Abstract
Objectives. To compare long-term angina pectoris relief of successful versus failed percutaneous coronary intervention of chronic total occlusions (CTO PCI). Background. Previous studies demonstrate better short-term angina pectoris relief of CTO PCI than with optimal medical treatment (OMT), however, data on the long-term effects are lacking. Methods. 295 patients undergoing CTO PCI were analyzed retrospectively, with a follow-up evaluation of symptoms of angina pectoris and all-cause death one to four years after the intervention. The primary outcome was long-term relief of symptoms of angina pectoris. Secondary outcomes included a composite of major adverse cardiovascular events (MACE), including all-cause death, myocardial infarction, stroke, and later target vessel revascularization (TVR). At follow-up, patients were interviewed for symptoms of angina pectoris at 1, 6, 12, and 22 to 48 months after the intervention. Results. CTO PCI was successful in 225 (76%) patients and failed in 70 (24%) patients. Short-term (six months) relief of angina pectoris was observed in both groups, but only the successful CTO PCI group showed long-term relief. The Kaplan–Meier curves of all-cause death did not differ between the groups (p = .715). The final follow-up was a mean (range) of 37 (25 to 44) months after the intervention in the successful CTO PCI group, and 33 (28 to 48) months in the failed CTO PCI group. Conclusions. Successful CTO PCI is associated with better long-term relief of symptoms of angina pectoris compared to failed CTO PCI.
Introduction
Coronary chronic total occlusion (CTO) is defined as a complete (100%) stenosis in a coronary artery, with Thrombolysis in Myocardial Infarction (TIMI) flow 0 in the occluded segment, and present for at least three months. Collateral circulation may provide antegrade flow beyond the occluded segment, but it is still considered a CTO [Citation1–3].
Several studies have evaluated the clinical outcomes and effect on angina pectoris of percutaneous coronary intervention for chronic total occlusion (CTO PCI). Two recent randomized control trials (RCT) compared the clinical outcomes between CTO PCI and optimal medical therapy (OMT), but both failed to show a difference in the incidence of major adverse cardiovascular events (MACE) between the two groups [Citation4,Citation5]. Angina pectoris relief was evaluated with the Seattle Angina Questionnaire (SAQ) at 12 months in the EUROCTO trial [Citation4], showing greater improvement in all SAQ subscales and more complete freedom of angina pectoris (71.6% versus 57.8%; p = .008) in the PCI group when compared to OMT. The DECISION-CTO trial evaluated quality of life (European Quality of Life-5 Dimensions score) and symptoms of angina pectoris (SAQ) up to 36 months [Citation5]. No difference between the CTO PCI and OMT groups were detected, although an increase in all values from baseline was seen in both groups and was sustained over the long-term follow-up period [Citation5]. However, the results of DECISION-CTO should be interpreted with caution as approximately 70% of patients had multivessel coronary artery disease (CAD), and nearly half of these patients received also PCI for non-CTO lesions [Citation5]. Additionally, crossover from the OMT to CTO PCI group was observed in 78/398 (19.6%) cases, and the quality of life and angina pectoris questionnaire response rate was only 59% at 36 months [Citation5]. Several observational studies report better symptom relief after successful CTO PCI when compared to failed CTO PCI [Citation6–9]. However, none of these studies continued beyond a year of follow-up, and the evidence of long-term effects on symptoms of angina pectoris of CTO PCI remains scarce.
The aim of this single-center, retrospective study was to compare the long-term effects on experienced symptoms of angina pectoris and clinical outcomes of successful versus failed CTO PCI.
