Abstract
Objective. Temporal trends in admissions for atrial fibrillation (AF) and severe bleeding associated with AF vary worldwide. We aimed to explore their temporal trends in England and their relation to the introduction of DOACs in 2014 in the UK. Design. This longitudinal ecological study utilised aggregated data that was extracted from the Hospital Episode Statistics database, which captured annual admissions for AF and severe bleeding associated with AF between 2001 and 2018. Trends in admissions over the study period and across age groups, gender and regions in England were assessed. Results. In total, there were 11,292,177 admissions for AF and 324,851 admissions for severe bleeding associated with AF. There was a steady rise in admissions for AF from 2001 to 2017 (204,808 to 1,109,295; p for trend<.001). A similar trend was observed for severe bleeding (4940 to 30,169; p for trend <.001), but the increase dropped slightly between 2013 and 2014 and continued thereafter. Conclusions. There was a rise in admissions for AF and severe bleeding in England between 2001 and 2018. There is little evidence that the slight drop in admissions for severe bleeding between 2013 and 2014 may have been caused by the introduction of DOACs in 2014. Contributors to these trends need urgent exploration.
Introduction
Atrial fibrillation (AF) is the most commonly encountered cardiac arrhythmia in clinical practice globally. It is associated with increased morbidity (measured by disability adjusted life years (DALYs)), mortality and healthcare resource utilisation [Citation1]. With increasing population ageing and survival with chronic diseases, there has been a proportionate increase in the prevalence and incidence of AF, which justifies the use of the term “global epidemic” [Citation2]. Among other clinical outcomes such as systemic embolism, AF is associated with about a five-fold increased risk of ischaemic stroke [Citation3]. Primary prevention of AF has always been the ultimate goal, but this approach has been met with challenges because of huge knowledge gaps with respect to the pathophysiology of AF [Citation1]. The role of anticoagulants for the prevention of stroke and systemic embolism in patients with AF is well established. The main challenges associated with anticoagulant therapy is achieving a balance between the threat of bleeding and the prevention of a disabling stroke. Vitamin K antagonists (VKAs) have been the first-choice treatment for stroke prevention in AF; warfarin remains the most commonly used anticoagulant and was the only one on the World Health Organization’s (WHO) Essential Medicines list (EML) [Citation4] till recently [Citation5]. Though VKAs are effective and cause substantial reductions in stroke, systemic embolism and all-cause mortality [Citation6], they are associated with limitations which include the need for frequent monitoring and dose-adjustment and an increased risk of major bleeding events [Citation7,Citation8]. Since 2009, several new oral anticoagulants – direct oral anticoagulants (DOACs) – have been developed and approved for use by regulatory authorities [Citation9–12]. The DOACs (comprising dabigatran, rivaroxaban, apixaban and edoxaban) have comparable efficacy and safety profiles with warfarin, but have several advantages such as rapid onset, fewer drug interactions, lower rates of bleeding and simplified treatment without the need for regular international normalised ratio (INR) monitoring [Citation13]. Their use has increased substantially because of their efficacy in stroke or systemic embolism prevention in AF among the general population [Citation14–16]. They have recently been successfully included in the 21st WHO Model List of Essential Medicines [Citation5]. In 2014, the UK National Institute for Health and Care Excellence (NICE) guidelines recommended the use of DOACs for stroke prevention in patients with non-valvular AF in the UK. Though the DOACs are generally associated with decreased risk of bleeding complications [Citation17], there have been recent concerns about bleeding risks attributed to them, especially with rivaroxaban [Citation18]. With the widespread uptake of DOACs into clinical practice, trends in admissions for severe bleeding in AF have varied worldwide. Some studies have reported an increase in hospital admissions for bleeding attributable to DOACs [Citation19,Citation20], others have reported no significant increases in admissions for bleeding complications [Citation21], whereas others have reported less admissions for bleeding complications [Citation22]. Given the uncertainty in the evidence, we aimed to explore temporal trends in admissions for AF and severe bleeding associated with AF in England and their relation to the introduction of DOACs in 2014 in the UK.
