Abstract
Objectives: The BASE-ACS trial demonstrated non-inferiority of titanium-nitride-oxide-coated bioactive stents (BAS), versus everolimus-eluting stents (EES), for major adverse cardiac events (MACE) at 1- and 2-year follow-up, in patients with acute coronary syndrome (ACS). We presented the 4-year outcome of the BASE-ACS trial.
Design: We randomized 827 patients with ACS to receive either BAS (417) or EES (410). MACE was a composite of cardiac death, non-fatal myocardial infarction (MI) or ischemia-driven target lesion revascularization (TLR) at 12-month follow-up. Analyses were performed by intention to treat. Follow-up was planned at 12 months, and yearly thereafter for 5 years.
Results: Four-year clinical follow-up was completed in 753 (91.1%) patients. At 4 years, BAS were non-inferior to EES for MACE (14.7% versus 17.8%, respectively; p = 0.24 for superiority; p = 0.001 for non-inferiority). Non-fatal MI was less frequent with BAS (5.0% versus 9.2%, respectively; p = 0.025). Cardiac death and ischemia-driven TLR were comparable (2.9% versus 3.5%, and 8.6% versus 9.2%; p = 0.62 and p = 0.80, respectively). Independent predictors of MACE were calcified lesions (HR 1.54, p = 0.021), the number of vessels treated (HR 1.53, p = 0.025), and reference vessel diameter (HR 0.54, p = 0.006).
Conclusions: In patients presenting with ACS, BAS was associated with a clinical outcome non-inferior to EES at 4-year follow-up.
Introduction
Evidence from meta-analyses and registries raised concerns about high risk of late – and very late – stent thrombosis (ST) following first-generation drug-eluting stents (DES).[Citation1,Citation2] And although second-generation everolimus-eluting stents (EES) improved safety outcomes compared with first-generation DES, definite/probable ST occurred at an appreciable rate with EES in an adequately powered real-world registry, at 4-year follow-up.[Citation3] Moreover, in approximately 10% of patients who need percutaneous coronary intervention (PCI) with stent implantation, the use of DES is discouraged because of an indication for long-term oral anticoagulation therapy.[Citation4]
The safety of titanium-nitride-oxide-coated bioactive stents (BAS) was established in several reports from real-world unselected populations,[Citation5,Citation6] as well as from randomized clinical trials in patients with acute coronary syndrome (ACS).[Citation7,Citation8] In this regard, the BASE-ACS randomized controlled trial, demonstrated non-inferiority of BAS, compared with EES, for the primary composite endpoint of major adverse cardiac events (MACE) in patients presenting with ACS, at 1- and 2-year follow-up.[Citation8,Citation9] We sought to present the 4-year clinical outcome of the BASE-ACS trial, with special emphasis upon the predictors of definite ST at long-term follow-up.
Materials and methods
Trial design and patient selection
The trial design was previously described elsewhere.[Citation8] In brief, the BASE-ACS trial was a prospective single-blinded multicenter randomized controlled trial conducted in 14 centers, in six (five European and one Southeast Asian) countries. From January 2009 to September 2010, we randomized 827 patients (1:1) presenting with ACS who underwent early PCI to receive either Titan-2® BAS (Hexacath, Paris, France) or Xience V® EES (Abbott Vascular, Santa Clara, CA). Follow-up was planned at 12 months, and yearly thereafter for 5 years.
Procedures and pharmacological intervention
Predilatation of the culprit lesion, PCI technique, selection of access site, antithrombotic agent, and use of glycoprotein IIb/IIIa inhibitors were all left to the operator’s discretion. In patients not maintained on aspirin, the trial protocol recommended premedication with aspirin at a loading dose of 100–500 mg orally, or 250–500 mg intravenously. Clopidogrel was administered at a loading dose of 300–600 mg orally immediately after the index procedure, if the patient was not already maintained on clopidogrel. At discharge, aspirin was prescribed at a dose of 100 mg daily orally, indefinitely, and clopidogrel at a dose of 75 mg daily orally, for at least 6 months. The trial investigators were by necessity not blinded to stent group allocation; however, those who performed data management and analysis, and patients were blinded.