Materials and methods
Patient selection
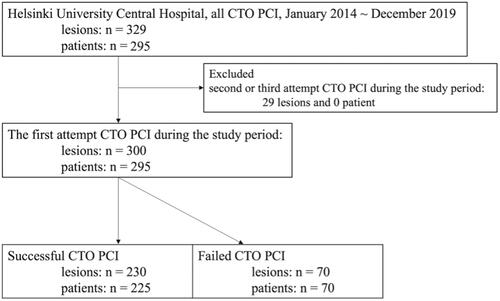
From January 2014 to December 2019 all patients in the province of Uusimaa with a coronary angiography confirmed CTO were evaluated by the heart team for different treatment strategies. All the patients were evaluated and treated in Helsinki University Central Hospital. Patients that proceeded to CTO PCI were evaluated for study inclusion. This resulted in total of 329 CTO lesions treated with CTO PCI in 295 consecutive patients (both stable and acute CAD). Patients with acute CAD treated with CTO PCI during the same hospitalization period were considered as emergency procedures. Only the first CTO PCI attempt was considered as index procedure. Of the 329 lesions, 29 were not the index procedure and the study included finally 300 CTO lesions in 295 patients (). The patients were divided into two groups depending on the revascularization success of the CTO PCI intervention, and analyzed for differences in baseline characteristics, clinical outcomes, and effect on symptoms of angina pectoris.
Definition and outcome measures
A CTO was defined as a 100% stenosis in a coronary artery stenosis with TIMI 0 antegrade flow, and an assumed duration of over three months [Citation10]. Procedural success was defined as a less than 20% residual angiographic stenosis with a TIMI flow grade 3, and without periprocedural MACE. All patients were on acetylsalicylic acid (ASA) treatment before the CTO PCI intervention, with dual antiplatelet therapy (DAPT) initiated using ADP receptor blockers (P2Y12 inhibitors, including clopidogrel, prasugrel, or ticagrelor) in all patients not receiving anticoagulant therapy. After successful CTO PCI, DAPT was recommended for at least 12 months for drug eluting stents. None of the patients were treated with a bare metal stent. After successful CTO PCI in anticoagulated patients DAPT and oral anticoagulation were continued for at least one month, after which the continuation of DAPT was according to the discretion of the treating cardiologist. The patients with a failed CTO PCI attempt received guideline-based OMT, without additional DAPT.
The primary outcome was long-term relief of symptoms of angina pectoris. Patients were evaluated by a single telephone interview by a dedicated study nurse or one of the investigators (LM) for the presence of symptoms of angina pectoris before the CTO PCI intervention, after PCI, and at 1 month, 6 months, 12 months, and at final follow-up (mean 33 months) using the Canadian Cardiovascular Society (CCS) functional classification scale and a study-specific symptom questionnaire. This questionnaire included four questions: ‘Do you experience chest pain or shortness of breath at rest (1), while walking (2), when climbing stairs (3), or when walking fast (4)?’. The secondary outcome was MACE incidents during follow-up, the composite outcome included all-cause death, myocardial infarction (MI), stroke, and target vessel revascularization (TVR). After the index procedure all other CTO PCI attempts, unplanned CABG and unplanned PCI to target vessel were considered as TVR outcomes. In-hospital and follow-up clinical data were retrospectively collected from electronic medical records (EMR). Mortality after discharge was determined by searching the EMR and the National Register System Records (Finnish Institute for Health and Welfare). The symptomatic questionnaire was administered by research nurses and LM by means of phone call.
Statistical analysis
Categorical variables are presented as frequencies or percentages and were compared using the chi-square or Fischer’s exact test. Continuous variables are presented as the mean and standard deviation (SD), and were analyzed using the Student’s t-test or the Wilcoxon rank-sum test based on their distributions. MACE and its subcomponents were estimated using the Kaplan–Meier curve and compared with the log-rank test. To assess possible TVR overestimation due to outcome definition, a second outcome analysis was performed with planned second or third attempt CTO PCI patients excluded from the cohort. To determine the predictors of MACE and improvement of symptoms of angina pectoris, a Cox hazard regression model and a logistic regression model were analyzed, including baseline and procedural covariates. Variables with a p value < .1 on univariate analysis were included for the multivariate model. All statistical tests were two-tailed with an alpha level of 0.05. To reduce selection bias the propensity score matching (PSM) method was used, using as covariates age, gender, a diagnosis of dyslipidemia, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, atrial fibrillation, chronic kidney disease (defined as GFR <60 ml/m2), heart failure, history of smoking, prior PCI, prior CABG, prior MI, prior stroke, abnormal left ventricular systolic function (LVEF <50%), and the use of retrograde approach for CTO PCI. To determine whether there were any differences in multiple dependent variables over time or between successful and failed CTO groups, repeated measures analysis of MANOVA was used. Statistical analyses were performed using JMP version 14.2 (SAS Institute Inc., Cary, NC, USA).
