Abstract
Objective. Most studies analyzing predictors of sudden cardiac death (SCD) after acute myocardial infarction included only high-risk patients or index reperfusion had not been performed in all patients. The aim of our study was to analyze the incidence of SCD and determine the predictors of SCD occurrence during 6-year follow-up of unselected patients with ST-elevation myocardial infarction (STEMI), treated with primary percutaneous coronary intervention (pPCI). Method. we analysed 3114 STEMI patients included included in the University Clinical Center of Serbia STEMI Register. Patients presenting with cardiogenic schock were excluded. Echocardiographic examination was performed before hospital discharge. Results. During 6-year follow-up, lethal outcome was registered in 297 (9.5%) patients, of whom 95 (31.9%) had SCD. The highest incidence of SCD was recorded in the first year of follow-up, when SCD was registered in 25 patients, which is 26.3% of the total number of patients who had had SCD, i.e. 0.8% of the patients analyzed. The independent predictors for the occurrence of SCD during 6-year follow-up were EF < 45% (HR 3.07, 95% 1.87–5.02), post-procedural TIMI flow <3 (HR 2.59, 95%CI 1.37–5.14), reduced baseline kidney function (HR 1.87, 95%CI 1.12–2.93) and Killip class >1 at admission (HR 1.69, 95%CI 1.23–2.97). Conclusion. There is a low incidence of SCD in unselected STEMI patients treated with primary PCI. Predictors of SCD occurence during long-term follow-up in analyzed patients are clinical variables that are easily recorded during index hospitalization and include: EF ≤45%, post-procedural flow TIMI < 3, Killip class >1, and reduced baseline kidney function.
Introduction
Sudden cardiac death (SCD) is responsible for 20–40% of all deaths in patients with acute myocardial infarction (AMI) after hospital discharge [Citation1,Citation2]. Primary percutaneous coronary intervention (pPCI) in patients with acute ST-elevation myocardial infarction (STEMI) significantly reduces short- and long-term cardiovascular mortality as well as the occurrence of SCD [Citation3,Citation4]. However, individual patients have a high risk of mortality and SCD despite timely and successful reperfusion as well as early application of secondary preventive pharmacotherapy with antiplatelet drugs, beta blockers, statins, ACE inhibitors/sartans and mineralocorticoid receptor antagonists [Citation1,Citation4–7]. It is known that the risk of the occurrence of SCD is particularly high in the first year following infarction and that it gradually decreases but never disappears [Citation1,Citation8]. One of the most important predictors for the occurrence of SCD is left ventricular systolic (dys)function, i.e. the value of the ejection fraction (EF), based on which the decision on the implantation of the implantable cardioverter defibrillator (ICD) for primary SCD prevention is made [Citation1]. However, there are also other predictors showing that, upon recovering from AMI, patients are at a higher risk of the occurrence of SCD, even in cases where EF is > 35% (i.e. it is above the cutoff value for ICD implantation in primary SCD prevention) [Citation1,Citation5,Citation8–13]. Most studies analyzing predictors of SCD in short-term and long-term follow-up after AMI included only so-called high-risk patients (patients with left ventricular systolic dysfunction and/or heart failure) and/or were studies where index reperfusion had not been performed in all patients [Citation1,Citation3,Citation8]. This is why we feel it may be important to define predictors of SCD in long-term follow-up, in unselected STEMI patients treated with pPCI.
The aim of our study was to analyze the incidence of SCD and determine the predictors of SCD occurrence during 6-year follow-up of patients with STEMI, treated with primary PCI.
Method
Study population, data collection and definitions
The present study enrolled 3,114 consecutive patients hospitalized between February 2006 and January 2012, who were included in the prospective University Clinical Center of Serbia STEMI Register. The purpose of the prospective University Clinical Center of Serbia STEMI Register has been published elsewhere [Citation14].
In brief, the objective of the Register is to gather complete and representative data on the management and short- and long-term outcomes of patients with STEMI, undergoing primary PCI in the Center. All consecutive patients with STEMI, aged 18 or older, who were admitted to the Coronary Care Unit after undergoing pPCI in the Center, were included in the Register. All patients for whom data were entered into the register have received written information of their participation in the registry and the long-term follow up, and their verbal consent for enrollment was obtained. For the purpose of this study, patients with cardiogenic shock at admission were excluded. Coronary angiography was performed via the femoral approach. Primary PCI and stenting of the infarct-related artery (IRA) was performed according to the standard technique. Aspirin, 300 mg, and clopidogrel, 600 mg, were administered to all eligible patients before pPCI. Selected patients, with visible intracoronary thrombi, were also given the GP IIb/IIIa receptor inhibitor during pPCI. Flow grades were assessed according to the Thrombolysis in Myocardial Infarction (TIMI) criteria. After pPCI, patients were treated according to current guidelines.
