Abstract
Objective. To analyze 10 years mortality in an all-comers registry of patients with left main coronary artery stenoses treated with either PCI or CABG. Design. Patients with left main coronary stenoses treated with either PCI or CABG in the period of the NOBLE trial (2010–2015) were all included in an institutional database and registry (University Hospital North Norway). Fifty-six of totally 429 patients were included in the randomized, multicenter and international NOBLE trail; 426 eligible patients from the trial and registry were analyzed for mortality on 20 October 2020. The longest observation time on this date was 3611 days (9.9 years); 205 patients were treated with PCI and 221 with CABG. The patients treated with PCI were 74 ± 10 years vs 68 ± 10 years (CABG). Also, on average the PCI patients had more comorbidities (lower ejection fraction, more peripheral artery disease, more often acute presentation). Results. Survival at the last observation date was 80% for CABG patients vs 48% for PCI. A Cox proportional hazards analysis found PCI to be the strongest independent predictor for mortality (HR = 2.5, 95% CI 1.7–3.7). Also, COPD, chronic kidney disease, age, peripheral vascular disease, cerebrovascular incidents and acute presentation were factor predicting increased 10 years mortality. Conclusion. These data indicate that life expectancy in the overall patient population is shorter for patient treated for left main disease with PCI than with CABG.
Introduction
The Noble study was designed as a non-inferiority study based on the results from the Syntax trial. In patients with reasonably uncomplicated coronary pathology the results from Syntax indicated that left coronary main stem stenosis and a low burden of overall coronary disease i.e. a Syntax score less than 22 [Citation1], could be treated with PCI without compromising outcome [Citation2].
Noble did not reach the predicted non-inferiority level designed for the study. A composite endpoint of mortality, myocardial infarction, stroke and repeat revascularization occurred in 29% of PCI-treated patients and 19% of patients treated with CABG at the first observation i.e. at a predicted five year follow up [Citation3,Citation4]. Despite this observation, PCI has become a core treatment of left main stem stenoses [Citation5]. Such a turn of therapy should be monitored from registries and observational studies, and registry-based studies are necessary to evaluate the impact of therapeutic choices on real life outcomes [Citation6,Citation7].
Noble was originally constructed similarly to the Syntax trial i.e. in order to evaluate the complete population of patients with left main coronary stenoses, an accompanying registry was planned to evaluate “all comers” for generalizability [Citation3]. However, being a low-budget study, this part of the trial was abandoned for lack of center follow up (see , an excerpt from the original Noble study).
Figure 1. Flow chart from the Noble study. As shown, 5 of the 36 centers including patients in the randomized trial assigned the planned registry for non-randomized patients. CABG: coronary artery bypass surgery; PCI: percutaneous coronary intervention; MI: myocardial infarction. *Six received CABG as index treatment and seven received no index treatment. †26 received PCI as index treatment and seven received no index treatment. Used with permission from the publisher (Elsevier Inc). Adapted from Lancet 2016; 388: 2743–2752 (https://doi.org/101016/50140-6786(16)32052-9).
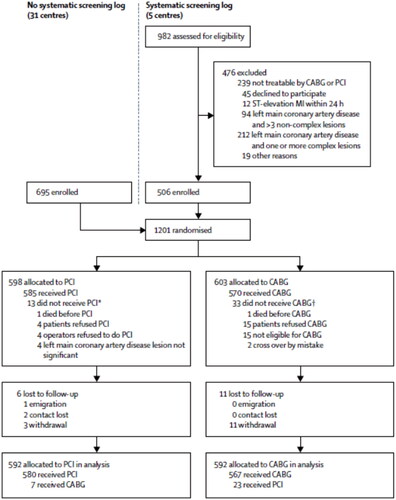
As one participating institution of the Noble trial, according to protocol we did establish a registry following all patients treated invasively for left main coronary artery stenoses in the period of the Noble inclusion. In this paper we present the approximately 10 years survival for all patients treated invasively for left main stenoses at our institution during this period.
Patients and methods
The Noble trial and registry
The handling of patient information complies with the Helsinki Declaration [Citation8]. The Noble trial and registry were approved by the Regional Ethics Committee of North Norway (REK 2459-5/2010). The study was also registered in clinicaltrial.gov: NCTT01496651. The data used in the present publication are pseudonymized and kept in an accessible file. Data are extracted both from the trial data and the individual patient electronic hospital records (using the data platform Dips – “Distributed Information and patient computer system for Hospitals”). These data are restricted from distribution by Norwegian law (https://lovdata.no/dokument/NL/lov/2014-06-20-43).
