Abstract
Objectives. To study the prevalence of active cytomegalovirus (CMV) infection in patients with stable and unstable conditions of coronary artery disease (CAD). Design. Forty patients with acute coronary syndrome (ACS), 50 patients with stable angina and angiographically verified CAD (SA) and 50 clinically healthy controls were included. Monocytes were isolated from peripheral blood and CMV-RNA expression was determined by a nested RT-PCR assay. CMV IgM and IgG antibodies, interleukin-(IL)-6, IL-10 and CRP were measured in serum. Results. The prevalence of active CMV infection was significantly higher in patients with ACS (15%) and in patients with SA (10%) compared with controls (2%) (p<0.001). The presence of an active CMV infection was associated with increased serum concentrations of IL-6. Conclusions. Active CMV infection was found to a larger extent in CAD patients than in healthy controls. The data indicate that CAD patients are more susceptible to reactivation of CMV and put new focus on the role of CMV in atherosclerosis.
Inflammation is a crucial component of atherosclerosis and there is evidence for a chronic antigen-driven immune stimulation in the vascular wall as well as in the circulation Citation1. Several microbial agents have been launched as potential atherosclerosis-related antigens, either as single-causing agents or as part of a total antigenic burden Citation2, Citation3.
CMV belongs to the herpesvirus family. After a primary infection, the virus establishes a life long latency in monocytes and/or myeloid linage cells Citation4. Data from histopathological studies of atherosclerotic lesions as well as experimental studies suggest that CMV infection is involved in atherogenesis Citation5–7. However, findings from clinical studies are contradictory regarding the association between CMV seropositivity and cardiovascular disease Citation8, Citation9. Lately, studies have been carried out to study the association between CMV-seropositivity and inflammatory status in CAD. An increased risk of cardiovascular events has been reported in patients who had the combination of CMV-seropositivity and elevated levels of CRP or IL-6 Citation10–12. Hydroxy-methylglutaryl coenzyme A reductase inhibitors (statins) that are widely used in cardiovascular patients provide not only cholesterol-lowering but also anti-inflammatory effects Citation13. Interestingly, a survival benefit of statins has been linked to the combination of CMV-seropositivity and high CRP levels Citation14.
So far, most clinical studies have used CMV seropositivity as a marker for CMV infection, which does not allow the discrimination between active and latent infection. In this study, we examined the prevalence of active CMV infection in monocytes obtained from patients with stable as well as unstable conditions of CAD and related the findings to systemic inflammatory activity.
Methods
Subjects
We studied a total of 90 patients with angiographically verified CAD; 40 with acute coronary syndrome (ACS) and 50 with stable angina (SA). All patients were recruited from the Heart Center, University Hospital of Linköping. The ACS patients were included if they had a diagnosis of unstable angina/non-ST elevation myocardial infarction on the basis of typical ECG-changes (ST-T segment depression and/or T-wave inversion) and/or elevated Troponin T. The SA patients had effort-related angina in accordance with the Canadian Cardiovascular Society functional class I and II without any worsening of the symptoms the latest 6 months. Patients were excluded if they were older than 70 years, or if they had diabetes, severe heart failure, immunologic disorders, neoplasmatic disease, evidence of acute or recent (<2 months) infection, recent major trauma, surgery or revascularization procedure or treatment with immunosuppressive or anti-inflammatory agents.
Fifty individuals of equivalent age, randomly selected from the population register in the same geographical area, served as controls. They had to be anamnestically healthy without taking any medication and with normal routine laboratory tests.
The research protocol was approved by the locally appointed ethical committee and informed consent was obtained from all subjects.
Blood was obtained in EDTA tubes by vein puncture in the morning after a 12-hour fast. In ACS patients, blood samples were drawn within 2–7 days (median of 6) after onset of symptoms and always prior to coronary intervention.
Monocyte cell isolation
Peripheral blood mononuclear cells were isolated by density gradient centrifugation on lymphoprep (Axis-Shield, Oslo, Norway) at 800×g for 20 min, washed and resuspended RPMI-1640 (Gibco BRL, Grand Island, NY), supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (Gibco) and 10% heat-inactivated fetal calf serum (FCS), plated on Primaria dishes (Primaria, Falcon, Becton Dickinson, San Jose, California, USA) at a total number of 0.5–1×106 cells/plate (35 mm2). The cells were incubated at 37°C for 2 h, non-adherent cells were removed, the adherent cells were harvested after addition of RLT buffer (RNeasy minikit, Qiagen Sciences, Maryland, USA) and stored at −70°C until use.
