Abstract
Objectives
Pilot studies have suggested the potential benefits of intravenous nicorandil for patients with acute decompensated heart failure (ADHF). However, clinical evidence remains limited. The aim of the study was to summarize the efficacy and safety of intravenous nicorandil for the treatment of ADHF.
Design
A systematic review and meta-analysis was performed. The search for relevant randomised controlled trials (RCTs) was conducted in PubMed, Embase, Cochrane’s Library, Wanfang, and CNKI databases. A random-effects model was employed to combine the results.
Results
Eight RCTs contributed to the meta-analysis. Pooled results showed that acute treatment with intravenous nicorandil could significantly improve the symptom of dyspnea at 24 h after treatment, as evidenced by the five-point Likert scale for dyspnea after treatment (mean difference [MD]: −0.26, 95% confidence interval [CI]: −0.40 to −0.13, p < 0.001). Furthermore, nicorandil significantly reduced serum B natriuretic peptide (MD: −30.03 ng/dl, 95% CI: −47.00 to −13.06, p < 0.001), and N-terminal proBNP (MD: −138.69, 95% CI: −248.06 to −29.31, p = 0.01). In addition, nicorandil significantly improved ultrasonic parameters including left ventricular ejection fraction and E/e’ at discharge. Moreover, during the follow-up duration of up to 90 days, intravenous nicorandil significantly reduced the incidence of major adverse cardiovascular events (risk ratio [RR]: 0.55, 95% CI: 0.32 to 0.93, p = 0.03). The incidence of treatment-related adverse events was not significantly different between nicorandil and controls (RR: 1.22, 95% CI: 0.69 to 2.15, p = 0.49).
Conclusions
Results of this study suggest that intravenous nicorandil may be an effective and safe treatment for patients with ADHF.
Introduction
Heart failure (HF) is the end-stage of various cardiovascular diseases (CVDs), which affects at least 26 million people globally [Citation1]. Due to the improved treatment strategies for CVDs and the ageing of the global population, the number of people suffering from HF is expected to continue growing in the upcoming decades [Citation2]. As suggested by the name, acute decompensated heart failure (ADHF) is characterized by a rapid onset or worsening of HF signs and symptoms which need immediate medical interventions [Citation3]. Pathophysiologically, acutely decreased cardiac function and related pulmonary edema and systemic congestion are key features of ADHF [Citation3]. Currently, despite the comprehensive treatment modalities recommended by the United and European guidelines [Citation4], the prognosis of patients with ADHF remains very poor, with rates of mortality and HF-related readmission of 14% and 25% within 3 months [Citation3]. Therefore, the identification of effective treatments for ADHF is of great significance.
Nicorandil is an anti-anginal drug which has been widely used in patients with coronary artery disease [Citation5]. As an adenosine-sensitive potassium channel opener and a nitrate-moiety nicotinamide ester, nicorandil stimulates cyclic guanosine monophosphate production, activates K ion channels, and promotes K ion outflow in vascular smooth muscle cells, thereby exerting the vasodilation efficacy [Citation6]. Besides, accumulating experimental analyses confirmed the benefits of nicorandil on the myocardium, which involve the improvement of microcirculation, anti-apoptosis, and the promoting energy metabolism etc. [Citation7,Citation8]. In view of these benefits, nicorandil has been suggested as an alternative treatment for patients with HF [Citation9]. However, for patients with ADHF, the clinical evidence for the use of intravenous nicorandil remains limited [Citation10]. In this study, we performed a systematic review and meta-analysis to summarize the results of previous randomized controlled trials (RCTs), and to systematically evaluate the efficacy and safety of intravenous nicorandil for the treatment of ADHF.
Methods
This study was designed and implemented according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [Citation11,Citation12] and Cochrane Handbook guidelines [Citation13].
