Abstract
Objectives. We explored the predictors and outcome of poor, versus good, initial TIMI flow in patients with acute coronary syndrome (ACS). Design. We performed post-hoc analysis of a randomized trial of patients presenting with ACS who received 2 comparative stents. Poor initial TIMI flow was defined as baseline TIMI flow grade 0/1 at the initial coronary angiography. The primary endpoint was major adverse cardiac events (MACE): a composite of cardiac death, non-fatal myocardial infarction or ischemia-driven target lesion revascularization. Stent thrombosis (ST) was adjudicated according to the criteria of definite ST described by the Academic Research Consortium. Propensity score-matched analysis was performed. We report data after 5-year follow-up. Results. Of 827 patients enrolled, 279 (33.7%) had initial TIMI 0/1 flow. Median follow-up duration was 5.0 years. Presentation by ST-elevation myocardial infarction and target vessel other than left anterior descending artery predicted initial TIMI 0/1 flow. MACE rate was comparable between the 2 subgroups (14% versus 15.9%, in patients with poor versus good initial TIMI flow, respectively, p = .46). Individual endpoints were comparable (p > .05 for all). Definite ST was more frequent in patients with initial TIMI 0/1 flow (3.6% versus 1.5%, respectively, p = .048). This was driven by more frequent early events (30 days) (p = .036); late/very late events were comparable (p = 1.0). Conclusions. Predictors of poor initial TIMI flow included presentation by ST-elevation myocardial infarction, and target vessel other than left anterior descending artery. Definite ST occurred more in patients with poor, versus good, initial TIMI flow, mainly driven by difference in early events.
Introduction
Previous studies demonstrated that infarct-related artery patency early before primary percutaneous coronary intervention (PCI) is associated with a better immediate angiographic outcome, and independently predicted better survival at mid-term follow-up, in patients presenting with acute ST-elevation myocardial infarction (STEMI) [Citation1–3]. Predictors of initial infarct-related artery patency in patients presenting with STEMI were previously reported [Citation4,Citation5]. Yet, predictors and outcome of poor initial TIMI flow in patients treated by early PCI for acute coronary syndrome (ACS) remain unclear. Several reports demonstrated safety of titanium-nitride-oxide-coated stents in unselected cohorts [Citation6,Citation7], and in randomized trials of patients with ACS [Citation8,Citation9]. BASE ACS trial, showed non-inferiority of titanium-nitride-oxide-coated versus everolimus-eluting stents, for the primary endpoint of major adverse cardiac events (MACE) in patients with ACS at mid- and long-term follow-up [Citation9–12]. In a post-hoc analysis of the trial, we explored the incidence, predictors, and long-term outcome of poor initial Thrombolysis In Myocardial Infarction (TIMI) flow in patients treated by early PCI for ACS.
Materials and methods
Patient selection and study design
The trial design was described previously [Citation9]. Briefly, BASE ACS trial was a prospective single-blinded randomized (1:1) trial in which 827 patients who underwent early PCI for ACS received either titanium-nitride-oxide-coated or everolimus-eluting stents. Early PCI entailed undertaking coronary angioplasty and stent implantation within 12 hours of symptom onset in patients presenting with STEMI, and within 72 hours in those presenting with non-ST-segment elevation ACS (NSTEACS). Follow-up was planned at 12 months, and yearly thereafter through 7 years. The trial was initiated by the investigators and conducted according to the ethical guidelines of the 1964 Declaration of Helsinki, as revised in 2013. Informed written consent was obtained from every patient after explanation of the trial protocol; the protocol was approved by the ethics committees of the participating centers. The trial is registered under ClinicalTrials.gov, with number NCT00819923.
Pharmacological interventions
Patients not previously maintained on aspirin were pretreated with aspirin at a loading dose of 250 mg orally or 250–500 mg intravenously, and continued at a dose of 75–150 mg daily indefinitely. Oral clopidogrel was initiated at a loading dose of 300–600 mg before or immediately after the procedure and continued at a dose of 75 mg daily. Patients in either group were prescribed clopidogrel for a minimum of 6 months, and thereafter, for extended periods (maximum 12 months) at operator’s discretion. During the procedure, low-molecular-weight (enoxaparin sodium 1 mg/kg every 12 hours subcutaneously) or unfractionated heparin (70–100 IU/kg intravenous bolus, or 50–70 IU/kg intravenous bolus if concomitant glycoprotein IIb IIIa inhibitors were used) was administered in the standard dosage. Use of glycoprotein IIb IIIa inhibitors or bivalirudin was left to operator’s discretion.