RNA isolation
RNA was obtained with RNeasy minikit according to the manufacturers protocol (Qiagen), the RNA samples were stored at −70°C until use.
Detection of CMV replication
cDNA was synthesized by the use of the Superscript First-Strand Synthesis System for Reverse Transcriptase PCR (RT-PCR) (Invitrogen, Stockholm, Sweden) and used as templates for CMV-specific primer pairs for the major immediate-early (MIE) gene in a nested RT-PCR assay (as previously described Citation15). As a positive control for the detection of cDNA, primers specific for the glucose-6-phosphatese dehydrogenase gene were used for each sample Citation15. cDNA samples from uninfected and CMV-infected human lung fibroblasts were included as positive and negative controls, respectively. The PCR products were visualized on 2% agarose gels. The sensitivity of the PCR assay was determined in a semi-quantitative way; a known DNA concentration of a vector containing the MIE gene (bluescript/MIE, kindly provided by Dr Jay A Nelson, Portland, Oregon) was serially diluted and used as a template in the PCR assay. The lowest concentration resulting in a positive PCR result gave the cut-off value for the sensitivity of this assay, which allowed for a detection of 1–10 genome copies of the MIE DNA.
CMV Serological assay
Serum samples were assayed for CMV IgM and IgG antibodies using a specific enzyme-linked immunoassay (ELISA) (performed by an “in house” ELISA at the Division of Clinical Virology, Karolinska Institutet, Huddinge University Hospital).
Serological assays
Serum samples were assayed for CRP by a highly sensitive latex-enhanced turbidimetric immunoassay with a lower detection limit of 0.03 mg/l (Roche Diagnostics GmbH, Vienna, Austria). Serum samples were also assayed for IL-6 and IL-10 levels, by using highly sensitive ELISA immunoassays according to the manufacturer's recommendations (Quantikine HS, R&D Systems Europe Ltd, Abingdon, Oxon, United Kingdom). The lower limits of detection for IL-6 and IL-10 were 0.039 pg/ml and 0.5 pg/ml, respectively.
Statistical analysis
Data were analyzed using the SPSSPC system (Norusis, 1986). For all base characteristics, the significance of any difference in means between patients and controls was tested by using Student's t-test. For all laboratory characteristics, data are presented as median (inter-quartile range) and the significance of differences was tested by using Mann-Whitney test. Chi-square tests were performed to evaluate differences in CMV-IgG and CMV RNA data. Correlation analysis was performed using Pearson r correlation coefficient. Two-tailed p-values < 0.05 were considered significant.
Results
The characteristics of the patients and controls are listed in . All patients were on various combinations of beta-blockers, nitrates and/or calcium antagonists. Ninety-six percent of the SA patients and 33% of the ACS patients were on long-term treatment, i.e > 2 months, with statins. The use of angiotensin-converting enzyme-inhibitors did not differ between SA patients and ACS patients (32% vs. 33%). In all ACS patients, therapy with aspirin, clopidogrel and low molecular heparin was introduced on admission. Seventy-six percent of the SA patients had low-dose aspirin, 6% had clopidogrel and 16% received a combination of aspirin and clopidogrel. Controls were all drug-free.
Table I. Characteristics of patients and controls. Values are given as mean (SD).
CMV-serological data
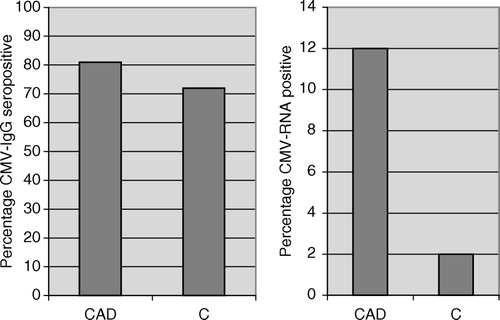
Eighty-five percent of the ACS patients, 78% of the SA patients, and 72% of the controls were CMV-IgG positive (A). None of the patients or controls had detectable serum levels of CMV IgM antibodies.
CMV RNA positivity
Detectable levels of CMV RNA in monocytes, as demonstrated by a nested RT-PCR assay using MIE specific primer pairs, were found in six of 45 (15%) patients with ACS, in five of 50 (10%) patients with SA and in one of 50 (2%) controls (p < 0.001). In total, 12% of all patients were CMV RNA positive (B). Among patients with active CMV infection, 2/11 were female, 2/11 were smokers and 9/11 were on long-term treatment with statins (4/6 ACS patients, 5/5 SA patients).
