Abstract
The standard Conventional Cold Storage (CCS) during heart transplantation procurement is associated with time-dependent ischemic injury to the graft, which is a significant independent risk factor for post-transplant early morbidity and mortality – especially when cold ischemic time exceeds four hours. Since 2018, Rigshospitalet (Copenhagen, Denmark) has been utilising ex vivo perfusion (Organ Care System, OCS) in selected cases. The objective of this study was to compare the short-term clinical outcomes of patients transplanted with OCS compared to CCS. Methods: This retrospective single-centre study was based on consecutive patients undergoing a heart transplant between January 2018 and April 2021. Patients were selected for the OCS group when the cold ischemic time was expected to exceed four hours. The primary outcome measure was six-month event-free survival. Results: In total, 48 patients were included in the study; nine were transplanted with an OCS heart. The two groups had no significant differences in baseline characteristics. Six-month event-free survival was 77.8% [95% CI: 54.9–100%] in the OCS group and 79.5% [95% CI: 67.8–93.2%] in the CCS group (p = 0.91). While the OCS group had a median out-of-body time that was 183 min longer (p < 0.0001), the cold ischemic time was reduced by 51 min (p = 0.007). Conclusion: In a Scandinavian setting, our data confirms that utilising OCS in heart procurement allows for a longer out-of-body time and a reduced cold ischemic time without negatively affecting safety or early post-transplant outcomes.
Introduction
Over the last five decades, survival and quality of life have steadily improved for heart transplant (HTx) recipients [Citation1]. HTx remains the gold standard surgical treatment for refractory, advanced and end-stage heart failure [Citation2]. At present, the standard method of donor heart preservation during organ procurement remains conventional cold storage (CCS), which is performed by arresting the donor heart using a cold cardioplegic solution and storing it in an icebox containing a crystalloid preservation solution [Citation3]. CCS is a simple, reliable and inexpensive preservation method but leads to time-dependent ischemic injury to the graft and subsequent ischemia–reperfusion injury upon reimplantation [Citation4]. A prolonged cold ischemic time (exceeding four hours) is an important independent risk factor for early morbidity and mortality [Citation5,Citation6], and it potentiates the detrimental effects of other risk factors such as older donor age and reduced left ventricle ejection fraction (LVEF) [Citation7]. Clinically, this results in an increased risk of primary graft dysfunction (PGD) and acute cellular rejection (ACR) early in the post-transplant period, thereby affecting both early and late survival [Citation8,Citation9]. Research into using ex vivo heart perfusion as an alternative method of graft preservation has been conducted since the 1960s, taking both hypothermic and normothermic approaches [Citation10].
The Organ Care System (OCS, developed by TransMedics, Andover, MA, USA), based on the Langendorff method [Citation11], preserves the graft in a normothermic, near-physiological and beating state inside a sterile chamber. The cold ischemic time is minimised, and the period of organ viability is extended [Citation12] – which may potentially improve post-transplant (Tx) survival [Citation13]. A total out of body time of as long as 12 h with recovery of graft function and preservation of endothelial cell function is feasible [Citation14]. The PROCEED II trial found that the OCS is a viable alternative to CCS with similar short- and intermediate-term clinical outcomes after transplantation [Citation15,Citation16]. Furthermore, a recent meta-analysis affirmed the safety and effectiveness of the technique, finding no significant differences in survival or secondary outcomes [Citation17].
The technology is relatively new in clinical use and has predominantly been tested in the USA [Citation17]. Scandinavia and USA differ considerably regarding donor age [Citation18], geographies and organ procurement organisation, making it essential to report outcomes from different regions. The aim of this study was to compare the first Scandinavian post-transplant short-term clinical outcomes of grafts preserved by CCS vs ex vivo normothermic perfusion (the Organ Care System). We hypothesise that the short-term clinical outcomes among patients transplanted with OCS hearts are similar to those transplanted with hearts preserved by CCS.