Ethics
The study was approved by the ethics committee of Helsinki University Central Hospital (approval number HUS/2738/2017). Informed consent was obtained from each patient prior to the beginning of the study.
Results
A total of 295 patients (successful CTO PCI n = 225, failed CTO PCI n = 70) and 300 CTO lesions (successful CTO PCI n = 230, failed CTO PCI n = 70) were included in the analyses (). The baseline characteristics are shown in . Mean age in the total cohort was 66.0 ± 11.0 years, 65.6 ± 11.1 in the successful CTO PCI group, and 67.6 ± 10.6 years old in the failed CTO PCI group (means of groups, p = .170). A failed CTO PCI attempt was more common in patients with HF (23% versus 36%; p = .041) and on dialysis (0.4% versus 4%; p = .043). The presentation differed between the two groups (successful CTO PCI 76% and failed CTO PCI 94% in elective procedures; p = .011). After a successful CTO PCI patients were more commonly treated with ASA or ADP receptor blocker (87% versus 98%; p = .001 and 60% versus 99%; p = < .001, respectively). No difference was detected in LVEF, hemoglobin, creatinine, eGFR, LDL-cholesterol, or Q waves on ECG. presents lesion and procedural characteristics. The Failed CTO PCI group had a higher J-CTO score (2.1 versus 3.4, p < .001). Femoral access and retrograde approach were more frequently employed in the failed CTO PCI group (68% versus 86%; p = .004 and 7% versus 43%; p < .001, respectively). Majority of the successful interventions were performed with antegrade wire escalation technique. Coronary artery stents were placed in almost all of the successful CTO PCI procedures (96%), with only 9 (4%) patients treated with drug-eluting balloons. A mean of 1.4 ± 0.8 stents were placed per intervention, with a mean combined length of stents of 48 ± 28 mm.
Table 1. Patient characteristics.
Table 2. Lesion characteristics.
shows CCS classification-based symptoms before and after the CTO PCI in the successful CTO PCI group () and failed CTO PCI group (). In the successful CTO PCI group a significant improvement of symptoms was perceived, an effect which endured beyond one year from the index procedure and up to the final follow-up call (75% experienced improvement of symptoms). The failed CTO PCI group showed similarly initial symptom improvement, but the effect was lost by 12 months (only 23% experienced improvement of symptoms). shows the improvement of symptoms at 12 months and at the last follow-up (median of 37 months in the successful CTO PCI group, and 33 months in the failed CTO PCI group).
Figure 2. Symptoms before and after the CTO PCI based on CCS classification. These figures showed the symptom based on CCS classification scale before PCI, after PCI, at 1 month, 6 months, 12 months, and the final follow-up in successful CTO PCI group (A) and failed CTO PCI group (B). The prevalence of symptoms before CTO PCI were compared to the prevalence at 12 months and at final follow up, and were classified as improved, no change, worsening, or TVR (C). CCS: Canadian Cardiovascular Society; CTO: chronic total occlusion; PCI: percutaneous coronary intervention; TVR, target vessel revascularization.
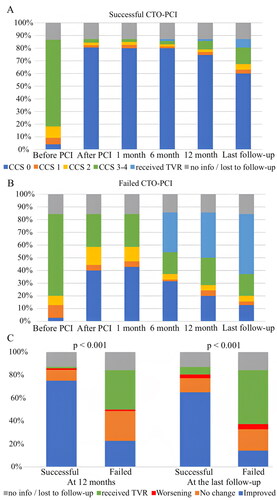
Predictors of symptom improvement at 12 months were analyzed with a multivariate model, revealing heart failure as a negative predictor (OR = 0.46; 95% CI = 0.23 to 0.90; p = .025), and successful CTO PCI as a positive predictor (OR = 10.4; 95% CI = 5.35 to 20.4; p < .001) (). Similarly the multivariate analysis of long-term improvement of symptoms at final follow-up () defined younger age (OR = 0.96; 95% CI = 0.94 to 0.99; p = .007) and successful CTO PCI as a positive predictor (OR = 10.4; 95% CI = 5.35 to 20.4; p < .001). The results of the study-specific symptom questionnaires are shown in . Similar trends to those based on CCS classification were observed in both groups. Symptom improvement (proportion of patients without symptoms) was seen in both groups after the intervention, but the effect was durable only in the successful CTO PCI group.