Inflammatory markers
The levels of IL-6 were significantly higher in the ACS patients compared to the SA patients and the control group whereas the levels of IL-10 did not differ between the groups (). The serum levels of IL-6 were higher in CMV IgG + /RNA+ patients compared to CMV IgG + /RNA- patients and CMV IgG + /RNA- controls (4.7 (1.4–5.6), 2.2 (1.6–3.4) and 2.0 (1.4–2.8) pg/ml, respectively, p < 0.05). Furthermore, the levels of CRP, were significantly higher in the ACS patients compared to the SA patients and the control group (). Although not statistically significant, the serum levels of CRP were higher in CMV + /RNA+ patients compared to CMV IgG + /RNA- patients and CMV IgG + /RNA- controls (2.5 (0.5–10.2), 1.4 (0.7–2.9), 1.7 (0.8–3.4) mg/l, respectively).
Table II. Cytokines in patients and controls. Values are given as medians (interquartile range).
Discussion
Several seroepidemiological studies have previously investigated the link between CMV seropositivity and atherosclerosis with contradictory results. Some have demonstrated an association between prior CMV infection and CAD Citation3, Citation12, whereas others have failed to demonstrate any association Citation10, Citation16. CMV seropositivity, however, indicates mainly previous exposure and does not discriminate between a latent or active infection. In CMV seropositive individuals, monocytes are thought to be responsible for dissemination of the virus and they are also the predominant cell type that harbors CMV in peripheral blood Citation17. The detection of CMV RNA transcripts in monocytes could therefore be considered a sensitive tool to study CMV reactivation in vivo. In this study, we found that 15% of patients with unstable CAD and 10% of patients with stable CAD had an active CMV infection, as assessed by RT-PCR from blood monocytes, compared with only 2% in the control group.
CMV infection is characterized by alternating periods of latency and reactivation. Reactivation of virus has been commonly reported in immunosuppressed individuals, e.g. patients with transplants, human immunodeficiency virus infection and chronic inflammatory diseases undergoing immunosuppressive therapy Citation18–20. Studies on CMV reactivation in healthy and immunocompetent persons are, however, rare. One study has shown CMV reactivation in 1.2% of healthy individuals as assessed by the shedding of CMV in urine Citation21. It has been argued that an already on-going activation of the immune system is necessary for the activation of latent CMV. Latent CMV is reactivated in macrophages from healthy donors through allogeneic stimulation of peripheral blood mononuclear cells and the inflammatory cytokines interferon-γ and tumor necrosis factor-α produced by allogeneically stimulated T cells have been shown to be important for the reactivation of latent virus Citation4, Citation22. Chronic inflammation is a major component of atherosclerosis and there is evidence for an excessive antigen load in the patients with CAD Citation2, Citation3. As we reported in a previous study, CAD is also associated with typical features of immunological aging Citation23. Hence, the persistent challenge of the immune system in CAD may explain why these CAD patients are more susceptible to CMV reactivation compared to healthy subjects.
In the present study, the systemic inflammatory response was, as expected, markedly higher in the ACS patients compared to the SA patients and the controls. The serum levels of IL-6 were also significantly increased in all CAD patients with an active CMV infection. On the other hand, CMV seropositivity without any detectable CMV RNA transcripts in monocytes was not associated with increased inflammatory activity, as assessed by IL-6 and CRP levels.
Some clinical studies on CAD patients have focused on the combined impact of inflammatory activity and CMV seropositivity demonstrating that the predictive power of CMV seropositivity is dependent on the inflammatory response Citation10–12, Citation14. Moreover, one of these studies showed that statin treatment reduced the increased mortality rates associated with CMV seropositivity Citation14. Statin has been found to inhibit the replication of CMV in human endothelial cells Citation24. In the present study, treatment with statin was extensively used and most of the CMV RNA positive patients were on long-term treatment with statins. However, this observation in a limited number of patients does not allow us to draw any conclusions about statin effects on CMV infection. Among several possibilities, factors like suboptimal doses of statins as well as variations in drug responsiveness must be considered.
Taken together, this is the first study to report an increased prevalence of active CMV infection in patients with stable as well as unstable conditions of CAD when compared to healthy subjects. The data indicate that CAD patients are more susceptible to reactivation of CMV. The active CMV infection in CAD patients was related to elevated serum levels of IL-6, a factor that may contribute to the local and/or systemic inflammatory reactions associated with plaque instability. However, further studies are needed to clarify the cause of CMV reactivation in CAD patients and, most importantly, the potential role of active CMV infection in atherogenesis and plaque instability.
Acknowledgements
This work was supported by grants from the Heart-Lung Foundation (20030830 (SG), 20020585(LJ), 199941305 (CSN)), King Gustaf V:s 80-Year Anniversary Foundation, Konung Gustaf Vs och Drottning Victorias Foundation, Groschinskys Foundation, and Pfizer.