Methods
Study design and population
This retrospective single-centre study was based on data from the Department of Cardiothoracic Surgery at Rigshospitalet (Copenhagen, Denmark) and included all patients who underwent HTx between January 2018 and April 2021. Data were extracted from patient medical records and the Scandiatransplant registry. This study was conducted with hospital management authorisation as part of a quality control and patient safety initiative at Rigshospitalet. Pursuant to the Danish Health Care Act, it did not necessitate ethics committee approval or patient informed consent. Documentation of the approval can be provided upon request.
Patients received an OCS heart when the cold ischemic time was expected to exceed 4 h, either on account of a long transport distance between donor and recipient or when complex recipient heart explantation (e.g. difficult surgical conditions due to prior cardiac surgery) was anticipated at the discretion of the coordinating transplant team. The primary outcome measure was six-month event-free survival (freedom from severe PGD ≤24 h, ACR (biopsy-proven, grade 2 R or more) or death within six months). Secondary outcome measures were total cold ischemic time, total out of body time, and length of stay in the intensive care unit (ICU) and hospital.
Definitions
Complications of PGD and ACR were diagnosed and graded based on ISHLT guidelines [Citation9,Citation19]. Inotrope requirement was defined as the maximal vasoactive-inotropic score (VISmax) [Citation20] during the first 24 h post-transplant.
Total cold ischemic time was defined as the time from aortic cross-clamping in the donor until cross-clamp removal in the recipient in the CCS group. The OCS group had two defined cold ischemic times: the time from cross-clamping in the donor until connection to the OCS (1st cold ischemic time) and the time from cardioplegic infusion inside the OCS until cross-clamp removal in the recipient (2nd cold ischemic time). Total out of body (preservation) time in the OCS group was defined as the cold ischemic time + duration of connection to the OCS.
Statistical analysis
Continuous variables are presented as the mean (with SD) or median (interquartile range) and compared with Student’s t-test, the Mann–Whitney U test, or the Kruskal–Wallis H test, depending on the normality of the distribution and the number of comparison groups. Categorical data are presented with numbers and percentages and were compared with the χ2 test or Fisher’s exact test (with the Freeman–Halton extension when comparing ≥3 subgroups). The overall and event-free survival was calculated and displayed as Kaplan–Meier plots and compared using the log–rank test. Follow-up was defined as the time from HTx until death (from any cause) or censoring (last search 16 May 2022). Results with a two-tailed p-value of ≤0.05 were considered statistically significant. All statistical analyses were conducted using R.
Results
Baseline characteristics
A total of 48 patients were included in the study, among which nine patients were transplanted with grafts preserved using OCS and 39 patients with grafts preserved using CCS. The main donor characteristics are presented in . The two groups had similar characteristics, with no significant differences. The overall median donor age was 45 years, and the majority were male donors (65%). There was one sex mismatch among donors/recipients in the OCS group (one female donor to male recipient), while there were nine mismatches in the CCS group (three female-to-male, six male-to-female) (p = 0.66). Most donors in the OCS group were located at another hospital in Denmark, while the largest proportion of donors in the CCS group was located at Rigshospitalet. Cerebrovascular events were the most frequent cause of death in both groups.
Table 1. Donor characteristics.
The recipient characteristics and preoperative risk factors () were similar in the two study groups, with a trend towards higher recipient age in the OCS group compared to the CCS group (59 [52–62] vs. 50 [31–58]; p = 0.09). The median time on the waiting list was 63 days [28–127 days] in the OCS group and 52 days [13–237 days] in the CCS group (p = 0.32), while five patients (13%) in the CCS group were transplanted from the urgent waiting list compared to none in the OCS group. There were no significant differences in preoperative risk factors between the groups. Pre-transplant mechanical circulatory support (temporary mechanical circulatory support (MCS) or durable left ventricular assist device (LVAD)) was less frequent in the OCS group (22% vs. 41%; p = 0.45). Adult congenital heart disease (ACHD) as the leading cause of heart failure was more prevalent in the OCS group (44% vs. 13%), but overall, no significant difference in indications for HTx was found.
Table 2. Pre-Tx Recipient characteristics.