Study endpoints and definitions
Diagnostic criteria for ST-elevation myocardial infarction (MI), non-ST-segment elevation MI, and unstable angina were previously described in detail.[Citation8] The primary endpoint was the first occurrence of MACE, defined as a composite of cardiac death, non-fatal MI (safety endpoints), or ischemia-driven target lesion revascularization (TLR) (efficacy endpoint). The definitions of these endpoints were also previously described.[Citation8] Secondary endpoints included all-cause death, a composite of cardiac death or non-fatal MI, and ST. For the adjudication of 4-year outcome, we adopted the “definite” category of ST as defined by the Academic Research consortium (ARC).[Citation10] An independent clinical event committee whose members were blinded to stent group allocation adjudicated the individual endpoints according to prespecified definitions. A Data and Safety Monitoring Committee reviewed safety data periodically and recommended each time that the trial continues without modification.
Ethical issues
The trial was initiated by the investigators and conducted according to the ethical guidelines of the 1964 Declaration of Helsinki, as revised in 2013. Informed written consent was obtained from every patient after full explanation of the trial protocol. The trial protocol was approved by the Ethics Committees of the co-ordinating center (Satakunta Central Hospital), and the other participating centers. The trial was registered as an international randomized controlled trial in www.clinicaltrials.gov, number NCT00819923.
Statistical analysis
Continuous variables are presented as mean ± SD, whereas categorical variables are described with absolute and relative (percentage) frequencies. Comparisons between the two groups were performed using the unpaired two-tailed t-test for continuous variables, and the Pearson chi-square test or Fisher’s exact test for categorical variables, as appropriate. Time-to-event curves were constructed using the Kaplan–Meier method, and data were compared using the log-rank test. In order to identify the independent predictors of adverse outcome (MACE and definite ST) at 4-year follow-up, initially, univariate logistic regression analysis was performed for each of the baseline, angiographic and procedural variables. Thereafter, the variables significantly associated with the adverse outcome (two-sided p < 0.1) in univariate analyses were included in a multivariable Cox proportional hazard model. All tests were two-sided and statistical significance was set at 5%. All data were analyzed with SPSS for Windows, Version 16.0 (SPSS Inc, Chicago, IL).
Results
Baseline clinical, angiographic, and procedural data
Between January 2009 and September 2010, a total of 827 patients were enrolled in 14 centers, in 6 countries (5 European and 1 Southeast Asian). Patients were randomly assigned to receive either BAS (417 patients, 480 lesions) or EES (410 patients, 484 lesions). The mean age was 63 ± 12 years; 76.1% were males. The baseline clinical, angiographic, and procedural characteristics of the 2 groups were well matched (). The frequency of complex lesions (AHA/ACC type B and C) was comparable between the 2 groups (p = 0.39).
Table 1. Baseline clinical, angiographic, and procedural data of the 2 study groups.