Search strategy
The Medline (PubMed), Embase (Ovid), CENTER (Cochrane Library), Wanfang, and China National Knowledge Infrastructure (CNKI) databases were searched for relevant studies with a combined strategy of: (1) “nicorandil” OR “2-Nicotinamidoethyl Nitrate” OR “2 Nicotinamidoethyl Nitrate” OR “Nitrate, 2-Nicotinamidoethyl” OR “2-Nicotinamidethyl Nitrate” OR “2 Nicotinamidethyl Nitrate” OR “Nitrate, 2-Nicotinamidethyl” OR “SG-75” OR “SG 75” OR “SG75” OR “KATP channel openers” OR “sigmart” OR “Ikorel”; and (2) “heart failure” OR “cardiac failure” OR “cardiac dysfunction”. Relevant studies were limited to those that included human subjects. Moreover, references of related reviews and original articles were also searched. Database searches were conducted on December 26, 2022.
Study selection
Included were studies with the following criteria: (1) full-length English or Chinese articles; (2) RCTs with parallel groups; (3) patients with ADHF were randomly assigned to an intervention group of intravenous nicorandil on the basis of conventional treatments, and a control group of conventional treatments only; and (4) (4) one or more of the following efficacy and/or safety outcomes were reported. In general, patients with gradual or rapid changes in HF signs and symptoms resulting in a need for urgent therapy who were diagnosed as ADHF according to the Framingham criteria were included [Citation14]. Patients with cardiac shock or hypotension requiring high doses of vasoactive drugs to maintain an SBP above 90 mmHg, acute coronary syndrome within 3 months, severe liver or kidney dysfunction, or severe infection were excluded from the included studies. The efficacy outcomes included the changes of the five-point Likert scale for dyspnea [Citation15] at 24 h after treatment, serum concentrations of B type natriuretic peptide (BNP) and N-terminal (NT)-pro hormone BNP NT-proBNP (NT-proBNP), and ultrasound cardiography (UCG) parameters of cardiac function at discharge, clinical outcomes and incidence of drug-related adverse events (AEs) during follow-up durations. Specifically, the UCG parameters involved left ventricular ejection fraction (LVEF), left ventricular end-systolic and diastolic volume (LVESV and LVEDV), and the ratio of peak early diastolic velocity to mitral annular velocity (E/e’). The clinical outcomes included all-cause mortality, HF-related rehospitalization, and the incidence of a composite outcome of major adverse cardiovascular events (MACEs). Non-randomized studies, studies including patients without ADHF, studies not in patients treated with intravenous nicorandil, studies comparing nicorandil with active controls, or studies that failed to report the outcome of interest were excluded. In cases of overlapped patient populations, the study with the largest sample size was included in the meta-analysis.
Data extraction and quality assessment
Data extraction, data mining, and quality evaluation were handled by two independent authors. If disagreements arose, discussions between the two authors were made to reach a consensus. Information regarding publication detail (first author, publication year, and study country), study design (blinded or open-label), patient characteristics (number of patients, mean age, sex, baseline LVEF, ischemic etiology, and background treatments), intervention (dosages and durations of intravenous nicorandil, regimens of controls), follow-up durations, and outcomes reported were extracted. We evaluated the quality of the study using Cochrane’s Risk of Bias Tool [Citation13] in accordance with the following criteria: (1) randomly generation of sequences; (2) concealing allocations; (3) blinding of participants and staff; (4) blinding outcome assessors; (5) presenting incomplete outcome data; (6) reporting selective results; and (7) other potential bias.
Statistical analysis
Differences in the changes of continuous variables after treatment between intervention and control groups were summarized as mean difference (MD) with a corresponding 95% confidence interval (CI). Influences of treatment on the outcomes of categorized variables were presented as risk ratios (RR) and corresponding CI. For the detection of heterogeneity, we used Cochrane’s Q test [Citation16]. A statistical analysis of heterogeneity was also conducted using the I2, and an I2 >50% confirmed significant heterogeneity [Citation17]. A random effect model was used in the pooled analyses to account for potential heterogeneity and provide a more general conclusion [Citation13]. An analysis of funnel plots and Egger’s regression asymmetry test was conducted when at least ten studies were included in order to determine publication bias [Citation18]. Statistically significant differences were defined as p < 0.05. Software RevMan (version 5.1; Cochrane, Oxford, UK) and Stata (version 12.0; Stata Corporation) were used.