Definitions and study endpoints
TIMI flow is graded as follows: grade 0 indicates no flow; grade 1, penetration without perfusion; grade 2, full perfusion with slow flow, and grade 3, full perfusion with normal flow. Poor initial TIMI flow was defined as baseline TIMI flow grade 0 or 1 at the initial coronary angiography. The diagnostic criteria for NSTEACS and STEMI were previously described [Citation9]. The primary endpoint was the first occurrence of MACE: a composite of cardiac death, non-fatal myocardial infarction (MI), or ischemia-driven target lesion revascularization (TLR). Secondary endpoints included non-cardiac death, and definite stent thrombosis (ST). Cardiac death was defined as death from cardiovascular causes or any death without known cause. ST was adjudicated according to the criteria of definite ST described by the Academic Research Consortium [Citation13]. An independent clinical events committee whose members were blinded to stent group allocation adjudicated all the individual endpoints according to the prespecified definitions.
Statistical analysis
Continuous variables were presented as mean ± standard deviation, whereas categorical variables were described with absolute and relative (percentage) frequencies. Comparisons between the 2 groups (patients with initial TIMI 0/1 flow versus those with initial TIMI 2/3 flow) were performed using the unpaired t-test for continuous variables, and Pearson chi-square test or Fisher’s exact test for categorical variables, as appropriate. In order to identify the independent predictors of initial TIMI 0/1 flow, univariate analysis was initially performed for baseline clinical, angiographic and procedural variables. Then, the variables significantly associated (2-sided p < 0.1) with initial TIMI 0/1 flow in univariate analysis were included as covariates in a multivariable logistic regression model in which the dependent variable was initial TIMI 0/1 flow. The results of multivariable regression were presented as hazard ratio (HR) with 95% confidence interval (CI). We observed significant differences between the 2 groups for baseline characteristics. Therefore, we performed propensity score-matched analysis of the 2 groups in order to estimate the impact of initial TIMI 0/1 flow on the clinical outcome. We calculated the propensity score using a logistic regression model in which we included – as covariates – the baseline variables with a difference between the 2 groups as indicated by a p < .1 in univariate analysis. The unmatched group variable (initial TIMI 0/1 versus initial TIMI 2/3) was entered in the model as the dependent variable. Probabilities predicted by the model were saved as a new variable: propensity score, which was then used to identify and select the matched pairs. Hosmer-Lemeshow test was used to assess the fit of the logistic regression model (chi-square: 10.11, p = .25). Finally, we employed the “Caliper and Radius” matching method for selection of the matched pairs. Matching was performed based on an estimated caliper width of 0.2 the standard deviation of the propensity score logit. Kaplan–Meier estimates of event rates were used to construct time-to-event curves, and were compared with the log-rank test. These estimates were based on all the available data for MACE, with follow-up data censored at the time of first event, or latest known follow-up. All tests were 2-sided and statistical significance was set at 5%. Data were analysed using SPSS v. 20 (SPSS IBM Inc., Chicago, IL).
Results
Baseline data
Of 827 patients enrolled in the trial, 279 (33.7%) had initial TIMI 0/1 flow. Median follow-up duration was 5.0 years; mean (SD) 4.2 (1.9) years. The prevalence of initial TIMI 0/1 flow was much higher in patients who presented with STEMI, compared with those who presented with NSTEACS (65.1% versus 13.9%, respectively, p < .001). Compared with those who had initial TIMI 2/3 flow, patients with initial TIMI 0/1 flow were younger, more often male smokers, but less likely to have hypertension and dyslipidemia. Expectedly, they presented more frequently with STEMI. They had less often prior coronary events, but more often complex, longer (but less calcified) lesions. Final TIMI flow grade 3 was less frequently observed in patients with initial TIMI 0/1 flow. Baseline data are summarized in .