Intraoperative and in-hospital outcomes
The median out-of-body time was significantly longer in the OCS group compared to the CCS group (379 [342–390] vs 196 [151–283], p = <0.0001); conversely, there was a significantly shorter median cold ischemic time in the OCS group (145 [134–150] vs 196 [151–283], p = 0.007). Total out-of-body time in the OCS group consisted of the 1st ischemic time, duration of OCS perfusion, and 2nd ischemic time (). Overall, 16 (41%) of patients in the CCS group had cold ischemic times of more than four hours compared to none in the OCS group (p = 0.02), comparative data between this group (CCS>4h) and the OCS group can be found in the supplemental material. Although not significant, patients in the OCS group had longer median cardio-pulmonary bypass and surgical times (). The groups had comparable volumes of intraoperative bleeding and blood transfusion requirements (). The median durations of inotropic support and mechanical ventilation were slightly longer in the OCS group, but the inotrope requirement was similar between the groups. The incidence of PGD was similar between groups (p = 0.71) and those without severe PGD. In-hospital acute cellular rejection at or above grade 2 R was only found in a single case in the OCS group. The median length of ICU stay was equal between groups, but it had a considerably longer upper quartile in the OCS group (11 vs. five days). The median length of hospital stay was longer in the OCS group. There were no in-hospital deaths in the OCS group, whereas one patient (3%) in the CCS group died one day post-transplant due to multiorgan failure.
Figure 1. The components of out of body time in the OCS group. Labels represent medians (interquartile range). OCS: organ care system.
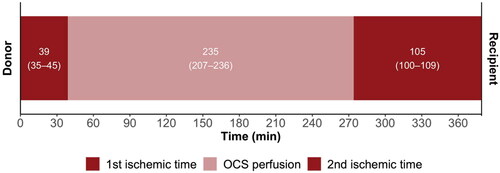
Table 3. Intraoperative and in-hospital outcomes.
Primary outcomes
Six-month follow-up data was available for all living patients, and the median follow-up time was 889 days (interquartile range: 708–968) and 916 days (interquartile range: 557–1156) in the OCS and CCS groups, respectively. The elements of the primary outcome measure are presented in , as well as in Kaplan–Meier plots of overall survival () and event-free survival (). The six-month overall survival was 100% vs. 92.3% in the OCS and CCS groups (log–rank test, p = 0.4). Causes of death in the CCS group were multiorgan failure, pulmonary mucormycosis, and unknown (1 case each). The primary outcome of event-free survival (severe PGD ≤24 h, acute cellular rejection ≥2 R or death within six months) was 77.8% [95% CI: 54.9–100%] in the OCS group and 79.5% [95% CI: 67.8–93.2%] (log–rank test, p = 0.91). When comparing the outcomes in the OCS group to the subgroup of CCS with cold ischemic times exceeding four hours (n = 16), six-month event-free survival was slightly lower at 75.0% [95% CI: 56.5–99.5%], while the difference remained insignificant with log–rank test p = 0.85 (see Supplemental Table D, Figure B).
Figure 2. Kaplan–meier plot of overall survival, stratified by preservation method. CCS: conventional cold storage; HTx : heart transplantation; OCS: organ care system.
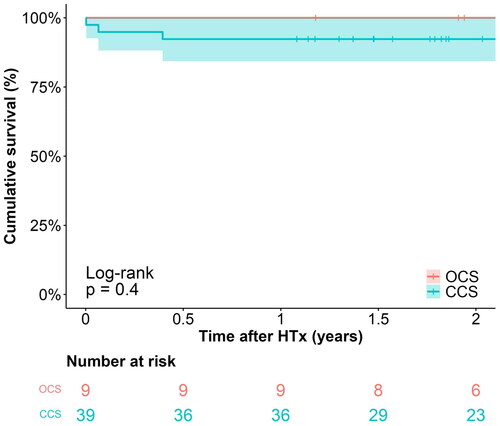
Figure 3. Kaplan–meier plot of event-free survival, stratified by preservation method. CCS: conventional cold storage; HTx: heart transplantation; OCS: organ care system.