Results
Search results
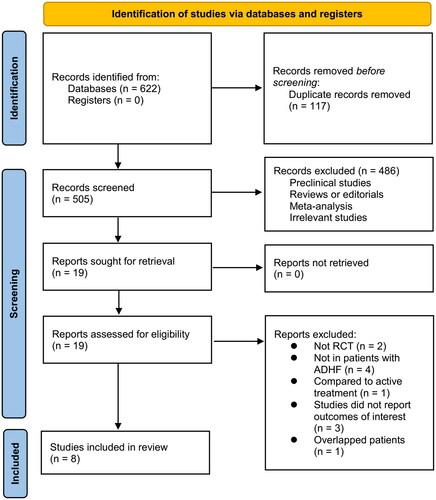
A diagram showing how to search databases and identify studies is shown in . By searching the database, 622 articles were obtained, and 505 were identified after excluding duplicates. Based on the title and abstract, 486 of them were subsequently excluded, mainly because their objectives were irrelevant. Nineteen articles were further excluded from the full-text review for the reasons illustrated in . The final analysis included eight RCTs [Citation19–26] in total.
Study characteristics and data quality
An overview of the included studies is presented in . Overall, eight RCTs [Citation19–26] including 764 patients with ADHF contributed to the meta-analysis. These studies were all open-label RCTs from Japan or China, which were published between 2010 and 2022. The mean ages of the patients were 56 to 75 years, and the mean LVEF at baseline was 33 to 46%. All the included patients received conventional treatments for ADHF, which included diuretics, beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, mineralocorticoid receptor antagonists, positive inotropic agents, carperitide, or dobutamine. For patients in the intervention group, intravenous nicorandil was administered for 1-5 days; while for patients in the control group, no other active treatment was administered on the basis of conventional therapy. The follow-up durations were hospitalization to up to 90 days. Details of study quality evaluation via Cochrane’s Risk of Bias Tool are shown in . No blindness was applied in either of the included studies. Details of random sequence generation were reported in three studies [Citation23–25], but none studies described how allocation concealment is achieved.
Table 1. Characteristics of the included studies.
Table 2. Quality evaluation of the included studies via Cochrane’s Risk of Bias Tool.
Symptoms at 24 h after treatment and cardiac function at discharge
Results of the meta-analysis showed that compared to conventional therapy alone, additional administration of intravenous nicorandil could significantly improve the symptom of dyspnea, as evidenced by the five-point Likert scale for dyspnea at 24 h after treatment (MD: −0.26, 95% CI: −0.40 to −0.13, p < 0.001; I2 = 0%; ). Moreover, nicorandil significantly reduced serum concentrations of BNP (MD: −30.03 ng/dl, 95% CI: −47.00 to −13.06, p < 0.001; I2 = 0%; ), and NT-proBNP (MD: −138.69, 95% CI: −248.06 to −29.31, p = 0.01; I2 = 0%; ) at discharge. For the included studies, standard transthoracic echocardiography was performed, with LVEF measured with Simpson’s method in two studies [Citation19,Citation26], with 2-D biplane method in one study [Citation20], and not specified in the other three studies [Citation23–25]. Nicorandil improved the UCG parameters for cardiac systolic and diastolic functions, including LVEF (MD: 4.16%, 95% CI: 2.73 to 5.60, p < 0.001; I2 = 0%; ), LVEDV (–24.88 ml, 95% CI: −33.04 to −16.72, p < 0.001; I2 = 0%; ), LVESD (–35.12 ml, 95% CI: −42.76 to −27.47, p < 0.001; I2 = 2%; ), and E/e’ (MD: −7.70, 95% CI: −9.85 to −5.56, p < 0.001; I2 = 0%; ) at discharge.
Figure 2. Forest plots for the meta-analysis of the influences of nicorandil on Likert scale at 24 h after treatment and serum concentrations of BNP and NT-proBNP at discharge in patients with ADHF; (A) Forest plots for the meta-analysis of Likert scale; (B) Forest plots for the meta-analysis of serum concentration of BNP; and (C) Forest plots for the meta-analysis of serum concentration of NT-proBNP.