Methods
Setting and data sources
Study design was a longitudinal ecological study. Aggregate data on annual admissions for AF and severe bleeding associated with AF between 2001 and 2018 were extracted from the Hospital Episode Statistics (HES) database. The HES database includes data on all NHS-funded admissions, outpatient appointments and emergency department attendances in England. All diagnoses were coded using the International Classification of Diseases version 10 codes (ICD-10). Severe bleeding included intracranial bleed, haematuria, haemoptysis, and upper and lower gastrointestinal bleed. The codes used in the classification of severe bleeding are listed in Supplementary Material 1. This study was registered with the local Clinical Audit Department and did not require ethics approval or patient consent. Data were used in line with the data sharing agreement with NHS Digital.
Statistical analysis
Descriptive statistics were used to summarise the aggregated data; participants’ characteristics were expressed as frequencies and percentages for categorical data. Temporal trends in admissions over the study period and across age groups, gender and regions in England were assessed. To better visualise the trends, line plots were generated. Trend analyses across the study years (2001–2004, 2005–2008, 2009–2013, and 2014–2017) were performed using Jonckheere-Terpstra test. All analyses were conducted using Stata version MP 17 (Stata Corp, College Station, Texas).
Results
Admissions for AF and severe bleeding during study period
During the period of data collection from 2001 to 2018, there were 11,292,177 admissions for AF and 324,851 admissions for severe bleeding with AF (). More males than females were admitted for AF (54.3%) and severe bleeding (59.1%) during the study period.
Table 1. Admissions for atrial fibrillation and severe bleeding by general characteristics of the population.
Temporal trends in admissions for AF
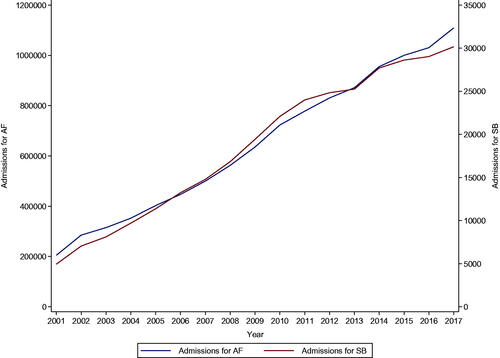
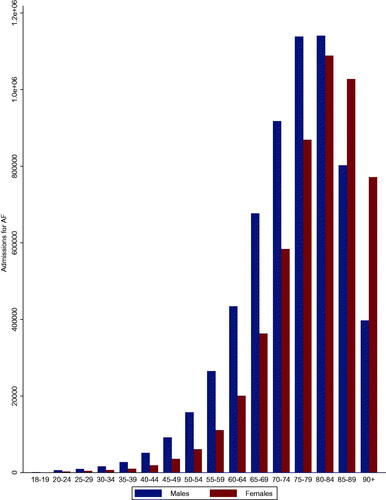
There was a steady increase in admissions for AF from 2001 to 2017 (204,808 to 1,109,295 cases; p for trend<.001) (). Admissions for AF increased with age from 18 to 19 and peaked at 80–84 with a slight drop in those aged 85–89 and 90+. Admissions for AF were higher in males than females across all age groups except for those aged 85–89 and 90+ ().