Clinical outcome
Four-year clinical follow-up was completed in 753 (91.1%) patients: 382 (91.6%) in the BAS group and 371 (90.5%) in the EES group. Clinical outcome data are presented in . At 4 years, BAS was non-inferior to EES with respect to the primary composite endpoint of MACE (14.7% versus 17.8%, respectively; hazard ratio (HR) for EES versus BAS, 1.26; 95% confidence interval (CI), 0.85–1.86; p = 0.24 for superiority; p = 0.001 for non-inferiority) (). During the period from 1 to 4 years, the incidence of MACE was lower in patients who received BAS versus those who received EES (4.8% versus 8.8%, respectively, p = 0.039). The rate of non-fatal MI at 4-year follow-up was lower in the BAS group (5.0% versus 9.2%, respectively; HR for EES versus BAS, 1.93; 95% CI, 1.08–3.45; p = 0.025). This was driven by fewer events during the first year (2.2% versus 5.9%, respectively, p = 0.007); yet, the rates were similar from 1 to 4 years (3.0% versus 3.0%, respectively, p = 0.97). At 4 years, the rates of ischemia-driven TLR were comparable between the 2 stent arms (8.6% versus 9.2%, respectively; HR for EES versus BAS, 1.07; 95% CI, 0.65–1.76; p = 0.80). During the first year, the rates were likewise comparable (6.5% versus 4.9%, respectively, p = 0.37); however, from 1 to 4 years ischemia-driven TLR events were fewer in the BAS arm (1.8% versus 4.2%, respectively, p = 0.064). Finally, at 4 years, the rates of all-cause death were comparable between the 2 stent arms (7.1% versus 6.7%, respectively; HR for EES versus BAS, 0.95; 95% CI, 0.54–1.67; p = 0.85).
Figure 1. Kaplan–Meier estimates for primary endpoints over 4 years of follow-up Kaplan–Meier curves show the cumulative incidence of major adverse cardiac events (the primary endpoint), a composite of cardiac death, non-fatal myocardial infarction, or ischemia-driven target lesion revascularization (Panel A); cardiac death (Panel B); non-fatal myocardial infarction (Panel C); and ischemia-driven target lesion revascularization (Panel D). BAS: bioactive stents; EES: everolimus-eluting stents; MACE: major adverse cardiac events; MI: myocardial infarction; TLR: target lesion revascularization.
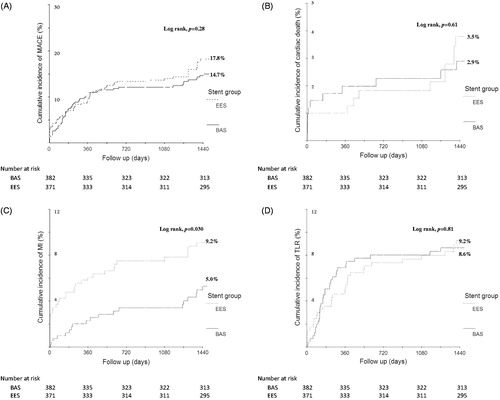
Table 2. Clinical outcome in the 2 study groups at 4-year follow-up.
Additionally, the incidence of ARC-definite ST at 4 years was lower in patients who received BAS (1.0% versus 3.5%, respectively; HR for EES versus BAS, 3.43; 95% CI, 1.11–10.62; p = 0.023) (). Again, this was driven by fewer events during the first year (0.7% versus 2.2%, respectively, p = 0.07); the rates were comparable from 1 to 4 years (0.3% versus 1.2%, respectively, p = 0.21). The timing of definite and definite/probable ST events is summarized in . During the first year, all definite ST events (12 events) occurred while the patients were on clopidogrel, whereas from 1 to 4 years, definite ST events (5 events) occurred after the patients discontinued clopidogrel. At 1-year follow-up, 51.3% of patients in the BAS arm were still maintained on clopidogrel compared with 68.3% of patients in the EES arm (p < 0.001). Yet, at 4 years, no patients in either group were still taking clopidogrel. The mean duration of clopidogrel use was 8.7 ± 3.6 versus 10.2 ± 3.0 months, in the BAS versus EES groups, respectively (p < 0.001).
Figure 2. Kaplan–Meier estimates for definite ST over 4 years of follow-up. BAS: bioactive stents; EES: everolimus-eluting stents; ST: stent thrombosis.
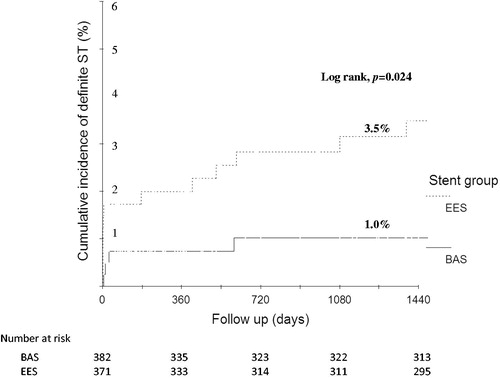
Table 3. Stent thrombosis in the 2 study groups according to the ARC definitions at 4-year follow-up.