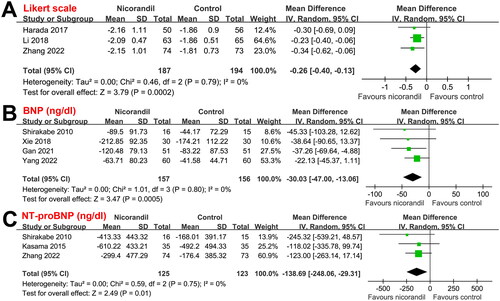
Figure 3. Forest plots for the meta-analysis of the influences of nicorandil on UCG parameters at discharge in patients with ADHF; (A) Forest plots for the meta-analysis of LVEF; (B) Forest plots for the meta-analysis of LVEDV; (C) Forest plots for the meta-analysis of LVESV; and (D) Forest plots for the meta-analysis of E/e’.
Clinical outcomes
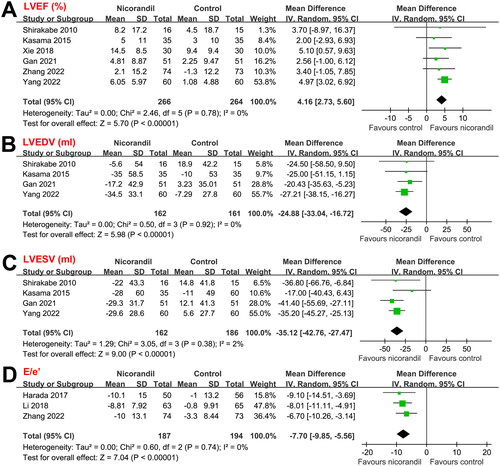
Follow-up durations among the included studies that reported the clinical outcomes of patients varied from during hospitalization to 90 days (during hospitalization in one study [Citation19], 60 days in one study [Citation21], and 90 days in another two studies [Citation22,Citation26]). Pooled results showed that intravenous nicorandil was not associated with a significantly reduced risk of all-cause mortality (RR: 0.52, 95% CI: 0.18 to 1.48, p = 0.22; I2 = 0%; ) or HF-rehospitalization (RR: 0.62, 95% CI: 0.31 to 1.26, p = 0.19; I2 = 1%; ). However, intravenous nicorandil significantly reduced the incidence of MACEs (RR: 0.55, 95% CI: 0.32 to 0.93, p = 0.03; I2 = 0%; ). Because limited studies were available for the above outcomes, we were unable to determine whether differences in follow-up duration may affect the results. However, since only mild heterogeneity was observed (I2 = 0% or 1%), variation in follow-up durations seems to have little impact on the outcomes.
Figure 4. Forest plots for the meta-analyses of the effects of nicorandil on clinical outcomes and drug-related AEs during follow-up duration up to 90 days; (A) Forest plots for the meta-analysis of all-cause mortality; (B) Forest plots for the meta-analysis of HF-rehospitalization; (C) Forest plots for the meta-analysis of MACEs; and (D) Forest plots for the meta-analysis of drug-related AEs.
Safety outcome
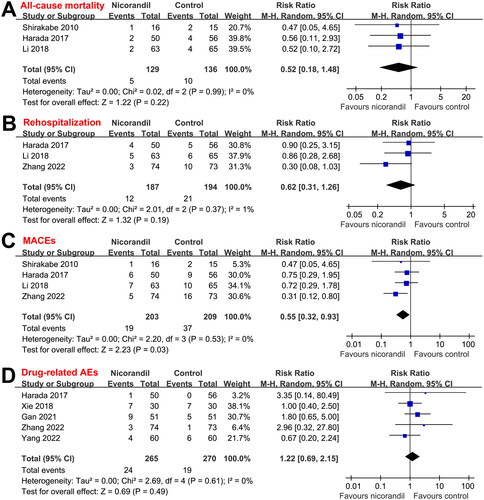
No severe AEs related to the treatment of nicorandil were reported among the included studies. Only mild AEs were reported, which typically included headache, tachycardia, gastrointestinal discomfort etc. Results showed that the incidence of treatment-related AEs was not significantly different between nicorandil and controls (RR: 1.22, 95% CI: 0.69 to 2.15, p = 0.49; I2 = 0%; ).
