Abstract
Background. It has been unclear whether simple atrial septal defect (ASD) is an independent risk factor for infective endocarditis (IE). This study aimed to untangle the risk of endocarditis in a large nationwide cohort. Methods. We acquired data from the Finnish hospital discharge register on all individuals with ASD diagnosis from 1969 to 2019. Patients with complex congenital cardiac abnormalities were ruled out. Five individualized controls from the general population were matched to the ASD patient’s birth year, sex, and residence at the index date. All the patients with ICD-8, −9, or −10 diagnosis codes for IE were gathered from the hospital discharge registry. Results. Altogether, 8322 patients with ASD and 39,237 individualized controls were enrolled in the study. Median follow-up was 21.6 years (IQR 11.8–36.9) from the first hospital contact. In total, 24 (16 male) cases of infective endocarditis among ASD patients and 10 (8 male) cases among controls were diagnosed during the follow-up. The incidence of endocarditis was 0.11 per 1000 person-years in the patients with ASD and 0.011 per 1000 person-years in the controls. The adjusted risk ratio for endocarditis was 13.51 (95% CI: 6.20–29.46) in patients with ASD compared to the control cohort. Patients with ASD and endocarditis had higher long-term mortality than individualized control patients (MRR 2.25, 95% CI: 1.23–4.11). Conclusions. The incidence of IE in patients with ASD was higher than in the general population. Mortality associated with IE was higher in patients with ASD compared to controls.
Introduction
Infective endocarditis (IE) is an inflammatory process of the endocardium caused by a pathogenic organism. It is a severe disease with a one-year mortality rate of as high as 10–30% [Citation1,Citation2]. The incidence of endocarditis in the general population is 0.08 cases/1000 patient-years, and men are twice as likely as women to be impacted [Citation2–4].
Congenital heart diseases are a major risk factor for endocarditis. It is estimated that 10% of all IE patients have an underlying congenital cardiac defect [Citation5]. Especially cyanotic or complex congenital heart disease (CHD) and ventricular septal defects are known to carry a significant risk for infective endocarditis [Citation6,Citation7]. European guidelines recommend prophylactic antibiotic use for CHD patients with any cyanotic CHD or any CHD repaired with prosthetic material in the previous six months [Citation8].
The overall mortality of CHD-related infective endocarditis is remarkable in both pediatric and adult patients [Citation9]. In adults, in-hospital mortality is estimated to be 10% [Citation10] and almost 20% during seven years of follow-up [Citation11]. Pediatric in-hospital mortality is 1.9%, and late mortality is 7.7% during over eight years of follow-up [Citation12].
Atrial septal defect (ASD) is a congenital heart disease that causes left to right shunt at the atrial level. If the shunt is significant and causes strain to the right heart, the defect is closed surgically or with a transcatheter closure device. ASD is thought not to be associated with endocarditis because of the slow shunt flow. Yet, extensive database studies have indicated that patients with ASD have a higher incidence of endocarditis than the general population [Citation2,Citation7,Citation13,Citation14]. However, the risk of endocarditis in ASD patients has not been compared to a matched general population. In this nationwide cohort study, we report the incidence and risk of endocarditis associated with simple ASD.
Material and methods
Study setting and data source
We obtained information on all patients with ASD diagnoses from 1969 to 2019 from the Finnish Hospital Discharge Register (FHDR) (Dnro THL/4814/14.02.00/2020). The FHDR is an old, individual-level data record commonly utilized in research. Since 1969, the registry has gathered nationwide hospital discharge data with personal identification numbers. The register includes all hospitalizations and outpatient contacts. In 1986, procedural codes were introduced to the registry.
First, all patients with ICD-8, 9 or 10 diagnoses for ASD were included. Secondly, patients having ICD-8, 9 or 10 codes for primum type ASD defect, more severe CHD, or patent ductus arteriosus were excluded, except for non-specific CHD codes (747.7–747.9 (ICD-8), 7474x–7479x (ICD-9), and Q26.5–Q26.6 (ICD-10). All patients included had important data accessible, and no patients were discarded due to missing data. Data from FHDR was combined with every Finnish tertiary center’s surgical register. The oldest surgical registers include patients from 1959.