Predictors of adverse clinical outcome
In univariate analyses, the predictors of MACE at 4-year follow-up were age above 65 (p = 0.006), diabetes (p = 0.014), hypertension (p < 0.001), smoking (p = 0.013), prior MI (p = 0.007), number of vessels treated (p = 0.014), reference vessel diameter (p = 0.01), and calcified lesions (p = 0.005). In multivariable analysis, the independent predictors of MACE were calcified lesions (HR 1.54, 95% CI 1.06–2.23, p = 0.021), the number of vessels treated (HR 1.53, 95% CI 1.05–2.24, p = 0.025), and reference vessel diameter (HR 0.54, 95% CI 0.35–0.84, p = 0.006).
Similarly, in univariate analyses, the predictors of definite ST at 4-year follow-up were hypertension (p = 0.086), calcified lesions (p = 0.018), and stent type (EES) (p = 0.025). In multivariable analysis, the independent predictors of definite ST were calcified lesions (HR 3.53, 95% CI 1.24–10.05, p = 0.017), and stent type (EES) (HR 3.46, 95% CI 1.12–10.62, p = 0.029).
Discussion
Major findings
The current 4-year report of the BASE-ACS trial demonstrated that in patients undergoing early PCI for ACS, BAS was associated with a rate of cumulative MACE at 4 years that was statistically non-inferior to EES. Moreover, non-fatal MI, and definite ST were lower with BAS versus EES, at 4-year follow-up. The rates of ischemia-driven TLR were statistically similar between the 2 stent arms. To the best of the authors’ knowledge, the BASE-ACS is the first trial to report a head-to-head randomized comparison of BAS versus EES in the setting of ACS, at long-term follow-up.
Safety endpoints
Evidently, the increase in the rate of cummulative MACE associated with EES at 4-year follow-up was largely driven by a substantial increase in non-fatal MI – and probably underlying definite ST. The rates of MACE amounted to an absolute risk reduction of 3.1% with BAS versus EES, which would translate into 31 major events avoided at 4-year follow-up per 1000 ACS patients treated with BAS rather than EES. Consequently, the number of ACS patients needed to treat with BAS rather than EES, to prevent one major event would be 32 patients, a rather small figure in terms of real-world practice. Moreover, the HR for definite ST (safety endpoint) associated with EES versus BAS at 1-year follow-up was 3.1 (p = 0.07). This HR slightly increased to 3.43 (p = 0.023) at 4-year follow-up. The rates of non-fatal MI and definite ST associated with EES at 4-year follow-up in the current report were higher than those reported in the RESOLUTE All-Comers 4-year report (9.2% versus 5.4%, and 3.5% versus 0.7%, respectively).[Citation11] Similarly, the rates of non-fatal MI were lower in the 5-year reports of the SPIRIT III and ISAR TEST 4 trials (3.1% and 5%, respectively); the rates of definite ST were also lower (1.1% and 0.6% respectively).[Citation12,Citation13] The lower rates of non-fatal MI and definite ST associated with EES in the aforementioned trials can be explained in view of the risk profile of enrolled patients. These trials enrolled all-comer populations with much lower risk profile than the current trial. The enrolment of high-risk patients presenting with ACS in the current trial most probably played a key role in the higher incidence of ischemic events in the EES arm, at similar time points of follow-up. Histopathological studies demonstrated that culprit sites in patients presenting with acute MI (underlying plaque rupture) had evidence of more delayed arterial healing (incomplete stent strut coverage and fibrin deposition) compared with culprit sites of patients presenting with stable angina who have underlying fibroatheroma and a thick fibrous cap; the prevalence of late ST was also higher in such patients.[Citation14] Nevertheless, data on “unrestricted” use of EES in real-world clinical practice showed that very late ST occurred at a steady rate of 0.2% per year at long-term follow-up.[Citation3] On the other hand, the incidence of non-fatal MI associated with BAS at 4-year follow-up was lower than that reported with BAS in the TITAX AMI trial at 5-year follow-up (5.0% versus 8.4%, respectively); the rate of definite ST was similar (1% versus 0.9%, respectively).[Citation7] The higher incidence of MI in the TITAX AMI trial might be attributed to slightly longer duration of follow-up, as well as the occurrence of non-target-vessel MI (as suggested by the low rate of definite ST at the same time point).