Publication bias
Due to the limited number of studies included, it was difficult to estimate the publication bias underlying the meta-analyses.
Discussion
In this systematic review and meta-analysis, by pooling the results of eight eligible RCTs, we found that in patients with ADHF, compared to a control group of conventional therapy alone, additional use of intravenous nicorandil on the basis of conventional therapy could significantly relieve the symptoms of dyspnea at 24 h after treatment, as evidenced by the improved Likert scale. Moreover, nicorandil significantly improved cardiac systolic and diastolic functions at discharge, reflected by significantly reduced serum levels of BNP and NTpro-BNP, and UCG parameters including LVEF, LVEDD, LVESD, and E/e’. In addition, although the incidence of all-cause mortality and HF-rehospitalization were not significantly reduced in patients with intravenous nicorandil as compared to control during a follow-up duration of up to 90 days, nicorandil was associated with a reduced incidence of MACEs. Finally, the incidences of drug-related AEs were not significantly different between intravenous nicorandil and controls. Taken together, these findings indicate that the use of intravenous nicorandil on the basis of conventional therapy may be an effective and safe treatment for patients with ADHF.
To the best of our knowledge, only one previous meta-analysis has observed the efficacy of nicorandil in patients with HF [Citation9]. However, this meta-analysis included both patients with chronic stable HF and ADHF, and included studies with oral and intravenous nicorandil [Citation9]. Besides, both the RCTs and observational studies were included in this meta-analysis, which may all confound the results and lead to significant heterogeneity [Citation9]. In the current meta-analysis, we focused on patients with ADHF, and enrolled RCTs with intravenous nicorandil only. The relatively strict inclusion criteria ensured the clinical relevance of the study and further reduced the heterogeneity of the included studies. We found that nicorandil on the basis of conventional therapy could significantly improve Likert scale 24 h after treatment. As the most commonly used tool to indicate the severity of dyspnea in HF patients, the Likert scale has been associated with the prognosis in patients with HF [Citation27]. Besides, serum levels of BNP and NT-proBNP were also significantly reduced following intravenous nicorandil at discharge, suggesting the benefits of the treatment on cardiac function [Citation28]. This was further supported by the UCG results at discharge, which showed that intravenous nicorandil significantly improved parameters of cardiac systolic function (LVEF, LVEDD, and LVESD) and parameters that reflect left ventricular [LV] filling pressure (E/e’) [Citation29]. Specifically, despite reduced LVEF that has been well-known to be correlated with the prognosis of patients with ADHF, increased E/e’ has also been associated with a higher risk of mortality and HF-rehospitalization in patients with ADHF, particularly for those with hypertension [Citation30] and preserved LVEF [Citation31]. The above findings may also explain the results that nicorandil reduced the risk of MACEs within 90-day follow-up. However, the individual outcome of all-cause mortality and HF-rehospitalization was not significantly reduced, which from our perspective may be due to the limited number of patients included and the small number of patients with these events. Results of the meta-analysis regarding the clinical outcomes were consistent with the finding of a previous large-scale retrospective study, which also showed that for patients with ADHF, the use of intravenous nicorandil was a significant predictor for the combined outcome of death and rehospitalization for HF [Citation32].
The mechanisms underlying the benefits of intravenous nicorandil for patients with ADHF may be multifactorial. First, as a vasodilator, intravenous nicorandil was shown to have significantly less hemodynamic tolerance over a 24-hour period compared with nitroglycerin, which may be a clinical advantage for short use in patients with ADHF, particularly for those with increased afterload [Citation33]. In addition, nicorandil could improve microcirculation of the myocardium, which has been shown to be important for maintaining cardiac function not only in patients with ischemic HF, but also for those with non-ischemic HF [Citation34,Citation35]. Besides, a previous experimental study has shown that nicorandil may reduce the incidence of ventricular arrhythmias in mice with progressive HF [Citation36], which may also contribute to the possible benefits of intravenous nicorandil for patients with ADHF. Studies are warranted in the future to determine the molecular mechanisms underlying the benefits of nicorandil for ADHF.