Table 1. Baseline clinical, angiographic and procedural characteristics of the 2 unmatched groups.
Predictors of initial TIMI 0/1
In univariate analysis, the predictors of initial TIMI 0/1 flow were younger age (r = −0.14, p < .001), male gender (r = −0.059, p = .089), smoking (r = .098, p = .005), presentation with STEMI (r = 0.52, p < .001), absence of dyslipidemia (r = −0.15, p < .001), absence of hypertension (r = −0.16, p < .001), absence of prior MI (r = −0.067, p = .053), absence of prior PCI (r = −0.092, p = .008), and target vessel other than the left anterior descending artery (r= −0.087, p = .012). In multivariable analysis, the independent predictors of initial TIMI 0/1 flow were presentation with STEMI (HR 12.0, 95% CI 8.47–17.01, p < .001), and target vessel other than the left anterior descending artery (HR 0.59, 95% CI 0.42–0.84, p = .003).
Long-term clinical outcome in crude groups
Five-year follow-up was complete in 458 (55.4%) patients: 153 (out of 279) patients in those with initial TIMI 0/1 flow, and 305 (out of 547) in those with initial TIMI 2/3 flow. The cumulative incidence of MACE at long-term follow-up was comparable between the 2 groups (14% versus 15.9%, in patients with initial TIMI 0/1 flow versus those with initial TIMI 2/3 flow respectively, p = .46) ( and ). The rates of cardiac death, non-fatal MI, and ischemia-driven TLR were comparable (p > .05 for all). Yet, the incidence of definite ST was higher in patients who presented with initial TIMI 0/1 flow (3.6% versus 1.5%, respectively, p = .048). This was mainly due to a difference in early definite ST events (within 30 days) (2.5% versus 0.5%, respectively, p = .036); the rates of definite ST events occurring after 30 days were comparable (1.1% versus 0.9%, respectively, p = 1.0) ().
Figure 1. Kaplan-Meier estimates of the primary endpoint (a composite of cardiac death, non-fatal myocardial infarction, or ischemia-driven target lesion revascularization) in the 2 groups at long-term follow-up. MACE: major adverse cardiac events.
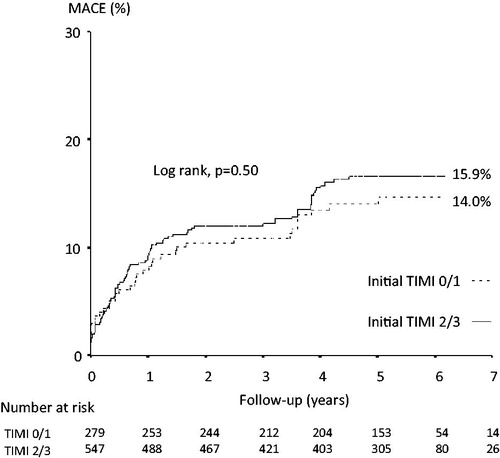
Table 2. Clinical outcome of the 2 unmatched groups at long-term follow-up.
Long-term clinical outcome in matched groups
Propensity score matching yielded 216 patients (108 pairs) with balanced baseline characteristics (). In the propensity score-matched pairs, MACE and all the individual endpoints were comparable between the 2 matched groups (p > .05 for all) ().
Table 3. Baseline clinical, angiographic and procedural characteristics of the 2 matched groups.
Table 4. Clinical outcome of the 2 matched groups at long-term follow-up.
Discussion
Main findings
In the current post-hoc analysis of a randomized trial in patients treated by early PCI for ACS, poor initial TIMI flow occurred in one-third of the patients. Predictors of poor initial TIMI flow included presentation with STEMI, and the target vessel not being the left anterior descending artery. The long-term cumulative incidence of definite ST was higher in patients who presented with poor, versus those who presented with good, initial TIMI flow in crude analysis; this was mainly due to a difference in early events (within 30 days). However, such incidence was comparable between the 2 groups in propensity score-matched analysis.
