulmonary bypass circuit was used: a Dideco (Mirandola-Modena) tubing set, which included a 40 micron filter, a Stockert roller pump (Stockert Instrumente, Munich, Germany) and a hollow fibre membrane oxygenator (Dideco D903 Avant, Mirandola (MO), Italy). Non pulsatile flow with an output of 2.4 l/m2 per min was used. Myocardial protection was achieved by using antegrade and retrograde warm blood cardioplegia Citation13. The left anterior descending artery (LAD) was always grafted using the pedicled left internal mammary artery. The right coronary was revascularized by single SVG or radial artery. The internal SV was harvested from the best side, as detected by preoperative Doppler scanning. Patients with a diseased CX, with at least two OM branches (diameter above 1.5 mm) were randomly allocated to receive single (45 patients, Group A) or sequential (45 patients, Group B) vein grafts. The distal anastomosis on a sequential graft was performed in end-to-side fashion, with the graft axis parallel to the native coronary vessel axis, the other one side-to-side in a diamond-shape.
Flowmetric analysis
Assessment of TTF was performed under stable hemodynamics, after protamine administration. Flowmetry of the grafts was performed with a transit-time flowmeter (HT313, Transonic, Transonic Systems Inc., Ithaca, NY), 2 cm from the proximal anastomosis. The curves were coupled with the EKG tracing to correctly differentiate the systolic from the diastolic flow. Measurements were interpreted as suggested by D'Ancona Citation11. Maximum, minimum and mean flow were reported as ml/min, P.I. as an absolute number Citation11.
Graft flow reserve
Intraaortic balloon pump (IABP) recruits graft flow reserve during assistance Citation14. In order to evaluate graft flow reserve, mean flow and P.I. were recorded in all patients undergoing preoperative intraaortic balloon pumping, both during IABP support and during temporary cessation. Graft flow reserve was calculated from the mean flow assessed during 1:1 IABP support divided by mean flow at baseline (IABP off).
Postoperative care
Inotropes were started immediately after aortic cross-clamp removal with enoximone at a dosage of 5 µg/Kg/min Citation13. Inotropes were defined as “low-dose” when enoximone was administered at a dosage ≤5 µg/kg/min, “medium-dose” when enoximone was between 6 and 10 µg/kg/min, or dobutamine added at a dosage between 5 and 10 µg/kg/min, and “high-dose” when enoximone or dobutamine were >10 µg/kg/min or epinephrine added at any dose.
Biochemical analysis and echocardiography
Determinations of blood concentration of cardiac troponin I (TnI) were conducted before anaesthetic induction, and at 12, 24, 48, and 72 h. All trans-thoracic echocardiograhic studies were performed in a blind manner, at hospital admission, before discharge, and at 12 months-follow-up. Analysis of wall motion score index (WMSI) was accomplished.
Statistical analysis
Statistical analysis was performed by the SPSS program for Windows, version 10.1 (SPSS Inc, Chicago, IL). Continuous variables are presented as mean±standard deviation (S.D.) and categorical variables presented as absolute numbers and percentages. Data were checked for normality before statistical analysis. Normally distributed continuous variables were compared using the unpaired t-test; the Mann-Whitney U test was used for not normally distributed variables. Categorical variables were analysed using either the χ2 test or Fischer's exact test. Comparison between and within groups was made using two-way analysis of variance for repeated measures. Estimates of freedom from treatment failure were determined with the method of Kaplan-Meier life table analysis. Log-rank test was performed to ascertain differences between the two groups. Comparisons were considered significant if p< 0.05.
Results
The two groups demonstrated comparable demographic data (). Intraoperative results and postoperative course are shown in . Perioperative troponin I leakage was comparable between the two groups (A).
Figure 1. Perioperative troponin I leakage (1A) and follow-up freedom from treatment failure (1B) between the two groups.
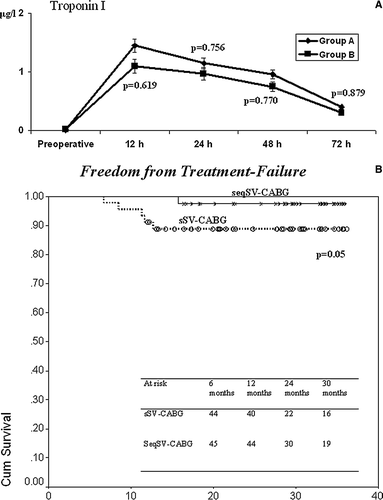
Table I. Demographic data.