Figure 3. Symptoms before and after the CTO PCI based on study original questionnaire. Last follow-up duration varied among the patients, with a median of 37 (25–44) months in the successful group (A) (n = 202) and 33 (28–48) months in the failed group (B) (n = 60). CTO: chronic total occlusion; PCI: percutaneous coronary intervention.
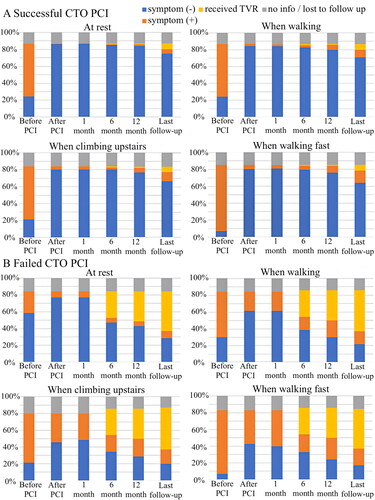
Table 3. Logistic regression model for symptom improvement at 12 months.
Table 4. Logistic regression model for improved symptoms at the final follow-up.
Intervention complications are presented in Supplementary Table 1. Coronary artery perforation occurred in 3% of failed CTO PCI cases, and in 0.4% of the successful CTO PCI cases (p = .141). None of the study patients died during PCI, required pericardiocentesis, or needed mechanical circulation support. Access site complication rates did not differ significantly between the successful and failed CTO PCI groups (4% and 1%; p = .691), and neither did in-hospital complications. Supplementary Table 1 presents the long-term clinical outcomes. The successful CTO PCI group developed less frequent MACE (23% vs 63%, p < .001), driven primarily by the less frequent TVR (6% vs 51%, p < .001), and unplanned TVR (6% vs 15%, p = .024). These subanalyses are presented in the Supplementary Figure 1.
The second analyses results’ (excluding patients with a planned second or third CTO PCI; n = 25) are presented in (Kaplan–Meier analysis for MACE , unplanned TVR ). Both MACE and unplanned TVR were more common in the failed CTO group (log-rank p = .004 and p < .001, respectively).
Figure 4. Kaplan–Meier curve for MACE and unplanned TVR among the patients without planned TVR. (A) Kaplan–Meier curve for MACE. (B) Kaplan–Meier curve for unplanned TVR. Patients with a planned second or third CTO PCI attempt (n = 25) were excluded from these analyses. MACE: major adverse cardiovascular event; TVR: target vessel revascularization; CTO: chronic total occlusion; PCI: percutaneous coronary intervention.
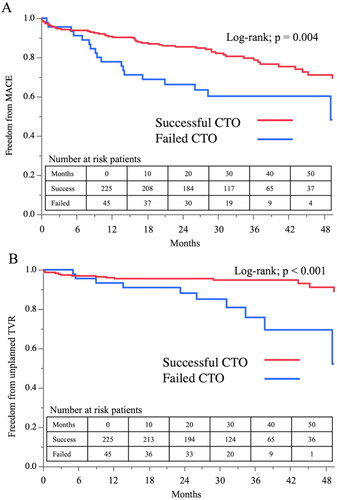
The multivariate analysis demonstrated that successful CTO PCI was an independent negative predictor of MACE up to 50 months (HR = 0.27; 95% CI = 0.17 to 0.44; p < .001) (Supplementary Table 2).
Propensity score matching resulted in 64 patients, however some differences remained as the standardized differences were more than 0.100 (). Kaplan–Meier analysis in the matched cohort demonstrated consistent results with the unmatched whole cohort analysis, showing no difference in all-cause mortality (log-rank p = .803) for the successful CTO PCI group (Supplementary Figure 2).
Table 5. Baseline characteristics of matched cohort.
Discussion
Successful CTO PCI improved symptoms with a lasting effect up to 37 months, with HF and a failed CTO PCI intervention as independent predictors of poor long-term symptom relief. In addition, the rate of in-hospital complications after CTO PCI was low.