Five individually matched controls from the general population were matched to the ASD patient’s birth year, residence (city or municipality) at the index date and gender. The index date was the date of the closure for closed ASDs and the date of the diagnosis for unclosed ASDs. Part of this cohort has been previously used to study the mortality of ASD patients [Citation15].
Endocarditis
All the patients with ICD-8, 9 or 10 diagnosis codes for infective endocarditis were gathered from the hospital discharge registry. Endocarditis cases that occurred during 1969 were excluded to prevent the inclusion of previous IE diagnoses.
A chart review was done from the surgical register on transcatheter-closed ASD patients (n = 1000) to validate that there were no missing endocarditis diagnoses. Incidences and even rates of endocarditis were calculated for patients with ASD and for the control population.
The mortality of the endocarditis patients was studied by comparing patients with ASD and endocarditis to their individualized controls.
Statistical analysis
The outcomes are presented as a mean (range) or median (interquartile range). Categorical variables were summarized using counts and percentages. Cumulative occurrences of distinct events were illustrated using Kaplan–Meier curves and log-rank statistics. When plotting the cumulative incidence of endocarditis, the follow-up was set to start from the first hospital contact. Based on first-event incidence rates, incidence risk ratios (RR) were calculated using Poisson regression with 95% confidence intervals. The risk ratio was further adjusted to sex, age at the start of the follow-up, and decade at the start of the follow-up. Mortality risk ratios were calculated using Poisson regression. Event rates were compared using the mid-P exact test. All analyses were performed using R software, version 4.2.1.
Results
Study cohort
In total 13 807 individuals were gathered from the surgical registries or had a diagnosis code for ASD in the FHDR. After the above-mentioned exclusions, 8322 patients with ASD were enrolled in the study. Follow-up started from the first hospital contact and the median follow-up was 21.6 years (IQR 11.8–36.9). The median age at the time of ASD diagnosis was 16.5 years (IQR 3.0–5.5, range 0–96.5 years). The size of the control cohort was 39,237 subjects.
In total, 2087 patients had ASD closed during the follow-up period. 63.8% (n = 1331) of the defects were closed surgically and the rest (n = 756) by transcatheter method. 3316 patients did not have ASD closure and in 2919 patients the closure status was undetermined (ASD diagnosed before 1986).
Patients with endocarditis
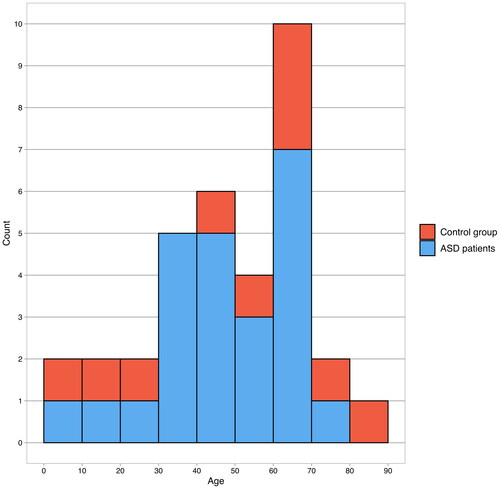
In total, 24 (16 male) cases of IE among the ASD patients and 10 (8 male) cases among the controls were diagnosed during the study period (). The mean age at the time of diagnosis was 47.8 (SD = 19.0, range 2.6–77.8) years in the patients with ASD and 49.8 (SD = 27.6, range 3.9–87.5) years in the controls. Patients with IE had more comorbidities compared to those without during the follow-up ().
Table 1. Descriptive characteristics and comorbidities during the follow-up.
The incidence of IE was 0.11 per 1000 person-years in patients with ASD and 0.011 per 1000 person-years in the controls. shows the cumulative incidence of IE from the first hospital contact. The adjusted risk ratio for endocarditis in ASD patients was 13.51 (95% CI: 6.20–29.46) ().
Figure 2. Cumulative incidence of endocarditis, follow-up starts from the study periods first hospital contact. p-value is calculated using the log-rank method.