References
- Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002; 91: 281–91
- Mayr M, Kiechl S, Willeit J, Wick G, Xu Q. Infections, immunity, and atherosclerosis: Associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation. 2000; 102: 833–9
- Zhu J, Nieto FJ, Horne BD, Anderson JL, Muhlestein JB, Epstein SE. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation. 2001; 103: 45–51
- Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997; 91: 119–26
- Melnick JL, Hu C, Burek J, Adam E, DeBakey ME. Cytomegalovirus DNA in arterial walls of patients with atherosclerosis. J Med Virol. 1994; 42: 170–4
- Hendrix MG, Salimans MM, van Boven CP, Bruggeman CA. High prevalence of latently present cytomegalovirus in arterial walls of patients suffering from grade III atherosclerosis. Am J Pathol. 1990; 136: 23–8
- Streblow DN, Soderberg-Naucler C, Vieira J, Smith P, Wakabayashi E, Ruchti F, et al. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 1999; 99: 511–20
- Zhou YF, Leon MB, Waclawiw MA, Popma JJ, Yu ZX, Finkel T, et al. Association between prior cytomegalovirus infection and the risk of restenosis after coronary atherectomy. N Engl J Med. 1996; 335: 624–30
- Ridker PM, Hennekens CH, Stampfer MJ, Wang F. Prospective study of herpes simplex virus, cytomegalovirus, and the risk of future myocardial infarction and stroke. Circulation. 1998; 98: 2796–9
- Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, et al. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000; 85: 140–6
- Blankenberg S, Rupprecht HJ, Bickel C, Espinola-Klein C, Rippin G, Hafner G, et al. Cytomegalovirus infection with interleukin-6 response predicts cardiac mortality in patients with coronary artery disease. Circulation. 2001; 103: 2915–21
- Muhlestein JB, Horne BD, Carlquist JF, Madsen TE, Bair TL, Pearson RR, et al. Cytomegalovirus seropositivity and C-reactive protein have independent and combined predictive value for mortality in patients with angiographically demonstrated coronary artery disease. Circulation. 2000; 102: 1917–23
- Halcox JP, Deanfield JE. Beyond the laboratory: clinical implications for statin pleiotropy. Circulation. 2004; 109: II42–8
- Horne BD, Muhlestein JB, Carlquist JF, Bair TL, Madsen TE, Hart NI, et al. Statin therapy interacts with cytomegalovirus seropositivity and high C-reactive protein in reducing mortality among patients with angiographically significant coronary disease. Circulation. 2003; 107: 258–63
- Soderberg C, Larsson S, Bergstedt-Lindqvist S, Moller E. Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection. J Virol. 1993; 67: 3166–75
- Haider AW, Wilson PW, Larson MG, Evans JC, Michelson EL, Wolf PA, et al. The association of seropositivity to Helicobacter pylori, Chlamydia pneumoniae, and cytomegalovirus with risk of cardiovascular disease: A prospective study. J Am Coll Cardiol. 2002; 40: 1408–13
- Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991; 72(Pt 9)2059–64
- Britt WA, Alford CA. Cytomegalovirus. Fields Virology. Lippincott-Raven Publishers, Philiadelphia 1996; 2: 2493–2523
- Mori T, Kameda H, Ogawa H, Iizuka A, Sekiguchi N, Takei H, et al. Incidence of cytomegalovirus reactivation in patients with inflammatory connective tissue diseases who are under immunosuppressive therapy. J Rheumatol. 2004; 31: 1349–51
- Cortez KJ, Fischer SH, Fahle GA, Calhoun LB, Childs RW, Barrett AJ, et al. Clinical trial of quantitative real-time polymerase chain reaction for detection of cytomegalovirus in peripheral blood of allogeneic hematopoietic stem-cell transplant recipients. J Infect Dis. 2003; 188: 967–72
- Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis. 2000; 182: 1761–4
- Soderberg-Naucler C, Streblow DN, Fish KN, Allan-Yorke J, Smith PP, Nelson JA. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J Virol. 2001; 75: 7543–54
- Jonasson L, Tompa A, Wikby A. Expansion of peripheral CD8+ T cells in patients with coronary artery disease: relation to cytomegalovirus infection. J Intern Med. 2003; 254: 472–8
- Potena L, Frascaroli G, Grigioni F, Lazzarotto T, Magnani G, Tomasi L, et al. Hydroxymethyl-glutaryl coenzyme a reductase inhibition limits cytomegalovirus infection in human endothelial cells. Circulation. 2004; 109: 532–6