Table II. Intraoperative data.
Graft flowmetry indicated that one patient in Group B (1/45–2.3%), but no one in Group A (p = 0.5) required graft revison. The patient demonstrated a “systolic” pattern of the curve with low mean flow value (4 ml/min) and high P.I. (7.8) of a single SV-to-right coronary (RX) graft. Graft revision demonstrated the recovery of the “diastolic” pattern of the curve together with the improvement of either mean flow (30.5 ml/min) and P.I. (1.7). Postoperative course was then uneventful, except for an isolated TnI peak at 12 h of 3.3 µg/l without ECG signs suggestive for perioperative AMI. On the other hand, three patients (6.7%) in group A and two patients in group B (4.5%; p = 0.5) demonstrated low mean flows (Group A: 10.7±0.5 ml/min; Group B: 11.0±2.6 ml/min) and high P.I.s (Group A: 4.6±0.3; Group B: 5.1±0.5) of singles SVG-to-posterior descending grafts, without systolic “spiky” pattern of the curves. The preserved diastolic flow pattern together with the angiographic findings of a “poor quality” target vessels, because of severe and diffuse atherosclerosis, did not led to graft revision. All these patients had an uncomplicated postoperative course, and were asymptomatic at the last ambulatory follow-up.
As far as TTF values on the CX territory were concerned, the maximum, mean, minimum flows and P.I. of the two groups always demonstrated better values in patients undergoing sequential SVG, as shown in . According to an institutional policy, 28 (62.2%) patients belonging to group A, and 25 (55.5%) belonging to group B underwent preoperative IABP insertion: this policy was due to the relatively low mean ejection fraction of the patient population, together with a high incidence of ongoing unstable angina despite i.v. nitrates and heparin, and with the aim to better protect the heart against life-threating acute ischemia during the preoperative induction of anesthesia in patients with hyper-critical coronary lesions. In order to rule out differences in graft flow reserve between the two techniques, these patients underwent intraoperative TTF analysis with either 1:1 IABP and temporary cessation. 1:1 IABP support recruited graft flow reserve in all these patients, but proved to be better in seqSV-CABG group ().
Table III. TTF results of sSV-CABG and seqSV-CABG on the circumflex territory at baseline and during IABP-induced recruitment of the graft flow reserve.
Echocardiographic recovery of WMSI was comparable (). Follow-up was 100% complete. There were no differences in mean follow-up between the two groups (sSV-CABG: 26.9±7.8 months vs. seqSV-CABG: 27.8±7.6; p = 0.573). During follow-up, five patients (11.1%) in Group A demonstrated treatment failure beacuse of AMI in 1, recurrence of angina in 4, and need for PCI in two of them, whereas only one patient (2.3%) in Group B had recurrent angina and need for a PCI. Follow-up freedom-from-treatment-failure was higher in seqSV-CABG (97.5±0.5% vs. 88.7±0.4%; p = 0.05 – B).
Table IV. Echocardiographic segmental Wall Motion Score Index results of sSV-CABG and seqSV-CABG on the circumflex territory.
Discussion
Despite the recent introduction and popularization of a variety of arterial conduits having superior long-term patency rates, and the recent enthusiasm for extensive arterial revascularization, SVG continue to be the backbone of daily coronary revascularization, being the second employed conduit in both STS National and EuroSCORE Databases Citation1, Citation2. In particular, the debate whether to use SVG as individual or sequential conduits, and whether the sequential technique may impair or improve early and late results following CABG is still open Citation3–6. This is particularly true considering that the majority of studies on sequential technique go back at least a decade, when TTF was unavailable Citation4–6. Moreover, it become critical to determine which operative technique may allow the best results when we consider that arterial grafting is still debated and the SVG are customarily used Citation1, Citation2 in elderly, who are increasingly referred to surgery Citation1, Citation2.