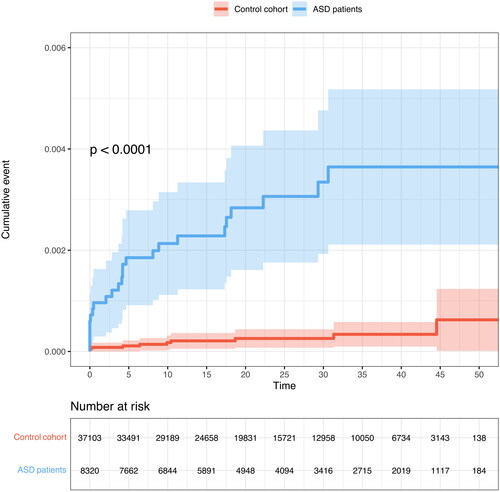
Table 2. Endocarditis in Patients with ASD and controls.
Nine of the 24 patients had no ASD closure. Five patients had ASD closed, one patient underwent transcatheter and two patients surgical closure after the IE. Two IE cases occurred after the surgical ASD closure and none of the endocarditis cases developed after transcatheter ASD closure. In 10 patients with ASD diagnosed before 1986, the closure status was undetermined.
Seven patients had an aortic valve procedure, and two patients had mitral valve surgery after the endocarditis. No procedural codes for the right heart valve surgery were noted.
Mortality of endocarditis patients
Patients with ASD and IE had higher long-term mortality (34.9 per 1000 person-years) than the individualized control patients (16.4 per 1000 person-years, p = .022) (). The crude long-term mortality risk ratio in patients with ASD compared to the controls was 2.25 (95% CI: 1.23–4.11). Fourteen (58.3%) patients with ASD and endocarditis deceased during the follow-up. Endocarditis was not the immediate or underlying cause of death in any of the patients. One (4.2%) death occurred in the first year following the endocarditis diagnosis.
Discussion
This nationwide study of ASD patients with long follow-up showed a high incidence of endocarditis in ASD patients. This is a novel finding and challenges the preceding sentiment of ASD not being a risk factor for endocarditis.
The risk of IE associated with ASD is often compared to other CHDs. In these studies, only a minority of CHD-associated endocarditis (0,4%) seems to be in ASD patients [Citation9]. However, compared to the matched general population, ASD itself seems to be a risk factor for endocarditis.
Incidence of IE
The incidence of endocarditis was 0.11 per 1000 person-years in patients with ASD in our material. The incidence is lower than in Quebec CHD (0.23/1000PY) and CONCOR databases (0.2/1000PY, estimated from the figure) [Citation13,Citation14].
The Quebec endocarditis study was conducted from 1988 to 2010 and included only pediatric patients. Their material had over 12 000 patients with ASD. They used ASD patients as the control population for other congenital defects. The risk of endocarditis in patients with ASD was not observed in their study. In a separate publication, the risk of endocarditis in adult patients was studied, and they found 37 IE cases in 9900 patients with ASD with an incidence risk ratio of 0.28 [Citation16].
The Dutch CONCOR register studied the incidence of endocarditis in adult CHD patients from 2001 to 2009. There were over 1500 patients with ASD with nine diagnoses of IE and the incidence rate of IE in ASD patients was higher than expected. It was speculated that the concomitant heart defects explain the high incidence of IE.
A more recent publication from the same registry with follow-up up to 2015 reported 8 IE events in over 2000 patients with ASD and over 12,000 follow-up patient-years. This corresponds to an incidence of 0.64 per 1000 per year. The higher incidence rate is probably explained by a more recent follow-up period.
In our study, there were no IE cases associated with ASD closure devices. This finding was further validated by chart review. The recommendation to use endocarditis prophylaxis six months after percutaneous ASD closure was followed in every institution. Closure device-associated endocarditis is reported to be extremely rare also in other materials [Citation17].