Improvement of symptoms of angina pectoris after successful CTO revascularization
When it comes to symptom relief and improvement of the quality of life (QoL), various observational studies, meta-analyses and RCTs have demonstrated the benefits of a successful CTO PCI when compared to either a failed CTO PCI intervention or OMT [Citation4,Citation7,Citation8,Citation11–15]. The DECISION-CTO trial on the other hand showed no difference in QoL improvement, although this may be partially due to study design (randomization before non-CTO PCI) [Citation5]. In this study both groups showed improvement of symptoms, as measured by the CCS classification and the study-specific symptom questionnaire, but only the successful CTO PCI group showed long-term improvement at 12 months and at the final follow-up (median of 37 months in the successful CTO PCI group, and 33 months in the failed CTO PCI group) ( and Citation3(A,B)). Short-term improvement of dyspnea after failed CTO PCI was observed in a previous study as well [Citation9]. The short-lasting improvement of symptoms is probably explained by partial revascularization (categorized as a failed intervention), study-controlled OMT, short-term limitations to movement due to femoral puncture, and the placebo effect of an intervention.
These findings lend support to clinicians contemplating CTO PCI interventions for symptomatic CTO patients. Similar results were shown by Grantham et al. [Citation8] and Quintar et al. [Citation9].
Latest guidelines for the management of HF [Citation16,Citation17] recommend CABG as the first-choice revascularization strategy for patients with CAD. Also in this study patients with HF at baseline more unlikely benefited from CTO PCI; HF was an independent negative predictor of symptom improvement at 12 months. Heart failure patients might present with irreversible heart dysfunction and myocardial scarring, thus reducing the effects of a successful CTO PCI. On the other hand a study by Perera et al. showed greater improvement in QoL in patients with HF after a successful non-CTO PCI compared to OMT at 6 and 12 months, but this effect had diminished at 24 months [Citation18]. In this study successful intervention was a strong positive predictor of symptom improvement at 12 months. Further studies focusing on the effect of patients’ baseline characteristics on symptomatic benefit from CTO PCI are warranted.
The safety and success rate of CTO PCI
CTO PCI is considered one of the most complex of coronary interventions, with a high risk of interventional failure and complications. Multiple attempts to pass guide wires, balloons, and other devices across the occluded lesion may result in coronary dissection, perforation, and cardiac tamponade. Several large registries reported increasing success rates of CTO PCI over time [Citation19–21] and a definite learning curve effect on the success rate [Citation22]. A detailed study by Brilakis et al. showed different success rates based on the yearly amount of procedures per CTO operator (success rate 53% with less than 5 procedures annually, 62% with 5 to 10 procedures annually, and 75% with over 10 procedures annually; p < .001) [Citation20]. The success rate in our study cohort was 75% (225/300; calculated per lesion), comparing well with previous study success rates [Citation5,Citation20,Citation23]. The average incidence of CTO PCI in the study institution during the study period was approximately 60 per year, and all interventions were performed by interventionalists with over three years of complex CTO PCI experience at the start of the study. Complication rates (in-hospital MACE, all-cause death, complications requiring invasive treatment) in this study were low, and comparable to rates reported in previous meta-analysis (5% in-hospital MACE, 0.7% in-hospital all-cause death) and the US National Cardiovascular Data Registry (successful CTO PCI in-hospital MACE 0% and all-cause death 0%, failed CTO-PCI in-hospital MACE 3.8% and all-cause death 1.1%) [Citation20,Citation24].
Clinical outcomes following CTO PCI
Several observational studies have suggested that successful CTO PCI improves mortality rates [Citation12,Citation25] and MACE [Citation12,Citation25,Citation26] when compared to failed CTO PCI, whereas one propensity score matched retrospective study revealed no difference in all-cause mortality [Citation26]. Any possible improvement in outcome could be attributed to changes in cardiac function and positive remodeling [Citation27,Citation28]. Evaluation of CTO PCI effects might be limited by bias, as patients with a failed CTO PCI intervention tend to be older, less healthy, and present with more complex CTOs, leading to higher rates of intervention failure, MACE, and mortality [Citation13,Citation23]. To overcome these factors RCTs have recently compared CTO PCI to OMT, reporting comparable rates of MACE and mortality [Citation4,Citation5,Citation29,Citation30], while successful CTO PCI is associated with an improvement in the myocardial ischemia burden [Citation14].