The Nordic-Baltic-British left main revascularization study (NOBLE) was an open label, randomized, multicenter non-inferiority study done in 36 centers between 2008 and 2015. Eligible candidates were assessed by at least one interventional cardiologist and one cardiac surgeon at each center. Patients with left main stenoses of 50% or more (or a fractional flow reserve of less than 80%), and no more than three additional non-complex coronary stenoses (length <25mm, no chronic total occlusions, no two-stent bifurcation, no calcified or tortuous vessel morphology) were potentially included in the study. Patients with both stable clinical presentation and acute coronary syndromes were included. Excluded were patients presenting within 24 h of an ST-segment elevation myocardial infarction [Citation3].
Patients in the registry
At the University Hospital North Norway the first patient was included in the NOBLE study on 1 October 2010 and the last was included 26 January 2015. During this period 56 patients were included in the randomized trial. In addition, 373 patients were treated with ether PCI or CABG for left main disease outside the RCT and these patients were allocated to the registry. Both the 56 patients in the RCT and the 373 patients in the registry only were included in the present analysis (n = 429).
Clinical characteristics
Definitions of clinical parameters included in the analysis are shown in , and distribution of these data among patients treated with PCI and CABG in . For patients not randomized to the Noble trial there was not a systematic Heart team discussion for every patient before recommending a treatment, but a unified cardiology/surgery assessment was done when deemed needed by the team performing the angiographies.
Table 1. Parameters used to define risk factors for mortality in the analysis.
Table 2. Risk factors in patients treated with PCI or CABG calculated difference between groupsa.
Analyses
All patients in this analysis were characterized by clinical data in addition to coronary anatomy ().
The Noble trial had a composite end point of mortality, myocardial infarction, stroke and repeat revascularization [Citation3]. In the present analysis we only assessed mortality. This endpoint was determined on 20 October 2020 by matching the patient database with the Norwegian mortality registry [Citation9]. This match was done at the Clinical Research Center at the University Hospital North Norway. Of the 429 patients in the complete registry, 3 were foreign citizens. These three were excluded from the study as they do not have data in the Norwegian death registry and therefore were considered lost to follow-up. The total number of patients eligible for mortality analysis was therefore 426. The longest observation period was 3611 days or 9.9 years.
Calculations and statistic
A prespecified separation in low and high-risk groups based on the clinical risk factors presented in was done as a screening. A Kaplan–Meier survival curve for patients treated with PCI and CABG was done with a corresponding log rank test. A set of clinical and treatment factors were then included in a univariable analysis (chi-square) of potential risk factors for mortality (). All factors with a p value of <.1 were entered in a multivariable Cox proportional hazard analysis to assess independent factors for increased mortality. Treatment factor (PCI or CABG) was entered as one factor. Proportional hazards were supported by the Kaplan–Meier curve and Wald statistics.
Results
The mortality for patients with less than six and more than five risk factors () is shown in . This crude assessment of overall mortality indicates an increased mortality in both low and high-risk patients undergoing PCI. To further analyze the distribution of risk factors among patients treated with either CABG or PCI, a univariable analysis of the distribution of these factors is presented in . As shown in this table, patients treated with PCI were significantly older, more often had an acute presentation, reduced ventricular function, peripheral vascular disease, stroke and previous revascularization with either PCI or CABG. On the other hand, the PCI patients had somewhat less complex coronary pathology.
Table 3. Mortality at the end of the 3611 day (9.9 years) observation period in patients with low (score table 1 < 6) or high (score table 1 > 5) clinical risk profile.
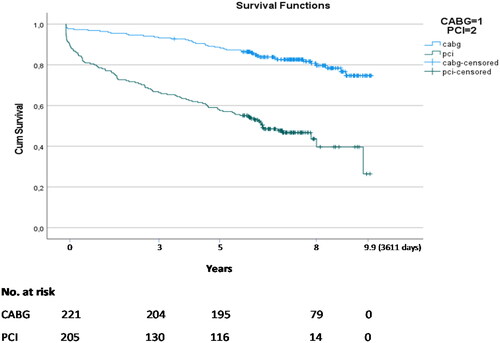
shows the survival curves for the two treatments. Patients treated with PCI had a particularly high initial mortality (within the first one to two years) and the difference was less pronounced, but still higher, during the following years (log rank test p < .001). The longest observation period was 3611 days.