Our study showed that the incidence of IE was 0.011 cases per 1000 person-years in the control population. The incidence of IE in the general population in Sweden has been reported to be 0.08 cases/1000 years [Citation2]. That cohort was based on the patients treated with IE during 1997–2007. Our cohort included endocarditis diagnosed during 1969–2019. The lower incidence in our study is mainly explained by the inclusion of earlier decades. In addition, the control population did not contain patients with congenital heart diseases.
All patients with ASD had echocardiography performed, while in the control population echocardiography was done only in patients with suspected heart symptoms. This probably explains the finding that patients with ASD had significantly more valvular diseases. Moreover, endocarditis patients had more comorbidities during follow-up in both ASD and control populations. Comorbidities like stroke and heart failure can be related to endocarditis. Also, endocarditis patients were older at the start of the follow-up, which can explain the larger comorbidity burden ().
Mortality of IE patients
Patients with ASD-related IE showed higher long-term mortality than the control population. The crude mortality risk ratio was 2.25 during the median follow-up of 18.8 years. One-year mortality after IE diagnosis was 4.2% in patients with ASD.
Tutarel et al. reported a 19.4% mortality during the median follow-up of 6.7 years in patients with congenital heart disease and endocarditis [Citation11], and Kuijpers et al. reported one-year mortality of 16% [Citation7]. In other studies with CHD-related endocarditis, the in-hospital mortality has been 6–7% [Citation11,Citation18]. The inclusion of all congenital heart defects can explain the higher mortality rates in other studies.
Mechanism
Infective endocarditis generally requires a damaged endothelium and bacteremia with microbes of sufficient virulence. The lung constitutes the largest epithelial surface area in direct contact with the external environment. Research on animal models has shown that endocardial damage is the first stage in vegetation development, followed by localized platelet and fibrin adhesion [Citation19]. The originally sterile platelet-fibrin vegetation gets secondarily contaminated by bacteria circulating in the blood, either because of a distant infection or temporary bacteremia from a mucosal or cutaneous source.
Increased blood flow in the lungs due to atrial shunt would increase the possibility of bacterial entry through pulmonary flow. Leukocyte and bacterial adhesion to the endothelium are increased when the endothelium is subjected to shear stress from turbulent flow [Citation9,Citation20,Citation21]. It is possible that atrial septal defects’ continuous flow can cause shear stress to the endocardium. Furthermore, endothelium trauma caused by surgery can predispose to endocarditis. It is also known that a significant source of bacteremia is the oral cavity, and several reports have identified poor oral health in CHD patients [Citation22,Citation23].
Limitations
The main limitation is the register-based nature of the study. We were only able to gather information available in the registers, and thus we lacked detailed information about the endocarditis patients. Since procedural codes were introduced to the FHDR only after 1986, we were not able to assess whether ASDs diagnosed before that were closed.
Although the Finnish Hospital Discharge Register has a high sensitivity in detecting all patients with IE, the specificity for IE discharge diagnoses in the register has not been independently confirmed. Diagnosis of endocarditis was more difficult before the echocardiography era, and thus our study might underestimate the modern incidence of IE. In addition, we were not able to differentiate which valves were affected by endocarditis.
The number of discovered IE cases is relatively small, and it cannot be ruled out that some results, such as the distribution of comorbidities between groups, are affected by the change. The danger of selection bias should be low because this study is based on a nationwide registry rather than single-center reports. Follow-up can be considered almost complete since the Finnish registries cover all hospital admissions and deaths in the whole country, and the emigration rate is low.
The control group did not include individuals who were diagnosed with congenital heart diseases. However, we were unable to identify and remove patients who may have had asymptomatic ASD. Nevertheless, considering that the estimated birth prevalence of ASD is 2–3 per 1000, it is likely that the number of symptomatic ASD patients in the control group is minimal [Citation24].
Conclusion
The incidence of IE in patients with ASD was higher than in the general population. In addition, mortality associated with IE was higher in ASD patients compared to controls.
Ethics statement
The Helsinki University hospitals ethics committee approved this study on 11.7.2019 (num. HUS/1820/2019), and it was conducted following the Declaration of Helsinki. Patient consent was not needed for register-based research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting our findings are available from Statistics Finland and the FHDR. Still, restrictions apply to data availability, which was used under license for the current research and is not publicly available. Data are, however, available from the authors upon reasonable request and with permission from Statistics Finland and the FHDR.