Demographic, baseline clinical, angiographic, and procedural data were collected and analyzed. Kidney function was assessed by estimating the glomerular filtration rate (eGFR) at admission, using the Modification of Diet in Renal Disease (MDRD) equation. The value < 60 ml/min/m2 was considered to be reduced baseline kidney function. Echocardiographic examination was performed within the first 3 days after pPCI. The left ventricular ejection fraction (EF) was assessed according to the biplane Simpson method, in classical two- and four-chamber apical projections. EF was missing in 10% of patients. The missing data were imputed via the single imputation method.
We considered SCD to be a non-traumatic, unexpected, fatal event, occurring one hour upon the onset of symptoms. If the death had not been witnessed, the definition applied when the patient had had no new symptoms 24 h before the event [Citation9].
Patients were followed-up at 6 years after enrolment. Follow-up data were obtained by scheduled telephone interviews and outpatient visits.
Ethics
The study protocol was approved by the ethics committee of University of Belgrade, Faculty of Medicine (approval number 470/II-4 of February 21, 2008) The study has been conducted in accordance with the principles set forth in the Helsinki Declaration. Written informed consent was obtained from all patients for their participation in the Registry.
Statistical Analysis
Categorical variables were expressed as frequency and percentage while the continuous variables were expressed as the median (med), with 25th and 75th quartiles (IQR). Analysis for normality of data was performed using the Kolmogorov-Smirnov test. Baseline differences between groups were analyzed using the one-way analysis of variance (ANOVA) for continuous variables with normal distribution, the Kruskal-Walis test for continuous variables and non-normal distribution, and the Pearson X2 test for categorical variables. The receiver operating characteristic (ROC) curve was used to test the predictive power of EF on SCD. Multiple cox analysis (backward method, with p < 0.10 for entrance into the model) was used for identifying independent risk factors for the occurrence of 6-year SCD. Two-tailed p values of < 0.05 were considered to indicate statistically significant difference. The SPSS statistical software, version 19, was applied (SPSS Inc, Chicago, IL).
Results
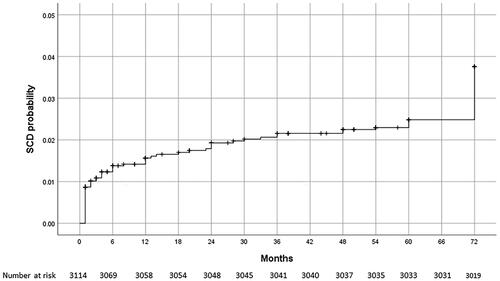
During 6-year follow-up, lethal outcome was registered in a total of 297 (9.5%) patients, of whom 95 (31.9%) had SCD. The highest incidence of SCD was recorded in the first year of follow-up, when SCD was registered in 25 patients, which is 26.3% of the total number of patients who had had SCD, i.e. 0.8% of the patients analyzed. The Kaplan-Meier curve showing the probability of SCD during 6-year follow-up is shown in .
Baseline characteristics of the patients analyzed are presented in .
Table 1. Baseline clinical, laboratory, angiographic, procedural characteristics, left ventricular ejection fraction, concomitant medical therapya and staged revacsularizationb of the study patients.
As compared to patients who were alive at the end of follow-up, patients who had had SCD were older, they were more frequently women, they more frequently had previous myocardial infarction and preexisting diabetes mellitus. At admission to hospital, patients with SCD more frequently had heart failure, atrial fibrillation, complete AV block, left bundle branch block, and anterior wall infarction. Also, patients with SCD more frequently had three-vessel coronary disease, stenosis of the left main coronary artery (LM) and preprocedural TIMI flow = 0 at the initial angiogram, as well as postprocedural TIMI flow < 3. In addition, patients with SCD had a lower baseline eGFR and a lower left ventricular ejection fraction (EF), as compared to those surviving until the end of follow-up.