Temporal trends in admissions for severe bleeding in people with AF
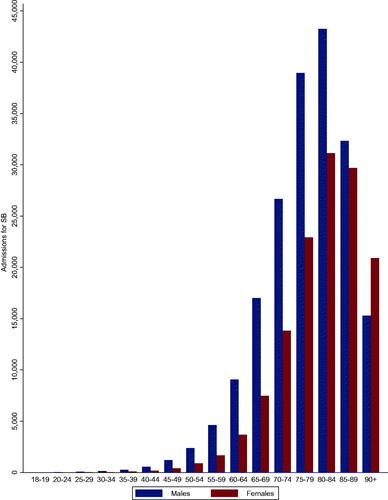
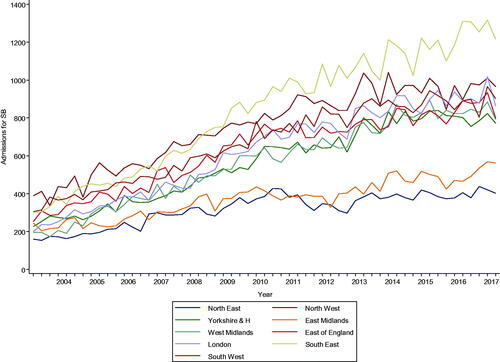
There was an increase in admissions for severe bleeding from 2001 to 2017 (4940 to 30,169 cases; p for trend<.001); however, there was a slight drop between 2013 and 2014 followed by a gradual increase thereafter (). Admissions for severe bleeding increased with age from 18 to 19 and peaked at 80–84 with a drop in those aged 85–89 and 90+. Admissions were higher in males across all age groups except for those aged 90+ (). Quarterly admissions for severe bleeding according to regions showed increases across all regions from 2004 to 2018 (p trend for all <.001), but the increases were steeper for the South East and North West regions, with more gradual increases for the East Midlands and North East regions ().
Discussion
Key findings
In this study, there was a steady increase in admissions for AF and severe bleeding over time between 2001 and 2017. For severe bleeding, there was a slight drop between the years 2013–2014, with a subsequent gradual increase thereafter. Admissions for AF and severe bleeding increased with age from 18–19 to 80–84 and were higher in males than females. Admissions for severe bleeding increased with time across all regions, but the increases were steeper for the South East and North West regions.
Comparisons with previous studies
Although the incidence and prevalence of AF vary from country to country, most studies have reported an increase in incidence and prevalence with time, with the increases projected to be substantial in the coming decades. Indeed, AF has been referred to as a global epidemic [Citation2]. In analysis of the UK Clinical Practice Research Datalink (CPRD) from 1998 to 2010 of patients with incident AF, it was reported that there was a temporal increase in AF over the period, followed by a plateau between 2007 and 2010, with a continued increase in patients over 75 years in both men and women [Citation23]. Among residents of Reykjavik, Iceland who were diagnosed with AF at a healthcare facility, the incidence of AF increased in women but not men from 1991 to 2008 [Citation24]. It was projected that the number of patients with AF was expected to triple in the next four decades. In a study of adult residents of Olmsted County, Minnesota, USA, who had ECG-confirmed first AF in the period 1980 to 2000, the age-adjusted incidence of AF increased significantly during the period, and it was projected that the number of persons with AF for the USA will exceed 10 million by 2050 [Citation25]. In a temporal trend analysis of emergency department visits of patients with AF using the Korean nationwide claims database between 2006 and 2017, the annual numbers of AF patients continuously increased during the 12 years [Citation26]. In contrast, worldwide data on temporal trends in admissions for severe bleeding associated with AF have not been consistent. In a UK study that aimed to evaluate changes in bleeding rates following an AF quality improvement initiative using hospital admissions data from 2011 to 2018, no significant changes were observed in bleeding admissions [Citation27]. In the temporal trend analysis of emergency department visits of patients with AF using the Korean nationwide claims database between 2006 and 2017, major bleeding events among the AF population increased slightly over the 12 years [Citation26]. In an analysis of an Italian administrative database of 12 million people to investigate changes in hospitalisations for AF and related outcomes between 2012 and 2016, in addition to the number of AF cases increasing over time, hospitalisations for major bleeds, mainly gastrointestinal, increased from 1.5% in 2012–2013 to 2.3% in 2015–2016 [Citation28].