Efficacy endpoint
In the BASE-ACS trial, the HR for ischemia-driven TLR (efficacy endpoint) associated with EES versus BAS at 1-year follow-up was 0.74 (p = 0.37). This HR was 1.07 (p = 0.80) in the current 4-year report. Obviously, there was a slightly higher rate of ischemia-driven TLR events in the EES arm of the trial during the period from 1-year to 4-year follow-up. This can be explained by the “late catch-up” phenomenon: secondary revascularization beyond 1 year after the index PCI.[Citation15] Late TLR continued to occur without attenuation up to 5 years following sirolimus-eluting stent implantation (2.2% per year) in the j-Cypher Registry.[Citation16] Predictors of late TLR after DES implantation include insulin-dependent diabetes mellitus, stent diameter, and first-generation DES.[Citation17] Neointimal tissue growth in patients with late (after 1 year) in-stent restenosis following sirolimus-eluting stent implantation is characterized by a higher frequency of lipid-laden neointimal, thin-cap fibroatherome-like neointimal, microchannels, and neointimal disruption, compared with that in patients with early (within 1 year) in-stent restenosis following the same stent.[Citation18] To date, the exact mechanism of late in-stent restenosis following second-generation DES remains unclear. Yet, the fact that no routine angiographic follow-up was performed may have influenced the relative rates of TLR between the two stent groups. It is well acknowledged that angiographic follow-up increases the absolute differences of TLR between stents beyond that which would otherwise be observed with clinical follow-up alone. Nevertheless, clinical follow-up more closely reflects real-life practice, avoiding repeat intervention for clinically “silent” angiographic lesions. Interestingly, the incidence of ischemia-driven TLR associated with EES in the current report at 4-year follow-up was slightly higher than that reported in the RESOLUTE All-Comers trial at a similar point of follow-up (9.2% versus 6.5%, respectively).[Citation11] Yet, the rate of ischemia-driven TLR associated with BAS in the current report is less than that reported in the TITAX AMI trial at 5-year follow-up (8.6% versus 11.2%, respectively).[Citation7]
Study limitations
Although the current trial was well powered to detect non-inferiority of BAS versus EES for the primary composite endpoint, it was not adequately powered to address the individual components of safety and efficacy, such as cardiac death, non-fatal MI, or ischemia-driven TLR, nor the secondary endpoints, such as definite ST. Second, the single-blinded nature of the trial is a weakness of the study design. Moreover, the current trial is not an all-comer trial; instead, some exclusion criteria existed in a background cohort of patients presenting with ACS. Exclusion criteria such as aorto-ostial lesions and lesions longer than 28 mm, might have, to some extent, favored the outcome of BAS, introducing selection bias. Another limitation is that 4-year follow-up was available for only 91.2% of the trial population. We also acknowledge the limitation that dual antiplatelet therapy was not extended for 12 months, as recommended by the last update of the guidelines for management of ACS. Whether the results of the current trial can be extrapolated to other second-generation DES would provide a potential avenue for future research. Finally, the TLR was clinically driven, and it may be that an angiographically driven protocol might well have had a different outcome.