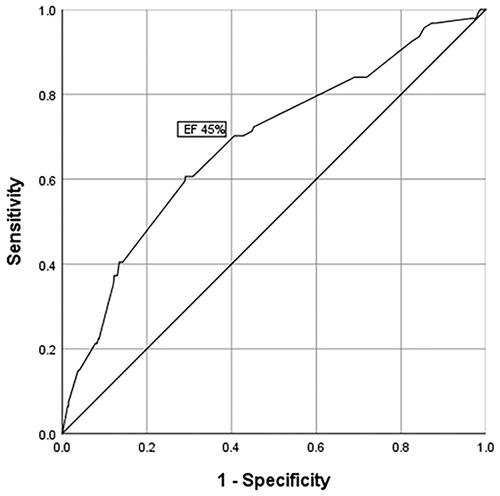
The receiver operating characteristics (ROC) curve for EF, predicting SCD, is presented in .
By applying the ROC curve, we found the cutoff value of EF for SCD occurrence to be 45%.
Independent predictors for the occurrence of SCD during 6-year patient follow-up are presented in .The following variables were entered into the Cox regression model: age (years), female sex, previous infarction, diabetes, Killip class >1 at admission, atrial fibrillation at admission, complete AV block at admission, anterior infarction, bundle branch block on initial ECG, baseline eGFR <60ml/min/m2, EF, 3- vessel disease, LM stenosis, pre-procedural flow TIMI = 0 and post-procedural flow TIMI < 3.
Table 2. Independent predictors for sudden cardiac death.
Discussion
In our study, we analyzed SCD incidence and defined the predictors for its occurrence during long-term follow-up in unselected STEMI patients treated with PCI. We found that the burden of SCD was the highest in the early period after STEMI (in the first year), and, despite becoming lower after that, it remained significant through the entire follow-up period of 6 years. The strongest and most significant independent predictor for the occurrence of SCD was left ventricular EF < 45, while the other independent predictors were: postprocedural TIMI flow < 3, baseline eGFR < 60 ml/min/m2, and Killip class > 1.
SCD in patients with AMI
There are not many studies analyzing SCD incidence during longer follow-up of unselected patients treated with primary PCI, which limits the possibility for direct comparison of our findings with the findings of other authors. In studies analyzing SCD predictors in high-risk patients with AMI, the incidence of SCD was higher than in our study [Citation1–5], which was to be expected since, in our study, we analyzed all STEMI patients, independently of EF values. On the other hand, in a study by Hooseiny et al. analyzing long-term mortality in consecutive STEMI patients treated with pPCI and hospitalized in a period of time (2006 - 2013) similar to the period that our patients were treated, the incidence of SCD during 1-year follow-up was 0.8%, which is identical to our result [Citation15]. Also, our finding that the risk of SCD was the highest during the first year of follow-up, decreasing after that, was in concordance with other findings [Citation1,Citation3,Citation5,Citation6]. In a study by Oikawa et al. patients with AMI, in whom myocardial revascularization was performed, were analyzed. In this study, 69% of the patients had STEMI and were treated with primary PCI. The independent predictors for the occurrence of lethal arrhythmias (VT/VF) in this study included: the duration of symptoms prior to admission, a high maximum value of creatinine kinase, Killip classes III and IV, preprocedural TIMI flow 0 – 1, and a more severe stage of chronic kidney disease (stages 3 - 5) [Citation7]. In this study, there was no statistically significant difference in EF value between patients with and without VT/VF, nor was there a difference in the postprocedural TIMI flow grade, which is different from our findings [Citation7]. In the study by Adabag et al. 31,286 patients with the diagnosis of AMI and post-IM EF > 35% were analyzed. In this study, coronarography was performed in all the patients, PCI was performed in 61% of the patients, and coronary artery bypass grafting was carried out in 13% of the patients. In the same study, the incidence of 1-year sudden cardiac arrest (SCA) was 1.8%, which is higher than our findings and can be explained by the fact that index reperfusion was performed in all our patients. In a study by Adabag et al. the absence of coronary revascularization at the time of incident MI was one of the strongest predictors for SCA within the period of 1 year, and, as far as EF is concerned, the independent predictor of SCA was EF < 50% [Citation8]. In a study by Docherty et al. 11 independent predictors for the occurrence of SCD were defined in high-risk patients following AMI, during 2-year follow-up. These predictors were incorporated into the risk score. In the same study, amongst other predictors, it was found that EF < 30% was an independent predictor for the occurrence of SCD, as was the no index of reperfusion, which indicates the significance of myocardial reperfusion in the prevention of SCD occurrence. Unlike our study, in a study by Docherty et al. data from randomized clinical trials (that had their own criteria for inclusion and exclusion of patients) were used [Citation1]. Also, in a study by Fan et al. it was shown that, in post-MI patients with EF ≤ 35%, increased risk of SCD may be predicted by age, EF ≤ 25%, and also by non-revascularization [Citation5].