Potential explanation of findings
The steady and temporal increase in admissions for AF is not unexpected. It is well known that the incidence of AF increases substantially with age and is higher in males than females [Citation29,Citation30]. Among adults aged 40 and above, the lifetime risk of developing AF is approximately 1 in 4 s [Citation29,Citation30]. Increasing population ageing and improved survival from cardiovascular conditions have contributed to the increased prevalence and incidence of AF. Increased awareness of AF and initiatives to improve the detection of AF [Citation31,Citation32], such as the UK Quality Outcomes Framework [Citation33], have also led to the steady increase in AF prevalence and incidence [Citation23]. The increased adoption of unhealthy lifestyles such as heavy alcohol intake, smoking, physical inactivity, which are AF-associated risk factors, have led to increases in the prevalence of obesity, CVD and diabetes, which substantially increase the risk of AF [Citation2]. With increasing admissions for AF, it is not surprising that this is associated with a concomitant increase in admissions for severe bleeding as bleeding-related complications are the most common side effects of anticoagulants, which are the main stay of treatment in preventing strokes in AF. There was a slight drop in admissions for severe bleeding between the years 2013–2014, the period during which UK NICE guidelines recommended the use of DOACs. This could just be a chance finding or the use of DOACs may have contributed to the drop in admissions for severe bleeding. However, it is unlikely that DOACS may have contributed to this slight drop given that in a recent study of the trends in anticoagulant prescribing for all NHS practices from 2014 to 2019 in England, it was reported that prescribing of DOACs increased from 9% of all anticoagulants in 2014 to 74% in 2019, while that of warfarin declined accordingly [Citation34]. In another study which evaluated the prescribing patterns of the DOACs and warfarin during 2009–2019 in primary care England, it was reported that the overall use of any DOACs increased from 16% in 2015 to 62% in 2019 with an average increase of 87% per year [Citation35]. Though serious and fatal events associated with DOACs were reported to have decreased by 6%, a breakdown by specific events such as bleeding was not provided [Citation35]. In contrast, in a recent UK based study which evaluated whether differences in the prescribing of DOACs compared with warfarin between General Practitioner practices across England was associated with variation of trends in emergency hospital admissions for bleeding and thromboembolic events between 2011 and 2016, it was reported that DOAC prescribing may have been associated with a higher rate of emergency admissions for bleeding conditions [Citation36] A number of similar studies based in other countries have either reported an increase in hospital admissions for severe bleeding [Citation19,Citation20], no changes in admissions for severe bleeding [Citation21] or less admissions for bleeding complications [Citation22].
Implications of findings
Our study findings show that admissions for severe bleeding in people with AF are on a steady increase and that the use of DOACs may be unlikely contributors to this trend. Though several studies have shown that DOACs are safer than warfarin in relation to major and intracranial bleeding [Citation37], a number of recent studies have also suggested that the DOACs are associated with the risk of bleeding complications [Citation18,Citation36,Citation38]. These recent concerns prompted an European Medicines Agency safety review on the risk of serious bleeding associated with apixaban, dabigatran etexilate and rivaroxaban in patients with non-valvular AF [Citation39]. The review, however, concluded that the pattern of serious bleeding seen in patients taking these medications was similar to that seen in the clinical trials on which the authorisation of the medicines were based [Citation9–12]. Subsequent studies have reported inconsistent findings - whereas, some studies have reported less bleeding rates in patients with AF [Citation40], others have reported higher risk of bleeding [Citation41]. It should be noted that a recent study reported increased bleeding with use of unadjusted doses of DOACs, when dose adjustment was indicated [Citation40]. It has been reported that the costs of DOACs are high compared with warfarin, which may have budget implications for the UK National Health Service and other healthcare systems [Citation42]. As a result of the potential consequences of increased bleeding and high costs due to DOACs, there have been calls to take these into account when assessing the benefits and costs of widespread DOAC use [Citation36]. It has been suggested that warfarin treatment could be optimised via genotype-guided dosing and point-of-care INR monitoring, to avoid the high costs associated with DOACs [Citation36]. Several arguments can be made for the continued used of DOACs in clinical practice. Though DOACs have comparable efficacy and safety profiles with warfarin, they have several advantages including fixed-dose administration and may have lower bleeding complications. Regarding the cost-effectiveness of DOACs, they have been shown to be of net benefit compared with warfarin [Citation37]. Furthermore, it is expected that the costs of DOACs will fall with patent expiry in 2022. Overall, our findings highlight the fact that admissions for severe bleeding in people with AF are still on a steady increase in England. This is very concerning given that the prescription of DOACs, which are claimed to be associated with lower rates of bleeding, have increased substantially from 2014 in England. Contributors to the upward trend in admissions for severe bleeding need urgent exploration. Furthermore, the clinical decision to use DOACs for anticoagulation in AF is a complex process and should involve a risk/benefit ratio assessment, be individualised, tailored to each patient’s health status, patient’s preference considered and the need for closer monitoring when being administered.