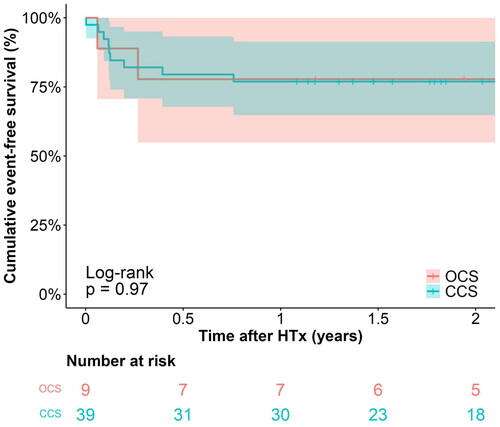
Table 4. Primary outcomes (at six-month follow-up).
Discussion
The OCS allowed for an increase in median out of body time of approximately three hours, while the median cold ischemic time was lowered by 51 min. Early outcomes, including length of stay in the ICU, inotrope requirement, and event-free survival, were similar between groups, augmenting the growing body of evidence showing that the OCS is a safe alternative to CCS [Citation15–17,Citation21–23]. It is worth noting that the median duration of hospital admission was six days longer in the OCS group, but since the complication rates (reoperations, haemodialysis, PGD) and length of stay in the ICU were similar, we ascribe this to small group sizes and statistical variation. Curiously, the age difference between recipient groups became more profound when comparing the OCS and CCS>4h groups – reaching significance when divided into age groups (p = 0.04). Rates of previous cardiac surgery, MCS and indications for HTx remained similar – making this finding challenging to interpret.
A recent meta-analysis by Langmuir et al. reviewed 12 studies of HTx utilising OCS, wherein nine were based on donation after brain death (DBD) donors, such as in our study [Citation17]. Our results were generally consistent with their findings, including similar patient groups. The only notable difference was the donor age in our OCS group (median 52 years) compared to the pooled mean of 39 years. Pooled 30-day survival among patients transplanted with an OCS heart (n = 172) was 97.4% (95% CI: 88.5–99.4%] compared to 95.7% (95% CI: 93.1–97.3%) in the CCS group (n = 481), similar to the findings in our study. No significant differences in PGD and rejection rates were found between preservation methods. Pooled data of cold ischemic time in OCS patients was shorter than in our study (102 min vs 145 min), but there was considerable between-study variance with mean times ranging between 52–361 min. The pooled duration of the OCS connection was 230 min, similar to the 235 min in our study.
To address the chronic shortage of donor's hearts [Citation24], a global trend to utilise hearts from marginal donors has developed in recent years [Citation2]. Several studies have found the combination of marginal donors and prolonged cold ischemic time to be particularly harmful [Citation7,Citation25–27]. The OCS has been suggested to be a viable and substantial method of expanding the donor pool by utilising extended donor criteria [Citation28]. However, the current capabilities of metabolic assessment of grafts are limited and require further study. Using lactate levels during OCS preservation as a metabolic marker of post-transplant outcomes has been suggested [Citation29], but its sensitivity remains debatable [Citation30–33]. Several studies have shown promising outcomes when using the OCS in marginal cases such as donation after circulatory Death (DCD) [Citation2,Citation30,Citation34,Citation35]. DCD is not currently employed in Denmark as national guidelines are being under development.
The OCS technology is costly [Citation36], making it relevant to identify which cases will benefit from the technology. Apart from the advantages regarding marginal donors, OCS extends the period of organ viability, allowing longer distances between donor and recipient and more time in complex surgical cases. When obtaining donor hearts located at the same centre or within a short transport distance of the recipient, the cons probably outweigh the pros of the OCS because the cold ischemic time will presumably be less than four hours, as observed in our study. When the cold ischemic time is expected to exceed the four-hour limit or when utilising hearts from marginal donors, the benefits of ex vivo perfusion become more substantial.
A significant knowledge gap exists, and further progress is needed to perfect the assessment and perfusion conditions of grafts preserved using the OCS. Since the OCS preserves grafts in a metabolically active state, it might provide a platform for therapeutic interventions. Specific treatments are being investigated [Citation37] and may potentially lead to ground-breaking improvements in long-term outcomes after HTx. Implementing the OCS in transplant programs may, in the future, improve donor/recipient matching (e.g. by human leukocyte antigen), provide a platform for novel therapies (e.g. immunomodulatory gene therapy) and enable HTx surgery to be deferred from nighttime to daytime.