Predictors of SCD
The first step in analyzing independent predictors of SCD is considering whether they are in concordance with the pathophysiological mechanisms that can be linked to the occurrence of SCD. It is well known that EF is a strong predictor of the occurrence of SCD amongst patients with AMI [Citation11]. In our patients, the cutoff value of EF for SCD occurrence was 45%, which is higher than the EF value that is considered to be an indication for ICD implantation in primary prevention of SCD. This is not an unusual finding, since other authors’ studies analyzing different patient groups with AMI found that most of the patients with SCD had an EF of 35–45% [Citation3,Citation8]. Also, in our study, the discriminative ability of EF to predict the occurrence of SCD was modest, which can also be found in literature [Citation13,Citation17]. A study by Exner et al. found that EF < 30% was an independent predictor of cardiac death or resuscitated cardiac arrest (HR 3.3, p = 0.005), however, the area under the ROC curve was also moderate (0.62), indicating the limitations of EF alone in the prediction of SCD [Citation11,Citation16]. Manifest heart failure is also a known independent predictor of the occurrence of SCD in patients with STEMI [Citation6,Citation18], and the risk increases when both factors are present – low EF and heart failure [Citation18]. In our patients, a strong predictor of SCD in long-term follow-up was postprocedural TIMI flow < 3. Relevant literature describes postprocedural TIMI flow = 3 as an independent predictor of favorable prognosis in patients with STEMI [Citation7]. SCD incidence is lower in patients with STEMI treated with pPCI, as compared with patients treated with thrombolysis, as well as with patients in whom reperfusion has not been carried out, which demonstrates a strong positive impact of successful reperfusion on patient prognosis [Citation3,Citation15]. Findings of the Zwolle study show that reperfusion of the myocardium through the application of pPCI reduced SCD incidence, even in patients with decreased EF; the authors of this study reported only eight instances of SCD over a period of 1 year, among 342 surviving STEMI patients with EF ≤ 30% [Citation19]. When considering the (primary) prevention of SCD upon AMI, comorbidities should also be taken into consideration. One of the frequent comorbidities in patients with AMI is kidney dysfunction. The finding that low eGFR in our patients was an independent predictor of SCD is in keeping with findings from literature, as it is known that, in different populations of patients with coronary disease, kidney dysfunction is a predictor of SCD (7, 12, 139. A study by Coiro et al. showed that there was a strong influence of kidney function (and diabetes) on the occurrence of SCD in patients with myocardial infarction and heart failure, during 2-year follow-up [Citation12]. In this study, the influence of decreased kidney function was particularly manifest in younger patients, aged < 55 years. The authors of the said study feel that the presence of low eGFR indicates the need for considering the implantation of ICD in patients with low eGFR, even if they have an EF > 35% [Citation12].
All independent predictors of SCD in our study are clearly linked to the development of myocardial scarring or the risk of repeated myocardial ischemia, and both of these represent strong substrates for the occurrence of malignant arrythmias. Kidney dysfunction in itself may lead to the development of cardiac fibrosis, and advanced stages of kidney dysfunction can also lead to electrolyte abnormalities, increased activity of the sympathetic nervous system, and probably to other, currently less known, abnormalities [Citation11,Citation12]. It is clear that the simultaneous presence of several predictors additionally increases the risk of SCD after AMI [Citation1].