Predictor of infarct-related artery patency
In the current study, presentation with STEMI independently predicted poor initial TIMI flow in patients treated by early PCI for ACS (within 12 hours of symptom onset in patients who presented with STEMI, and within 72 hours in those who presented with NSTEACS). The prevalence of poor initial TIMI flow in patients who presented with STEMI the current study was similar to prior reports [Citation1–3,Citation5]. Previous studies reported the predictors of infarct-related artery patency in patients presenting with STEMI. In a pooled analysis of 2 large trials, infarct-related artery patency (TIMI flow grade 3) was independently associated with diabetes mellitus, smoking, extent of coronary artery disease, and longer door-to-device time [Citation2]. In an observational study of patients who received prehospital thrombolytic therapy, the independent predictors of initial TIMI 3 flow in the infarct-related artery included smoking, Killip class I, ST-segment elevation in no more than 5 leads before thrombolysis, chest pain relief and ST-segment resolution after thrombolysis [Citation4]. In recent registry data, the independent predictors of infarct-related artery patency (TIMI flow grade 2/3) included pre-hospital use of rapid-acting potent antiplatelet agents (glycoprotein IIb IIIa inhibitors, and prasugrel), shorter time from symptom onset to ECG diagnosis, longer time from ECG diagnosis to angiography, beta blocker use before the index MI, and the absence of prior PCI [Citation5]. Other studies highlighted the role of blood indices such as the neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, leucocytic count, and mean platelet volume as negative predictors of infarct-related artery patency [Citation14–17]. Moreover, biochemical markers such as uric acid and homocysteine were independently associated with infarct-related artery occlusion [Citation14,Citation15,Citation18]. Infarct-related artery other than the left anterior descending artery was independently associated with poor initial TIMI flow in ACS patients in the current study. Similarly, the prevalence of left anterior descending artery as the infarct-related artery was lower in patients with poor (grade 0/1), versus those with good (grade 2/3) initial TIMI flow in a post-hoc analysis of the HORIZONS AMI trial (2 × 2 factorial design randomized trial that compared bivalirudin with provisional glycoprotein IIb IIIa inhibitors versus unfractionated heparin with routine glycoprotein IIb IIIa inhibitors, and paclitaxel-eluting stents versus bare-metal stents, in patients who underwent primary PCI for acute STEMI) [Citation1]. Comparably, in patients presenting with non-STEMI, the prevalence of infarct-related artery patency (TIMI flow grade 2/3) was higher in those with pre-MI angina (p = .03) [Citation19]; unfortunately, data on pre-MI angina are not available in the current report.
Outcome of patients with initial infarct-related artery occlusion
In the current study, patients who presented with ACS and had poor initial TIMI flow developed more frequent definite ST events at long-term follow-up, versus those who had good flow, clearly driven by more frequent early (30 days) events. Occluded infarct-related artery at initial coronary angiography implies greater thrombus burden, which might be associated with more residual thrombus after PCI, or might lead to thrombus compression by the stent, followed thereafter by abluminal thrombus resolution and “late acquired” stent strut malapposition; both are known to portend the risk of ST. In a post-hoc analysis of patients presenting with NSTEACS from the ACUITY trial (randomized trial that compared 3 antithrombotic regimens: heparin plus a glycoprotein IIb IIIa inhibitor, bivalirudin plus a glycoprotein IIb IIIa inhibitor, or bivalirudin monotherapy, in patients presenting with moderate- or high-risk NSTEACS), the presence of thrombus on baseline angiography predicted definite/probable ST, as well as death and MI at 30 days following PCI [Citation20]. Yet, similar to the current report, the rates of “late” (30 days to 12 months) ST and MI were comparable between patients with and without baseline thrombus; “late” death rate was even lower in patients with thrombus in landmark analysis [Citation20]. Lower-risk profile of patients who had baseline thrombus probably explains why the risk of early events imposed by the presence of thrombus was not persistent at longer follow-up. Similarly, in the current report, patients with poor initial TIMI flow were younger, had less frequent risk factors and prior coronary events, and less calcified target vessels, versus those with good initial TIMI flow. Likewise, in a retrospective