Figure 2. Kaplan–Meier survival curves for patients treated with PCI or CABG. All patients in the registry are identified in the Norwegian Registry for Public Health (Folkeregisteret) with a complete track of death dates in the population. Censored data (vertical bars) therefore mean observation time for patients still alive at the assessment date of 20 October 2020. The longest recorded observation period was 3611 days or 9.9 years. Log Rank test: p < .001.
Finally, the independent factors related to an increased mortality were analyzed using the Cox proportional hazard model. The highest independent predictor of mortality in the observed 10 years period was PCI with a 2.5 times increased mortality compared to CABG ().
Table 4. Cox proportional hazard model showing independent variables with significant effect on mortality with their corresponding hazard ratios (Exp(B)).
Discussion
The Noble study was initiated in our institution on 1 October 2010. On 20 October 2020 there was an 80% survival among 221 patients with left main stenosis treated with CABG and 48% survival among the 205 patients treated with PCI. These patients are all-comers treated during the five years study period of NOBLE and thus represent a very heterogeneous patient population with an extensive spectrum of risk factors.
The design of the NOBLE study was influenced by the design and results from the Syntax-trial [Citation2]. The Syntax trial had a carefully built registry based on a collective evaluation from both cardiac interventionalists and cardiac surgeons. Of the 3075 patients screened for the Syntax trial, 35% were allocated CABG without eligibility for randomization and 6% were assigned PCI directly. These PCI patients were on average 71 years of age and the CABG patients in the Syntax Registry were on average 65 years. These populations are thus age-wise similar to our patient population. In the Syntax registry, death at three years were 18% for the PCI patients and 7% for the CABG patients. The authors presenting the Syntax Registry by protocol made no effort to statistically compare these cohorts [Citation10].
Our registry data from the NOBLE era reveal a change of therapy during the course of the trial period. At the start of the study period (2010), the ratio of CABG/PCI was overall 2.36. In 2015, this ratio was 0.63 developed from a gradual increase in the use of PCI at the expense of CABG. It is of interest to observe that this change of therapy occurred before the analysis of the NOBLE study was completed. The change of therapy was not based on a prespecified protocol and could in theory reflect both an increased acceptance of high-risk patients for PCI and/or an anticipation of non-inferiority for PCI as a revascularization strategy for left main disease. Such a change of therapy is not unique to our institution but is a universal observation [Citation4].
The patients treated with PCI at our institution were older than the CABG patients, and when evaluating mortality up to 10 years, one can anticipate an influence of older age on mortality. Our analysis not surprisingly did find age to be a significant predictor of mortality. However, a separate analysis of the NOBLE data [Citation11] found that CABG has an advantage also in patients over 70. This observation has been confirmed in a recent meta-analysis of the four large left main randomized trials [Citation12]. Of importance, despite the influence of age, our multivariable analysis found PCI to be the strongest predictor of mortality unrelated to the age of the patient. This observation has to be interpreted with caution since the grouping of very heterogeneous patients is hampered with possible unforeseen confounders. However, the observation demonstrated in i.e. a relative increase in PCI-related mortality also in low-risk patient, do raise concerns.
As demonstrated, patients with left main stenosis treated with PCI at our institution had a clearly increased risk profile with more acute presentation and an overall comorbidity. It is particularly concerning that the initial mortality was increased among PCI treated patients, and this observation points to the impression that very sick and multimorbid patients are offered PCI as a less risky last-option treatment for patients with end-stage coronary disease or complex intercurrent medical problems. Such a treatment strategy will drive mortality.
We found that approximately half of both patient groups had 3 -vessel disease. Such a complex pathology is less than optimal for PCI [Citation13] and probably is partly an explanation for the high long-term mortality related to this procedure.
From the point of trial design, the selective RCT with the lack of an “all-comers” design in NOBLE sets the stage for a potentially skewed comparison between the two treatment groups. Only a registry for all-comers can guarantee that the complete outcome can be analyzed. The “Ideal-principle” [Citation14] demonstrate that an RCT should be used in the research phase of a treatment introduction to demonstrate the effect of the treatment, but the overall effect on all patient groups must be followed in registries to assess treatment-effects in a “real world” scenario.