There are also some limitations in this study. First and foremost, all the RCTs were conducted in Japan and China. Further studies in other countries are required to demonstrate consistent benefits of the combined treatment. In addition, a lack of high-quality RCTs is evident, as well as smaller sample sizes in included studies. Also, all of the included studies were open-label RCTs, which were not blind to the patients or outcome assessors. These are associated with a higher risk of bias as compared to double-blind RCTs. There is a need to validate the results of the meta-analysis in large-scale double-blind RCTs in the future. Also, due to the limited studies included, we were unable to determine if the benefits of intravenous nicorandil for ADHF could be modified by patient or study characteristics, such as age, sex, baseline LVEF, etiology of HF, and dose and duration of intravenous nicorandil treatment. Moreover, the follow-up lengths are relatively short. The long-term efficacy of intravenous nicorandil on clinical outcomes of patients with ADHF remains to be investigated. Finally, due to the limited number of studies in each outcome of the meta-analysis, we could not exclude the possibility of publication biases, which may affect the findings of the meta-analysis.
Conclusions
To sum up, this systematic review and meta-analysis for the first time proposed that combined treatment with intravenous nicorandil and conventional treatment could significantly relieve the symptom of dyspnea 24 h after treatment, and improve the cardiac function of patients with ADHF at discharge, without increasing the risk of drug-related AEs. Moreover, intravenous nicorandil may also reduce the incidence of MACEs during a follow-up duration of up to 90 days. Taken together, the results of this study suggest that intravenous nicorandil may be an effective and safe treatment for patients with ADHF.
Author contributions
Yan Zhu and Shanshan Xie designed the study, performed database search, literature review, data collection, study quality evaluation, performed statistical analyses and interpreted the results. Yan Zhu drafted the manuscript and both of the authors critically revised the manuscript and approved the submission of the manuscript.
Ethical statement
The manuscript does not contain clinical studies or patient data (it is a meta-analysis of previously published studies).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Savarese G, Becher PM, Lund LH, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023; 118(17):3272–3287.
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. 2022; 145(8):e153–e639.
- Arrigo M, Jessup M, Mullens W, et al. Acute heart failure. Nat Rev Dis Primers. 2020; 6(1):16.
- Behnoush AH, Khalaji A, Naderi N, et al. ACC/AHA/HFSA 2022 and ESC 2021 guidelines on heart failure comparison. ESC Heart Fail. 2023; 10(3):1531–1544.
- Frampton J, Buckley MM, Fitton A. Nicorandil. A review of its pharmacology and therapeutic efficacy in angina pectoris. Drugs. 1992; 44(4):625–655.
- Gvishiani M, Gabunia L, Makharadze T, et al. Nicorandil efficacy in the treatment of ischemic heart disease (review). Georgian Med News. 2018; (280-281):152–155.
- Ahmed LA. Nicorandil: a drug with ongoing benefits and different mechanisms in various diseased conditions. Indian J Pharmacol. 2019; 51(5):296–301.
- Horinaka S. Use of nicorandil in cardiovascular disease and its optimization. Drugs. 2011; 71(9):1105–1119.
- Zhao F, Chaugai S, Chen P, et al. Effect of nicorandil in patients with heart failure: a systematic review and meta-analysis. Cardiovasc Ther. 2014; 32(6):283–296.
- Singh A, Laribi S, Teerlink JR, et al. Agents with vasodilator properties in acute heart failure. Eur Heart J. 2017; 38(5):317–325.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021; 372:n160.
- Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.2. London, UK: The Cochrane Collaboration. 2021. www.training.cochrane.org/handbook.
- McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the framingham study. N Engl J Med. 1971; 285(26):1441–1446.