Sequential grafting was introduced in the early ‘70s by Flemma, with the supposed advantage of a decreased total resistance to the graft flow, a minimized impedance mismatch, and a complete revascularization with a shorter vein segment Citation15. On the other hand, its main drawback is that proximal conduit failure compromises blood flow to a large mass of myocardium Citation16. It has been demonstrated that the principal determinant of the patency rate of CABG in the late postoperative period is the flow rate throughout the graft: Faulkner reported a strict association between an increased flow rate and a less intimal proliferation Citation17; Rittgers demonstrated a reverse relationship between the flow rate and intimal proliferation Citation18. According to the fact that the variables used in estimating the flow rate are SVG diameter, graft resistance, and the resistance posed by the native coronary vessel, and considering that the diameters of SVG are relatively constant for a given patient, and the resistance posed by a SVG is negligible when compared to that of its coronary counterpart, the resistance of the native coronary vessels remains the principal determinant of the flow rate Citation16. Therefore, if individual resistances of the grafted coronaries are assumed to be equivalent, a double sequential graft poses only half of the resistance of an individual graft. Thus, the individual SVGs are more resistant than sequential conduits, and are expected to have a worse patency rate compared to the sequential ones Citation4, Citation6, Citation16. This also explains the results by O'Neill Citation4 and Grondin Citation5 who found proximal anastomoses of a sequential graft to have better patency rate compared to the individual grafts. Accordingly, we found better flowmetric results in patients undergoing sequential SVGs compared to single SVG. Moreover, it is well known that with the aid of TTF method, not only the absolute flow values, but the P.I. indicated the quality of the graft Citation11. Again, we found better results in patients undergoing sequential grafting. Finally, keeping in mind that TTF results predicted graft patency at angiohgraphic follow-up Citation9, Citation10, we can speculate that, giving the higher TTF values and the better P.I.s of sequential grafts, these may have better patency rate at follow-up. In fact, Vural found sequential grafts to have a 5-year patency of 85%, higher than single anastomoses (76%) Citation16. In this way we can interpret the higher freedom-from-treatment-failure demonstrated in the seqSV-CABG.
On the other hand, we found a comparable recovery of the segmental left ventricular wall motion in the two groups. It is well known that ventricular segments with ischemic dysfunction before grafting demonstrated a significant increase in quantitative indices of regional motion after grafting Citation17. Kozower demonstrated that complete revascularization correlated with improved hospital results and long-term survival, elderly patients as well Citation18. Our findings demonstrated that either sSVG-CABG achieves a complete revascularization. Accordingly, perioperative TnI was comparable, demonstrating a similar completeness of revascularization and quality of myocardial protection: Fellahi showed TnI to be the most sensitive and specific marker of perioperative myocardial ischemia and of the adequacy of myocardial protection, being furthermore prognostic for cardiac events during follow-up Citation12. Therefore, it can be speculated that the differences in follow-up outcome cannot be considered secondary to a different quality of myocardial protection, nor to differences in the completeness of myocardial revascularization, but, together with the demonstrated correlation between graft flow and intimal hyperplasia Citation19, Citation20, to the differences in intraoperative TTF results.
Limitations of the study
The main limitation of the study is related to the small sample size of the patients enrolled. This is a result of the single-center design of the study, which however guarantees uniformity of the perioperative management of the patient population. Moreover, on an intention-to-treat basis, we enrolled patients with the most similar coronary and systemic risk profile, as patients with isolated proximal CX critical stenosis, without severe organ co-morbidities, which may mislead the results.
It has to be kept in mind that the population of the study has a very high mean age, together with a very high percentage of diabetes, COPD, and poor left ventricular ejection fraction, as a result of the growing interventional coronary practice observed at our hospital, as well as worldwide. This furthermore determines a very high risk profile – also suggested by the high EUROscore of the two groups – and is the whitness of a generally poor coronary bed encountered by surgeons in the daily practice: this can be a limitation of the study according to the fact that coronary bed is a main determinant of TTF results. Therefore our results can not be certainly extrapolated to low-risk profile coronary patients, as, as example, those with isolated proximal lesions and a good quality of the distal coronary bed.
Finally, the study lacks follow-up angiography. Coronary angiogram is still the “gold standard” to assess graft patency. However, this was not the purpose of our study, in which intraoperative TTF as well as major perioperative and mid-term follow-up clinical and sub-clinical results were investigated.
Conclusions
The results of our study confirmed previously published reports demonstrating a clear advantage of seqSV-CABG Citation10. Despite slightly longer cross-clamp times, seqSV-CABG on a diseased circumflex artery achieves better flowmetric results, in terms of either maximum, mean, minimum flow, PI, and graft flow reserve, together with a higher freedom from treatment failure at 2-year follow-up. Therefore patients, in whom SV has been chosen as a CABG conduit, should undergo sequential grafting on all the graftable branches encountered on the surgical pit.