Clinical significance of the study
We feel that our findings add to the existing knowledge on the incidence of SCD and the predictors of its occurrence, in patients with AMI. First, this study is a Register analysis and presents the incidence of SCD and the independent predictors of SCD, during six-year follow-up, in unselected STEMI patients treated with primary PCI. Second, in assessing the risk of SCD occurrence in each STEMI patient, multiple predictors must always be taken into consideration, as there is no single predictor that would, on its own, be strong enough to indicate the risk of SCD occurrence. All independent predictors obtained in our study are patient characteristics that are recorded during index hospitalization, they are easily attainable and based on these characteristics, clinicians, in their day-to-day work, usually make decisions on implementing further measures, i.e. decisions on further patient treatment (more frequent check-ups after hospital discharge, possible repeated 24h/48h ECG Holter monitoring, and in individual patients, the implantation of an ILR or electrophysiological testing), as well as on primary prevention of SCD [Citation8].
We should not forget the importance of secondary prevention. There are studies showing that patients’ knowledge about cardiovascular prevention is insufficient and that they have usually poor knowledge and awareness of their coronary artery disease (CAD) risk [Citation20,Citation21]. This especially applies to patients treated with (primary) PCI [Citation20]. Changing in these patients’ lifestyle, increase in their knowledge about secondary prevention of CAD (at hospital discharge or from general practitioner after hospital discharge) and stationary rehabilitation may be also important in primary prevention of SCD in the long-term follow-up.
Study limitations
The study is observational, but it is controlled, prospective and has included consecutive patients, thus limiting possible selection bias. Patients enrolled in the study are hospitalized between 2006 and 2012. The number of subjects with SCD in the study is relatively low. The longitudinal measurement of EF was not performed in order to evaluate the improvement in left ventricular function after STEMI [Citation22]. Also, contemporary imaging modalities, such as echocardiographic speckle-tracking based strain imaging, could have improved the assessment of left ventricular systolic function. We did not perform ECG analysis (e.g. arrhythmic risk stratification), which might have been important, especially in patients with preserved EF [Citation23]. The exact mechanism for every SCD remains unknown. In the present study, patients were treated with clopidogrel; there were no patients treated with more recently developed antiplatelet drugs (prasugrel and/or ticagrelor), and pPCI was predominantly performed using bare metal stents. Ticagrelor, prasugrel and/or the new generation of drug-eluting stents or biodegradable polymers were not available for routine administration to patients at the time of their enrollment into the Register, and this may have influenced the prognosis of the analyzed patients. Coronary angiography and concomitant PCI were performed via the femoral approach [Citation24]. Radial approach was not used in routine clinical practice to patients at the time of their enrollment into the Register. The study was not designed to evaluate whether changing pharmacological treatment (or any other treatment) would have had impact on the long-term outcome or SCD incidence in the patients analyzed.
Conclusion
There is a low incidence of SCD in unselected STEMI patients treated with primary PCI. The independent predictors of SCD occurence during long-term follow-up in analyzed patients are clinical variables that are easily recorded during index hospitalization and include: EF ≤ 45%, post-procedural flow TIMI < 3, Killip class > 1, and reduced baseline kidney function.
Author contributions
LS and IM conceived of the study and participated in its design, acquisition of data and coordination. LS performed statistical analysis. MA, SS, GK, RL and DS participated in the design of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors express their gratitude to the physicians and nurses of the Coronary Unit and the Catheterization Laboratory participating in the primary PCI program
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Docherty KF, Ferreira JP, Sharma A, et al. Predictors of sudden cardiac death in high-risk patients following a myocardial infarction. Eur J Heart Fail. 2020;22(5):848–855.
- Hui SK, Sharma A, Docherty K, et al. Non-fatal cardiovascular events preceding sudden cardiac death in patients with an acute myocardial infarction complicated by heart failure: insights from the high-risk myocardial infarction database. Eur Heart J Acute Cardiovasc Care. 2021;10(2):127–131.
- Shiga T, Hagiwara N, Ogawa H, Heart Institute of Japan Acute Myocardial Infarction-II (HIJAMI-II) Investigators, et al. Sudden cardiac death and left ventricular ejection fraction during long-term follow-up after acute myocardial infarction in the primary percutaneous coronary intervention era: results from the HIJAMI-II registry. Heart. 2009;95(3):216–220.
- Hall TS, von Lueder TG, Zannad F, High-Risk Myocardial Infarction Database Initiative investigators, et al. Relationship between left ventricular ejection fraction and mortality after myocardial infarction complicated by heart failure or left ventricular dysfunction. Int J Cardiol. 2018;272:260–266.
- Fan X, Hua W, Xu Y, et al. Incidence and predictors of sudden cardiac death in patients with reduced left ventricular ejection fraction after myocardial infarction in an era of revascularisation. Heart. 2014;100(16):1242–1249.