- Weber CK, Miglioranza MH, Moraes MA, et al. The five-point Likert scale for dyspnea can properly assess the degree of pulmonary congestion and predict adverse events in heart failure outpatients. Clinics. 2014;69(5):341–346.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21(11):1539–1558.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003; Sep 6327(7414):557–560.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315(7109):629–634.
- Shirakabe A, Hata N, Yokoyama S, et al. Efficacy and safety of nicorandil therapy in patients with acute heart failure. J Cardiol. 2010; 56(3):339–347.
- Kasama S, Toyama T, Funada R, et al. Effects of adding intravenous nicorandil to standard therapy on cardiac sympathetic nerve activity and myocyte dysfunction in patients with acute decompensated heart failure. Eur J Nucl Med Mol Imaging. 2015; 42(5):761–770.
- Harada K, Yamamoto T, Okumura T, et al. Intravenous nicorandil for treatment of the urgent phase acute heart failure syndromes: a randomized, controlled trial. Eur Heart J Acute Cardiovasc Care. 2017; 6(4):329–338.
- Li YD, Zhang ML, Wang X, et al. Efficacy and safety of intravenous nicorandil combined with conventional therapy in patients with acute heart failure. Sandong Med J. 2018;58(17):49–51.
- Xie YS, Zou L. Therapeutic effect of nicorandil combined with conventional therapy in patients with acute heart failure. J Qiqihar Med Univ. 2018;39(19):2290–2292.
- Gan YH. The study of nicorandil combined with recombinant human brain natriuretic peptide in the treatment of acute heart failure. Pract Clin J Integr Trad Chin West Med. 2021;2021(21):6.
- Yang CZ, Li J, Ren ZL, et al. Clinical effect of nicorandil combined with dopamine in the treatment of elderly patients with acute heart failure. Clin J Med Offic. 2022;50(7):745–750.
- Zhang Y, Cai Z, Ke X, et al. Effectiveness and safety of intravenous nicorandil application in patients with acute heart failure with low baseline blood pressure. Heart Lung Circ. 2022; 31(1):95–100.
- Zhang X, Zhao C, Zhang H, et al. Dyspnea measurement in acute heart failure: a systematic review and evidence map of randomized controlled trials. Front Med . 2021;8:728772.
- Hendricks S, Dykun I, Balcer B, et al. Higher BNP/NT-pro BNP levels stratify prognosis equally well in patients with and without heart failure: a meta-analysis. ESC Heart Fail. 2022; 9(5):3198–3209.
- Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997; 30(6):1527–1533.
- Matsushita K, Minamishima T, Sakata K, et al. Differences in predictors of one-year mortality between patients with hypertensive and non-hypertensive acute heart failure: usefulness of E/E' in hypertensive heart failure. Eur J Intern Med. 2017; 38:e13–e14.
- Zamfirescu MB, Ghilencea LN, Popescu MR, et al. The E/e’ ratio-role in risk stratification of acute heart failure with preserved ejection fraction. Medicina. 2021; 57(4):375.
- Ishihara S, Koga T, Kaseda S, et al. Effects of intravenous nicorandil on the mid-term prognosis of patients with acute heart failure syndrome. Circ J. 2012;76(5):1169–1176.
- Larsen AI, Goransson L, Aarsland T, et al. Comparison of the degree of hemodynamic tolerance during intravenous infusion of nitroglycerin versus nicorandil in patients with congestive heart failure. Am Heart J. 1997; 134(3):435–441.
- Bravo PE, Di Carli MF, Dorbala S. Role of PET to evaluate coronary microvascular dysfunction in non-ischemic cardiomyopathies. Heart Fail Rev. 2017; 22(4):455–464.
- Tona F, Montisci R, Iop L, et al. Role of coronary microvascular dysfunction in heart failure with preserved ejection fraction. Rev Cardiovasc Med. 2021; 22(1):97–104.
- Hirose M, Takeishi Y, Nakada T, et al. Nicorandil prevents galphaq-induced progressive heart failure and ventricular arrhythmias in transgenic mice. PLoS One. 2012;7(12):e52667.