Conclusion
In the current 4-year report of the prospective randomized BASE-ACS trial, in patients presenting with ACS who underwent early PCI, BAS achieved a rate of cumulative MACE at 4-year follow-up that was statistically non-inferior to EES. Moreover, non-fatal MI, and definite ST were lower with BAS versus EES, at 4-year follow-up.
Funding information
The study was supported by grants from the Finnish Foundation for Cardiovascular Research, Helsinki, Finland. This work was also supported by unrestricted institutional grant From Hexacath, Paris, France; however, the company had no role in study design, data collection, data analysis, and interpretation, or manuscript writing and submission for publication.
Acknowledgements
The authors thank Tuija Vasankari, RN, Eija Niemelä, RN, and Minna Ampio, RN, for their support in the conduct of this study.
Disclosure statement
All authors state that no conflict of interest exists.
References
- Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs. bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J. 2006;27:2784–2814.
- Lagerqvist B, James SK, Stenestrand U, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–1019.
- Räber L, Magro M, Stefanini GG, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125:1110–1121.
- May AE, Geisler T, Gawaz M. Individualized antithrombotic therapy in high risk patients after coronary stenting. A double-edged sword between thrombosis and bleeding. Thromb Haemost. 2008;99:487–493.
- Mosseri M, Miller H, Tamari I, et al. The titanium-NO stent: results of a multicenter registry. Eurointervention. 2006;2:192–196.
- Karjalainen PP, Ylitalo A, Airaksinen KEJ, et al. Five-year clinical outcome of titanium nitride-oxide-coated bioactive stent implantation in a real world population: a comparison with paclitaxel-eluting stents: The PORI registry. J Interv Cardiol. 2011;24:1–8.
- Tuomainen PO, Ylitalo A, Niemelä M, et al. Five-year clinical outcome of titanium-nitride-oxide-coated bioactive stents versus paclitaxel-eluting stents in patients with acute myocardial infarction: long-term follow-up from the TITAX AMI trial. Int J Cardiol. 2013;168:1214–1219.
- Karjalainen PP, Niemelä M, Airaksinen JK, et al. A prospective randomised comparison of titanium-nitride-oxide-coated bioactive stents with everolimus-eluting stents in acute coronary syndrome: the BASE-ACS trial. EuroIntervention. 2012;8:306–315.
- Romppanen H, Nammas W, Kervinen K, et al. Stent-oriented versus patient-oriented outcome in patients undergoing early percutaneous coronary intervention for acute coronary syndrome: 2-year report from the BASE-ACS trial. Ann Med. 2013;45:488–493.
- Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351.
- Taniwaki M, Stefanini GG, Silber S, et al. 4-year clinical outcomes and predictors of repeat revascularization in patients treated with new-generation drug-eluting stents: a report from the RESOLUTE All-Comers trial (a randomized comparison of a zotarolimus-eluting stent with an everolimus-eluting stent for percutaneous coronary intervention). J Am Coll Cardiol. 2014;63:1617–1625.
- Gada H, Kirtane AJ, Newman W, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6:1263–1266.
- Kufner S, Byrne RA, Valeskini M, et al. Five-year outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: final results from the ISAR-TEST 4 randomised trial. EuroIntervention. 2016;11:1372–1379.
- Nakazawa G, Finn AV, Joner M, et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138–1145.
- Iijima R, Araki T, Nagashima Y, et al. Incidence and predictors of the late catch-up phenomenon after drug-eluting stent implantation. Int J Cardiol. 2013;168:2588–2592.
- Kimura T, Morimoto T, Nakagawa Y, et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation. 2012;125:584–591.
- Choi IJ, Park HJ, Seo SM, et al. Predictors of early and late target lesion revascularization after drug-eluting stent implantation. J Interv Cardiol. 2013;26:137–144.
- Ino Y, Kubo T, Kitabata H, et al. Difference in neointimal appearance between early and late restenosis after sirolimus-eluting stent implantation assessed by optical coherence tomography. Coron Artery Dis. 2013;24:95–101.