- Saito K, Kondo Y, Takahashi M, et al. Factors that predict ventricular arrhythmias in the late phase after acute myocardial infarction. ESC Heart Fail. 2021;8(5):4152–4160.
- Oikawa J, Fukaya H, Ako J, on behalf the J-MINUET Investigators, et al. Risk factors of in-Hospital lethal arrhythmia following acute myocardial infarction in patients undergoing primary percutaneous coronary Intervention - Insight from the J-MINUET study. Circ Rep. 2019;2(1):17–23.
- Adabag S, Zimmerman P, Lexcen D, et al. Predictors of sudden cardiac arrest among patients with Post-Myocardial infarction ejection fraction greater than 35. J Am Heart Assoc. 2021;10(14):e020993.
- Priori SG, Blomström-Lundqvist C, Mazzanti A, ESC Scientific Document Group, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the european society of cardiology (ESC). endorsed by: association for european paediatric and congenital cardiology (AEPC). Eur Heart J. 2015;36:2793–2867.
- McDonagh TA, Metra M, Adamo M, ESC Scientific Document Group, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726.
- Dagres N, Hindricks G. Risk stratification after myocardial infarction: is left ventricular ejection fraction enough to prevent sudden cardiac death? Eur Heart J. 2013;34(26):1964–1971.
- Coiro S, Girerd N, Sharma A, et al. Association of diabetes and kidney function according to age and systolic function with the incidence of sudden cardiac death and non-sudden cardiac death in myocardial infarction survivors with heart failure. Eur J Heart Fail. 2019;21(10):1248–1258.
- Deo R, Vittinghoff E, Lin F, et al. Risk factor and prediction modeling for sudden cardiac death in women with coronary artery disease. Arch Intern Med. 2011;171(19):1703–1709.
- Mrdovic I, Savic L, Lasica R, et al. Efficacy and safety of tirofiban-supported primary percutaneous coronary intervention in patients pretreated with 600 mg clopidogrel: results of propensity analysis using the clinical center of serbia STEMI register. Eur Heart J Acute Cardiovasc Care. 2014;3(1):56–66.
- Doost Hosseiny A, Moloi S, Chandrasekhar J, et al. Mortality pattern and cause of death in a long-term follow-up of patients with STEMI treated with primary PCI. Open Heart. 2016;3(1):e000405.
- Exner DV, Kavanagh KM, Slawnych MP, REFINE Investigators, et al. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J Am Coll Cardiol. 2007;50(24):2275–2284.
- Gehi A, Haas D, Fuster V. Primary prophylaxis with the implantable cardioverter-defibrillator: the need for improved risk stratification. JAMA. 2005;294(8):958–960.
- Kondo Y, Noda T, Sato Y, et al. Comparison of 2-year outcomes between primary and secondary prophylactic use of defibrillators in patients with coronary artery disease: a prospective propensity score-matched analysis from the nippon storm study. Heart Rhythm O2. 2021;2(1):5–11.
- Ottervanger JP, Ramdat Misier AR, Dambrink JH, et al. Zwolle myocardial infarction study group. Mortality in patients with left ventricular ejection fraction ≤30% after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol. 2007;100(5):793–797.
- Wójcicki K, Krycińska R, Tokarek T, et al. Knowledge and prevalence of risk factors for coronary artery disease in patients after the first and repeated percutaneous coronary intervention. Kardiol Pol. 2020;78(2):147–153.
- Kowalewska E, Komnacka K, Wójcicki K, et al. Sources of patients’ knowledge about cardiovascular disease prevention in Poland - a pilot study. Postepy Kardiol Interwencyjnej. 2022;18(1):27–33.
- Søholm H, Lønborg J, Andersen MJ, et al. Repeated echocardiography after first ever ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention–is it necessary? Eur Heart J Acute Cardiovasc Care. 2015;4(6):528–536.
- Gatzoulis KA, Tsiachris D, Arsenos P, et al. Arrhythmic risk stratification in post-myocardial infarction patients with preserved ejection fraction: the PRESERVE EF study. Eur Heart J. 2019;40(35):2940–2949.
- Tokarek T, Dziewierz A, Plens K, et al. Radial approach expertise and clinical outcomes of percutanous coronary interventions performed using femoral approach. JCM. 2019;8(9):1484.