What are the potential explanations for the discrepancies between mortality data observed in RCTs and their meta-analyses compared to the present registry data? Most importantly, patients included in the RCTs are highly selected with a low coronary complexity. In the NOBLE study no more than 3 non-complex stenoses in addition to a LMC was allowed for inclusion [Citation3] opposed to the approximately 50% three-vessel disease in the registry. Of particular interest, an increased observation period could demask long term excessive PCI-related mortality also in these selected RCT groups. This is now observed in the Excel trial despite the fact that this trial was not powered for mortality [Citation15]. A recent analysis from an “all-comers” database in Europe [Citation16] confirms the trend in selection of patients treated with PCI. Patients with a “non-Excel like profile,” being 61% of the patients, had a significant 10% higher MACCE than patient complying with the Excel criteria [Citation16]. Thus, extending data from a carefully conducted RCT to the every-day clinical scenario is challenging.
To what extent an increased mortality observed in the PCI population will have an impact on patient’s choice of treatment will be individual. The most important for practicing physicians and guideline committees will be to understand accumulated data and not to extrapolate indications to patient-groups other that those were data are in fact available.
Acknowledgements
The authors are grateful to Veronika Nordskag at the Clinical Research Center, University Hospital North Norway, for doing the matching of patients to the Norwegian Death Registry.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are partly from the Noble Study. Data used in the present publication are pseudonymized and kept in an accessible file at the hospital research server. Data from electronic patient records are restricted from distribution by Norwegian law (https://lovdata.no/dokument/NL/lov/2014-06-20-43). Approval was obtained from the Regional Ethics Committee (REK 2459-5/2010).
Additional information
Funding
References
- Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219–227.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary interventions in patients with three-vessel disease and main coronary artery disease: 5-year follow-up of the randomized, clinical SYNTAX trial. Lancet. 2013;381(9867):629–638.
- Mäkikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis (NOBLE): a prospective, randomized, open-label, non-inferiority trial. Lancet. 2016;388(10061):2743–2752.
- Holm NR, Mäkikallio T, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomized, non-inferiority NOBLE trial. Lancet. 2020;395(10219):191–199.
- Ben-Dor I, Walkman R. Treatmentof left main disease. J Am Heart Assoc. 2021;10(14):e021990.
- Franzone A, Heg D, Räber L, et al. External validity of the «all-comers» design: insights from the BIOSCIENCE trial. Clin Res Cardiol. 2016;105(9):744–754.
- Batra G, Wallentin L. Do we need to reconsider how we design and conduct randomized controlled trials? Eur Heart J Qual Care Clin Outcomes. 2022;8(4):374–376.
- Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- Available from: https://www.skatteetaten.no/person/folkeregister/attester-og-opplysninger/folkeregisteropplysninger/
- Head SJ, Holmes DR, Mack MJ, et al. Risk profile and 3 -year outcomes from the SYNTAX percutaneous coronary intervention and coronary artery bypass, grafting nested registries. JACC Cardiovasc Interv. 2012;5(6):618–625.
- Steigen T, Holm NR, Myrmel T, et al. Age-stratified outcome in treatment of left main coronary artery stenosis; a NOBLE trial substudy. Cardiology. 2021;146(4):409–418.
- D, Ascenzo F, De Filippo O, Elia E, et al. Percutaneous vs surgical revascularization for patients with unprotected left main stenosis: a meta-analysis of 5-year follow-up randomized controlled trials. Eur Heart J Qual Care Clin Outcomes. 2021;7(5):476–485.
- Marui A, Kimura T, Nishiwaki N, et al. Comparison of five-year outcomes of coronary artery bypass grafting versus percutaneous coronary interventions in patients with left ventricular ejection fractions <50% versus >50% (CREDO-Kyoto PCI/CABG registry cohort-2). Am J Cardiol. 2014;114(7):988–996.
- Dimik JB, Sedrahyan A, McCulloch P. The IDEAL framework for evaluating surgical innovations. How can it be used to improve the quality of evidence. JAMA. 2019;154:685–686.
- Stone GW, Kappetein AP, Sabik JF, et al. Five-year outcome after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381(19):1820–1830.
- Castaldi G, Vermeersch P, Zivelonghi C, et al. Applicability of the EXCEL trial criteria to an all-comers real-world of unprotected left main percutaneous coronary intervention. Am J Cardiol. 2023;0:1–9.