analysis of patients treated with drug-eluting stents for STEMI, large thrombus burden (TIMI thrombus grade 4 versus grade <4) was the strongest predictor of ST at 2-year follow-up [Citation21]. Moreover, in a post-hoc analysis of patients treated by primary PCI for STEMI in a randomized trial, initial TIMI flow grade 0/1 was associated with higher rates of 1-year mortality and definite/probable ST; infarct-related artery patency was an independent predictor of reduced mortality at 1 year [Citation1]. Similar to the current report, the higher cumulative rate of definite/probable ST was driven by more “acute” (≤24 hours, p = .001) and “subacute” (1–30 days, p = .050) events; the rates of “late” (30 day to 1 year) events were comparable between the 2 groups (p = .9) [Citation1]. On the other hand, in an evaluation by intravascular ultrasound at long-term follow-up, late acquired strut malapposition could be attributed to positive vessel remodelling and/or plaque/thrombus resolution [Citation22]. In a meta-analysis of 17 studies with intravascular ultrasound performed at 6–9 months, the risk of (very) late ST was 6-fold higher in patients with, versus those without, late stent malapposition [Citation23]. Variations in the timing and dosage of antithrombotic medications could account, at least in part, for the different rates of early ST between groups, and could, therefore, confound the results. In general, the above data highlight the importance of more potent antiplatelet and antithrombotic therapy in patients presenting with ACS who have poor initial TIMI flow at baseline angiography. However, it should be noted that the current study is underpowered for comparison of ST events even at long-term follow-up; hence, we cannot rule out the possibility of type I statistical error underlying this finding. The current findings should, therefore, be taken as hypothesis-generating, rather than conclusive.
Limitations of the study
The trial was not designed a priori to explore differences in outcome based on initial TIMI flow. Due to the retrospective nature of this post-hoc analysis, some data relevant to the outcome might have been missed. Although relevant for the occurrence of ST, information is lacking on the medical treatment received prior to the index event, as well as on the timing and actual dose of antithrombotic medications received in the trial. Additionally, the trial is underpowered for specific subgroup analysis. Moreover, analysis of patient data in one subgroup with different stent designs should also be interpreted with caution. Finally, the current post-hoc analysis was a non-randomised subgroup analysis; this might limit the conclusiveness of the results.
Conclusions
In patients treated by early PCI for ACS, poor initial TIMI flow occurred in one-third of the patients; predictors of poor initial TIMI flow included presentation with STEMI, and the target vessel not being the left anterior descending artery. The long-term incidence of definite ST was higher in patients with poor, versus those with good, initial TIMI flow, mainly driven by a difference in early events.
Acknowledgements
The authors thank Tuija Vasankari, Eija Niemelä, and Minna Ampio, for their support in the conduct of this study.
Disclosure statement
The authors report no conflicts of interest.
Funding
The current post-hoc analysis received no grants from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Rakowski T, Dudek D, Dziewierz A, et al. Impact of infarct-related artery patency before primary PCI on outcome in patients with ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. EuroIntervention. 2013;8:1307–1314.
- Brener SJ, Mehran R, Brodie BR, et al. Predictors and implications of coronary infarct artery patency at initial angiography in patients with acute myocardial infarction (from the CADILLAC and HORIZONS-AMI Trials). Am J Cardiol. 2011;108:918–923.
- Rakowski T, Siudak Z, Dziewierz A, et al. Impact of infarct related artery patency after early abciximab administration on one-year mortality in patients with ST-segment elevation myocardial infarction (data from the EUROTRANSFER Registry). Kardiol Pol. 2012;70:215–221.
- Bongard V, Puel J, Savary D, et al. Predictors of infarct artery patency after prehospital thrombolysis: the multicentre, prospective, observational OPTIMAL study. Heart. 2009;95:799–806.
- Bailleul C, Puymirat E, Aissaoui N, et al. Factors associated with infarct-related artery patency before primary percutaneous coronary intervention for st-elevation myocardial infarction (from the FAST-MI 2010 registry). Am J Cardiol. 2016;117:17–21.
- Mosseri M, Miller H, Tamari I, et al. The titanium-NO stent: results of a multicenter registry. Eurointervention. 2006;2:192–196.