Strengths and limitations
The main strength of our study is the utilisation of nationwide data on admissions from 2001 to 2018, which allowed for the evaluation of national trends in admissions for AF and severe bleeding associated with AF and the period in which guideline recommendations were made for the use of DOACs. There were several major limitations such as the lack of data on total admissions, prevalence of AF, sociodemographic characteristics, comorbidities and anticoagulant prescriptions, which precluded estimation of incidence rates including age- and sex-adjusted rates, and evaluation of the factors that might have influenced the trends in admissions. Due to lack of data on DOAC prescribing practices and adherence to DOAC medications, we were unable to prove if the introduction of DOACs might have actually contributed to the slight drop in severe bleeding admissions during the period 2013–2014; HES does not contain drug and prescribing information. There was absence of data on the specific bleeding complications. For the year 2018, data was only available for the first quarter. The ecological design meant that it was not possible to link information on specific individuals to particular hospital admissions. Hence, any relationships at the aggregate level may not reflect relationships at the level of individual patients. Given the limitations, these findings need to be interpreted with caution.
Conclusions
There has been a steady increase in admission for AF and severe bleeding in England between the years 2001–2018. Though there was a slight drop in admissions for severe bleeding between 2013 and 2014, there is little evidence that this may have been caused by the introduction of DOACs in 2014. Contributors to these trends need urgent exploration.
Supplemental Material
Download MS Word (12.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Morillo CA, Banerjee A, Perel P, et al. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14(3):195–203.
- Kornej J, Borschel CS, Benjamin EJ, et al. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127(1):4–20.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham study. Arch Intern Med. 1987;147(9):1561–1564.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J. 2015;36(11):653–656.
- Zaidel EJ, Leng X, Adeoye AM, et al. Inclusion in the World Health Organization model list of essential medicines of non-vitamin K anticoagulants for treatment of non-valvular atrial fibrillation: a step towards reducing the burden of cardiovascular morbidity and mortality. Glob Heart. 2020;15(1):52.
- Hart RG, Pearce LA, Aguilar MI. Adjusted-dose warfarin versus aspirin for preventing stroke in patients with atrial fibrillation. Ann Intern Med. 2007;147(8):590–592.
- Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2005;2005(3):CD001927.
- Blin P, Dureau-Pournin C, Lassalle R, et al. A population database study of outcomes associated with vitamin K antagonists in atrial fibrillation before DOAC. Br J Clin Pharmacol. 2016;81(3):569–578.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104.
- Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
- Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128(12):1300–1305 e1302.
- Marzec LN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69(20):2475–2484.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962.
- Vinogradova Y, Coupland C, Hill T, et al. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ. 2018;362:k2505.
- Mueller T, Alvarez-Madrazo S, Robertson C, et al. Comparative safety and effectiveness of direct oral anticoagulants in patients with atrial fibrillation in clinical practice in Scotland. Br J Clin Pharmacol. 2019;85(2):422–431.