Additional information
Funding
References
- Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882–893.
- Ternhag A, Cederström A, Törner A, et al. A nationwide cohort study of mortality risk and Long-Term prognosis in infective endocarditis in Sweden. PLOS One. 2013;8(7):e67519.
- Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet. 2015;385(9974):1219–1228.
- Shah ASV, McAllister DA, Gallacher P, et al. Incidence, microbiology, and outcomes in patients hospitalized with infective endocarditis. Circulation. 2020;141(25):2067–2077.
- Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med. 1998;129(10):761–769.
- Berglund E, Johansson B, Dellborg M, et al. High incidence of infective endocarditis in adults with congenital ventricular septal defect. Heart. 2016;102(22):1835–1839.
- Kuijpers JM, Koolbergen DR, Groenink M, et al. Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: focus on the use of prosthetic material. Eur Heart J. 2017;38(26):2048–2056.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–3128.
- Knirsch W, Nadal D. Infective endocarditis in congenital heart disease. Eur J Pediatr. 2011;170(9):1111–1127.
- Yoshinaga M, Niwa K, Niwa A, et al. Risk factors for in-hospital mortality during infective endocarditis in patients with congenital heart disease. Am J Cardiol. 2008;101(1):114–118.
- Tutarel O, Alonso-Gonzalez R, Montanaro C, et al. Infective endocarditis in adults with congenital heart disease remains a lethal disease. Heart. 2018;104(2):161–165.
- Knirsch W, Haas NA, Uhlemann F, et al. Clinical course and complications of infective endocarditis in patients growing up with congenital heart disease. Int J Cardiol. 2005;101(2):285–291.
- Rushani D, Kaufman JS, Ionescu-Ittu R, et al. Infective endocarditis in children with congenital heart disease: cumulative incidence and predictors. Circulation. 2013;128(13):1412–1419.
- Verheugt CL, Uiterwaal CSPM, van der Velde ET, et al. Turning 18 with congenital heart disease: prediction of infective endocarditis based on a large population. Eur Heart J. 2011;32(15):1926–1934.
- Muroke V, Jalanko M, Haukka J, et al. Cause-specific mortality of patients with atrial septal defect and up to 50 years of follow-up. J Am Heart Assoc. 2023;12(2):e027635.
- Mylotte D, Rushani D, Therrien J, et al. Incidence, predictors, and mortality of infective endocarditis in adults with congenital heart disease without prosthetic valves. Am J Cardiol. 2017;120(12):2278–2283.
- Abaci A, Unlu S, Alsancak Y, et al. Short and long term complications of device closure of atrial septal defect and patent foramen ovale: meta-analysis of 28,142 patients from 203 studies. Catheter Cardiovasc Interv. 2013;82(7):1123–1138.
- Maser M, Freisinger E, Bronstein L, et al. Frequency, mortality, and predictors of adverse outcomes for endocarditis in patients with congenital heart disease: results of a nationwide analysis including 2512 endocarditis cases. JCM. 2021;10(21):5071.
- Garrison PK, Freedman LR. Experimental endocarditis I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med. 1970;42(6):394–410.
- Shive MS, Hasan SM, Anderson JM. Shear stress effects on bacterial adhesion, leukocyte adhesion, and leukocyte oxidative capacity on a polyetherurethane. J Biomed Mater Res. 1999;46(4):511–519.
- Langille BL. Morphologic responses of endothelium to shear stress: reorganization of the adherens junction. UMIC. 2001;8(3):195–206.
- Rosén L, Stecksén-Blicks C. Experience of dental care for children with congenital heart disease among swedish dentists. Swed Dent J. 2007;31(2):85–90.
- Wilson WR, Gewitz M, Lockhart PB, et al. Prevention of viridans group streptococcal infective endocarditis: a scientific statement from the American Heart Association. Circulation. 2021;143(20):e963–e978.
- van der Linde D, Konings EEM, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–2247.