- Karjalainen PP, Ylitalo A, Airaksinen KEJ, et al. Five-year clinical outcome of titanium nitride-oxide-coated bioactive stent implantation in a real world population: a comparison with paclitaxel-eluting stents: The PORI registry. J Interv Cardiol. 2011;24:1–8.
- Tuomainen PO, Ylitalo A, Niemelä M, et al. Five-year clinical outcome of titanium-nitride-oxide-coated bioactive stents versus paclitaxel-eluting stents in patients with acute myocardial infarction: long-term follow-up from the TITAX AMI trial. Int J Cardiol. 2013;168:1214–1219.
- Karjalainen PP, Niemelä M, Airaksinen JK, et al. A prospective randomised comparison of titanium-nitride-oxide-coated bioactive stents with everolimus-eluting stents in acute coronary syndrome: the BASE-ACS trial. EuroIntervention. 2012;8:306–315.
- Romppanen H, Nammas W, Kervinen K, et al. Karjalainen PP, Stent-oriented versus patient-oriented outcome in patients undergoing early percutaneous coronary intervention for acute coronary syndrome: 2-year report from the BASE-ACS trial. Ann Med. 2013;45:488–493.
- Karjalainen PP, Niemelä M, Pietilä M, et al. Clinical outcome of titanium-nitride-oxide-coated bioactive stents versus everolimus-eluting stents in acute coronary syndrome: 4-year report of the BASE-ACS trial. Scand Cardiovasc J. 2016;50:218–223.
- Karjalainen PP, Nammas W, Ylitalo A, et al. Long-term clinical outcome of titanium-nitride-oxide-coated stents versus everolimus-eluting stents in acute coronary syndrome: Final report of the BASE ACS trial. Int J Cardiol. 2016;222:275–280.
- Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351.
- Sahin DY, Gür M, Elbasan Z, et al. Predictors of preinterventional patency of infarct-related artery in patients with ST-segment elevation myocardial infarction: importance of neutrophil to lymphocyte ratio and uric acid level. Exp Clin Cardiol. 2013;18:e77–e81.
- Acet H, Ertaş F, Akıl MA, et al. Novel predictors of infarct-related artery patency for ST-segment elevation myocardial infarction: platelet-to-lymphocyte ratio, uric acid, and neutrophil-to-lymphocyte ratio. Anatol J Cardiol. 2015;15:648–656.
- Yayla Ç, Akboğa MK, Canpolat U, et al. Platelet to Lymphocyte ratio can be a predictor of infarct-related artery patency in patients with ST-segment elevation myocardial infarction. Angiology. 2015;66:831–836.
- Maden O, Kacmaz F, Selcuk MT, et al. Relationship of admission haematological indices with infarct-related artery patency in patients with acute ST-segment elevation myocardial infarction treated with primary angioplasty. Coron Artery Dis. 2007;18:639–644.
- Keleş T, Yeter E, Durmaz T, et al. Relation of homocysteine levels with patency and flow rate of infarct-related artery in patients receiving fibrinolytic therapy. Anadolu Kardiyol Derg. 2010;10:410–415.
- Misumida N, Kobayashi A, Saeed M, et al. Association between preinfarction angina and angiographic findings in non-ST-segment elevation myocardial infarction. Clin Cardiol. 2015;38:535–541.
- Goto K, Lansky AJ, Nikolsky E, et al. Prognostic significance of coronary thrombus in patients undergoing percutaneous coronary intervention for acute coronary syndromes: a subanalysis of the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. JACC Cardiovasc Interv. 2011;4:769–777.
- Sianos G, Papafaklis MI, Daemen J, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol. 2007;50:573–583.
- Hong MK, Mintz GS, Lee CW, et al. Late stent malapposition after drug-eluting stent implantation: an intravascular ultrasound analysis with long-term follow-up. Circulation. 2006;113:414–419.
- Hassan AK, Bergheanu SC, Stijnen T, et al. Late stent malapposition risk is higher after drug-eluting stent compared with bare-metal stent implantation and associates with late stent thrombosis. Eur Heart J. 2010;31:1172–1180.