- Bouget J, Oger E. Emergency admissions for major haemorrhage associated with direct oral anticoagulants. Thromb Res. 2015;136(6):1190–1194.
- Marco Garbayo JL, Koninckx Canada M, Perez Castello I, et al. Hospital admissions for bleeding events associated with treatment with apixaban, dabigatran and rivaroxaban. Eur J Hosp Pharm. 2019;26(2):106–112.
- Badal M, Aryal MR, Mege J, et al. Evaluation of trends of inpatient hospitalisation for significant haemorrhage in patients anticoagulated for atrial fibrillation before and after the release of novel anticoagulants. Heart Lung Circ. 2015;24(1):94–97.
- Ganetsky M. Trends and characteristics of emergency department patients prescribed novel oral anticoagulants. J Emerg Med. 2015;49(5):693–697.
- Lane DA, Skjoth F, Lip GYH, et al. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc. 2017;6.
- Stefansdottir H, Aspelund T, Gudnason V, et al. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011;13(8):1110–1117.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted county, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125.
- Lee SR, Choi EK, Lee SY, et al. Temporal trends of emergency department visits of patients with atrial fibrillation: a nationwide Population-Based study. J Clin Med. 2020;9(5):1485.
- Wong KY, Davies B, Adeleke Y, et al. Hospital admissions for stroke and bleeding in Hounslow following a quality improvement initiative. Open Heart. 2021;8(1):e001558.
- Maggioni AP, Dondi L, Andreotti F, et al. Four-year trends in oral anticoagulant use and declining rates of ischemic stroke among 194,030 atrial fibrillation patients drawn from a sample of 12 million people. Am Heart J. 2020;220:12–19.
- Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–953.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham heart study. Circulation. 2004;110(9):1042–1046.
- Fitzmaurice DA, Hobbs FD, Jowett S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335(7616):383.
- Lowres N, Neubeck L, Salkeld G, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost. 2014;111(6):1167–1176.
- Forbes LJ, Marchand C, Doran T, et al. The role of the quality and outcomes framework in the care of long-term conditions: a systematic review. Br J Gen Pract. 2017;67(664):e775–e784.
- Ho KH, van Hove M, Leng G. Trends in anticoagulant prescribing: a review of local policies in English primary care. BMC Health Serv Res. 2020;20(1):279.
- Afzal S, Zaidi STR, Merchant HA, et al. Prescribing trends of oral anticoagulants in England over the last decade: a focus on new and old drugs and adverse events reporting. J Thromb Thrombolysis. 2021;52(2):646–653.
- Alfirevic A, Downing J, Daras K, et al. Has the introduction of direct oral anticoagulants (DOACs) in England increased emergency admissions for bleeding conditions? A longitudinal ecological study. BMJ Open. 2020;10(5):e033357.
- Lopez-Lopez JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058.
- Jun M, Lix LM, Durand M, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ. 2017;359:j4323.
- European Medicines Agency. [cited 2020 Mar 27; assessed 2021 May 24]. Amsterdam, The Netherlands: European Medicines Agency. Available from: https://www.ema.europa.eu/en/documents/press-release/no-change-needed-use-direct-oral-anticoagulants-following-ema-funded-study_en.pdf
- Jackevicius CA, Lu L, Ghaznavi Z, et al. Bleeding risk of direct oral anticoagulants in patients with heart failure and atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2021;14(2):e007230.
- Radadiya D, Devani K, Brahmbhatt B, et al. Major gastrointestinal bleeding risk with direct oral anticoagulants: does type and dose matter? - a systematic review and network meta-analysis. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e50–e58.
- Protty MB, Hayes J. Dawn of the direct-acting oral anticoagulants: trends in oral anticoagulant prescribing in Wales 2009-2015. J Clin Pharm Ther. 2017;42(2):132–134.