All-cause mortality in our study was similar in both groups, both in the full cohort and the propensity score matched subanalysis. During the analysis of results structural bias in study design was identified due to inclusion of second or third planned CTO PCI attempt to TVR outcome. We conducted a secondary analysis that excluded all such attempts, but significant difference in TVR was still observed between the groups. This is primarily a result of patients with unsuccessful procedure often eventually receiving CABG. This difference in MACE driven by TVR is statistically significant, but is mainly a result of TVR definition and the clinical practice where failed CTO often leads to CABG. Importantly, no difference was observed between the groups in all-cause mortality, MI or stroke rates.
A secondary analysis which excluded all patients with a planned second or third CTO PCI attempt (25 patients, 29 CTO lesions) showed a significant difference in one-year MACE that favored the successful CTO PCI group. In the full cohort the difference in MACE between successful and unsuccessful CTO PCI group was even more distinct, driven primarily by TVR. This is due to a bias in study design, as 36% of failed CTO PCI patients underwent an additional, planned CTO PCI intervention within three months.
Limitations
Firstly, the typical limitations of a single-center retrospective study should be observed, as well as the small number of patients in the propensity-score matched cohort. Secondly, CTO definition was based on typical angiographic appearance, as many patients lacked earlier angiographic results. Thirdly, our study design determined second or third CTO PCI attempts and CABG as TVR, regardless of the results. This leads to a significant bias overestimating the number of MACE, which includes TVR as a component, and has an impact on the study results. While a second analysis excluding all such patients confirmed the primary study results, a study design bias effect remains. Therefore, this inherent bias should be considered when evaluating critically the positive MACE results. Despite the bias, no difference was detected in all-cause death, MI or stroke. A further limitation was that the questionnaire data of experienced symptoms of angina pectoris were collected during a single phone interview, basing the results on patients’ recollection of symptoms at each time point. Data loss due to patients not remembering their symptoms causes a slight recall bias, and as usual, all patients who died before the follow-up interview were lost from analysis (successful CTO PCI group 11%, failed CTO PCI group 9.3%). Finally, the set of questionnaires used in our study was not standardized.
Conclusions
In a single center retrospective study, CTO PCI was safe and improved long-term symptoms of angina pectoris.
Supplemental Material
Download MS Word (9.9 MB)Acknowledgment
The authors thank Kenneth Quek for English language editing, and study nurse Johanna Purjo for her dedicated work. Authors Hirokazu and Mansikkaniemi have equally contributed to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, LM, upon reasonable request.
Additional information
Funding
References
- Di Mario C, Werner GS, Sianos G, et al. European perspective in the recanalisation of chronic total occlusions (CTO): consensus document from the EuroCTO club. EuroIntervention. 2007;3(1):30–43.
- Galassi AR, Werner GS, Boukhris M, et al. Percutaneous recanalisation of chronic total occlusions: 2019 consensus document from the EuroCTO club. EuroIntervention. 2019;15(2):198–208.
- Sianos G, Werner GS, Galassi AR, et al. Recanalisation of chronic total coronary occlusions: 2012 consensus document from the EuroCTO club. EuroIntervention. 2012;8(1):139–145.
- Werner GS, Martin-Yuste V, Hildick-Smith D, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39(26):2484–2493.
- Lee SW, Lee PH, Ahn JM, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation. 2019;139(14):1674–1683.
- Sapontis J, Salisbury AC, Yeh RW, et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO registry (outcomes, patient health status, and efficiency in chronic total occlusion hybrid procedures). JACC Cardiovasc Interv. 2017;10(15):1523–1534.
- Hirai T, Grantham JA, Sapontis J, et al. Quality of life changes after chronic total occlusion angioplasty in patients with baseline refractory angina. Circ Cardiovasc Interv. 2019;12(3):e007558.
- Grantham JA, Jones PG, Cannon L, et al. Quantifying the early health status benefits of successful chronic total occlusion recanalization: results from the FlowCardia’s approach to chronic total occlusion recanalization (FACTOR) trial. Circ Cardiovasc Qual Outcomes. 2010;3(3):284–290.