Conflict of interest
None of the authors have any conflicts to disclose
References
- Ferguson TB, Dziuban SW, Edwards FH, Eiken MC, Shroyer ALW, Pairolero PC, et al. The STS National Database: Current changes and challenges for the new millennium. Ann Thorac Surg. 2000; 69: 680–91
- Nashef SAM, Roques F, Michel P, Cortina J, Faichney A, Gams E, et al. Coronary surgery in Europe: Comparison of the national subsets of the European System for Cardiac Operative Risk Evaluation database. Eur J Cardiothorac Surg. 2000; 17: 396–9
- Meeter K, Veldkamp R, Tijssen JGP, van Herwerden LL, Bos E. Clinical outcome of single versus sequential grafts in coronary bypass operations at ten years follow-up. J Thorac Cardiovasc Surg. 1991; 101: 1076–81
- O'Neill MJ Jr, Wolf PD, O'Neill TK, Montesano RM, Waldhausen JA. A rationale for the use of sequential coronary artery bypass grafts J Thorac Cardiovasc Surg. 1981;81:686–90.
- Grondin CM, Limet R. Sequential anastomoses in coronary artery grafting. Technical aspects and early and late angiographic results. Ann Thorac Surg. 1977; 23: 1–8
- Dion R, Glineur D, Derouck D, Verhelst R, Noirhomme P, El Khoury G, et al. Complementary saphenous grafting: Long-term follow-up. J Thorac Cardiovasc Surg. 2001; 122: 296–304
- Scott R, Blackstone EH, McCarthy PM, Lytle BW, Loop FD, White JA, et al. Isolated bypass grafting of the left internal thoracic artery to the left anterior descending coronary artery: Late consequences of incomplete revascularization. J Thorac Cardiovasc Surg. 2000; 120: 173–84
- Osswald BR, Blackstone EH, Tochtermann U, Schweiger P, Thomas G, Vahl CF, et al. Does the completeness of revascularization affect early survival after coronary artery bypass grafting in elderly patients?. Eur J Cardiothorac Surg. 2001; 20: 120–6
- Walpoth BH, Bosshard A, Genyk T, et al. Transit time flow measurement for detection of early graft failure during myocardial revascularisation. Ann Thorac Surg. 1998; 66: 1097–100
- Takami Y, Ina H. Relation of intraoperative flow measurement with postoperative quantitative angiographic assessment of coronary artery bypass grafting. Ann Thorac Surg. 2001; 72: 1270–4
- D'Ancona G, Karamanoukian HL, Ricci M, Schmid S, Bergsland J, Salerno TA. Graft revision after transit time flow measurement in off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg. 2000; 17: 287–93
- Fellahi JL, Gue X, Richomme X, Monier E, Guillou L, Riou B. Short and long-term prognostic value of postoperative cardiac troponin I concentration in patients undergoing coronary artery bypass grafting. Anesthesiology. 2003; 99: 270–4
- Onorati F, Renzulli A, De Feo M, et al. Perioperative enoximone infusion improves cardiac release after CABG. J Cardiothorac Vasc Anesth. 2004; 18: 409–14
- Tedoriya T, Kawasuji M, Sakakibara N, Takemura H, Watanabe Y, Hetzer R. Coronary bypass flow during use of intraaortic balloon pumping and left ventricular assist device. Ann Thorac Surg. 1998; 66: 477–81
- Flemma RJ, Johnson WD, Lepley D., Jr Triple aorto-coronary bypass as treatment for coronary insufficiency Arch Surg. 1971;103:82.
- Vural KM, Sener E, Tasdemir O. Long term patency of sequential and individual saphenous vein coronary bypass grafts. Eur J Cardiothorac Surg. 2001; 19: 140–4
- Paluszkiewicz L, Kwinecki P, Jemielity M, Szyszka A, Dyszkiewicz W, Cieslinski A. Myocardial perfusion correlates with improvement of systolic function of the left ventricle after CABG. Dobutamine echocardiography and Tc-99m-MIBI SPECT study. Eur J Cardiothorac Surg. 2002; 21: 32–5
- Kozower BD, Moon MR, Barner HB, Moazami N, Lawton JS, Pasque MK, et al. Impact of complete revascularization on long-term survival after coronary artery bypass grafting in octogenarians. Ann Thorac Surg 2005; 80: 112–7
- Faulkner SL, Fisher RD, Conkle DM, Page DL, Bender HW, Jr Effect of blood flow rate on subendothelial proliferation in venous autografts used as arterial substitutes Circulation. 1975;52((Suppl I)):163–72.
- Rittgers SE, Karayannacos PE, Guy JF, Nerem RM, Shaw GM, Hostetler JR, et al. Velocity distribution and intimal proliferation in autologous vein grafts in dogs. Circ Res. 1978; 42: 792–801