- Qintar M, Grantham JA, Sapontis J, et al. Dyspnea among patients with chronic total occlusions undergoing percutaneous coronary intervention: prevalence and predictors of improvement. Circ Cardiovasc Qual Outcomes. 2017;10(12):e003665.
- Ybarra LF, Rinfret S, Brilakis ES, et al. Definitions and clinical trial design principles for coronary artery chronic total occlusion therapies: CTO-ARC consensus recommendations. Circulation. 2021;143(5):479–500.
- Abuzeid W, Zivkovic N, Elbaz-Greener G, et al. Association between revascularization and quality of life in patients with coronary chronic total occlusions: a systematic review. Cardiovasc Revasc Med. 2021;25:47–54.
- Christakopoulos GE, Christopoulos G, Carlino M, et al. Meta-analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am J Cardiol. 2015;115(10):1367–1375.
- van Veelen A, Elias J, van Dongen IM, et al. Percutaneous coronary intervention versus medical therapy for chronic total coronary occlusions: a systematic review and meta-analysis of randomised trials. Neth Heart J. 2021;29(1):30–41.
- Obedinskiy AA, Kretov EI, Boukhris M, et al. The IMPACTOR-CTO trial. JACC Cardiovasc Interv. 2018;11(13):1309–1311.
- Jaguszewski M, Targonski R, Fijalkowski M, et al. Recanalization of isolated chronic total occlusions in patients with stable angina. Int J Cardiol. 2013;167(4):1542–1546.
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24(1):4–131.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–e1032.
- Perera D, Clayton T, O'Kane PD, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387(15):1351–1360.
- Patel VG, Brayton KM, Tamayo A, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6(2):128–136.
- Brilakis ES, Banerjee S, Karmpaliotis D, et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8(2):245–253.
- Othman H, Seth M, Zein R, et al. Percutaneous coronary intervention for chronic total occlusion-the Michigan experience: insights from the BMC2 registry. JACC Cardiovasc Interv. 2020;13(11):1357–1368.
- Young MN, Secemsky EA, Kaltenbach LA, et al. Examining the operator learning curve for percutaneous coronary intervention of chronic total occlusions. Circ Cardiovasc Interv. 2019;12(8):e007877.
- Christopoulos G, Menon RV, Karmpaliotis D, et al. The efficacy and safety of the "hybrid" approach to coronary chronic total occlusions: insights from a contemporary multicenter US registry and comparison with prior studies. J Invasive Cardiol. 2014;26(9):427–432.
- Khan MF, Brilakis ES, Wendel CS, et al. Comparison of procedural complications and in-hospital clinical outcomes between patients with successful and failed percutaneous intervention of coronary chronic total occlusions: a meta-analysis of observational studies. Catheter Cardiovasc Interv. 2015;85(5):781–794.
- Jones DA, Weerackody R, Rathod K, et al. Successful recanalization of chronic total occlusions is associated with improved long-term survival. JACC Cardiovasc Interv. 2012;5(4):380–388.
- Jaguszewski M, Ciecwierz D, Gilis-Malinowska N, et al. Successful versus unsuccessful antegrade recanalization of single chronic coronary occlusion: eight-year experience and outcomes by a propensity score ascertainment. Catheter Cardiovasc Interv. 2015;86(2):E49–E57.
- Hoebers LP, Claessen BE, Elias J, et al. Meta-analysis on the impact of percutaneous coronary intervention of chronic total occlusions on left ventricular function and clinical outcome. Int J Cardiol. 2015;187:90–96.
- Megaly M, Saad M, Tajti P, et al. Meta-analysis of the impact of successful chronic total occlusion percutaneous coronary intervention on left ventricular systolic function and reverse remodeling. J Interv Cardiol. 2018;31(5):562–571.
- Elias J, van Dongen IM, Råmunddal T, et al. Long-term impact of chronic total occlusion recanalisation in patients with ST-elevation myocardial infarction. Heart. 2018;104(17):1432–1438.
- Juricic SA, Tesic MB, Galassi AR, et al. Randomized controlled comparison of optimal medical therapy with percutaneous recanalization of chronic total occlusion (COMET-CTO). Int Heart J. 2021;62(1):16–22.