Hypothermic ex-vivo perfusion (non-ischemic heart preservation, NIHP) has been proposed as an alternative approach to solving the challenges during conventional HTx-procurement. In NIHP, the graft is kept arrested in a hypothermic state but is continuously perfused using a cold nutrition- and hormone-enriched cardioplegic solution at 8 °C. Only a single clinical trial has been published to date, including six patients transplanted with grafts procured using NIHP [Citation38]. Although the trial presented promising short-term outcomes regarding both survival and post-transplant adverse events, the method is not as well established as the OCS and requires further study.
To address the limitations of our study, the design was retrospective with a small single-centre population. Patients were selected for the OCS group when the coordinating transplant team expected prolonged cold ischemic time, which warranted ex vivo perfusion in the first place. Less than half of the patients in the CCS group had cold ischemic times ≥4 h, which might have negated some of the positive effects of the OCS, but outcomes remained similar during subgroup analysis (supplemental material). The prevalence of pre-transplant cardiac surgery and MCS were similar between groups, and median surgical time was only slightly longer in the OCS group. Since 16 of the 17 CCS donors located outside Rigshospitalet had cold ischemic times above 4 h, donor location appears to be the most essential criterion for OCS selection in our population.
In conclusion, this is the first investigation of the short-term outcomes of HTx using hearts preserved with the OCS in Scandinavia. We have shown that by utilising the OCS during heart procurement, the cold ischemic time of the graft can be significantly reduced, while the out-of-body time can be increased without compromising safety or short-term outcomes after HTx.
Supplemental Material
Download MS Word (246.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data from this study are available upon reasonable request to the corresponding author (WHN). Public availability is restricted to respect patient privacy and ethical considerations.
Additional information
Funding
References
- Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult heart transplantation report—2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1056–1066. doi: 10.1016/j.healun.2019.08.004.
- Sponga S, Bonetti A, Ferrara V, et al. Preservation by cold storage vs ex vivo normothermic perfusion of marginal donor hearts: clinical, histopathologic, and ultrastructural features. J Heart Lung Transplant. 2020;39(12):1408–1416. doi: 10.1016/j.healun.2020.08.021.
- Minasian SM, Galagudza MM, Dmitriev Y V, et al. Preservation of the donor heart: from basic science to clinical studies. Interact Cardiovasc Thorac Surg. 2015;20(4):510–519. doi: 10.1093/icvts/ivu432.
- Jernryd V, Metzsch C, Andersson B, et al. The influence of ischemia and reperfusion time on outcome in heart transplantation. Clin Transplant. 2020;34(5):e13840. doi: 10.1111/ctr.13840.
- Lund LH, Khush KK, Cherikh WS, et al. The registry of the international society for heart and lung transplantation: thirty-fourth adult heart transplantation report—2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36(10):1037–1046. doi: 10.1016/j.healun.2017.07.019.
- Banner NR, Thomas HL, Curnow E, et al. The importance of cold and warm cardiac ischemia for survival after heart transplantation. Transplantation. 2008;86(4):542–547. doi: 10.1097/TP.0b013e31818149b9.
- Russo MJ, Chen JM, Sorabella RA, et al. The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the united network for organ sharing database. J Thorac Cardiovasc Surg. 2007;133(2):554–559. doi: 10.1016/j.jtcvs.2006.09.019.
- Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation. 2012;93(1):1–10. doi: 10.1097/TP.0b013e31823cab44.
- Kobashigawa J, Zuckermann A, Macdonald P, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant. 2014;33(4):327–340. doi: 10.1016/j.healun.2014.02.027.
- Wang L, MacGowan GA, Ali S, et al. Ex situ heart perfusion: the past, the present, and the future. J Heart Lung Transplant. 2021;40(1):69–86. doi: 10.1016/j.healun.2020.10.004.
- Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the langendorff technique of isolated heart perfusion. J Mol Cell Cardiol. 2011;50(6):940–950. doi: 10.1016/j.yjmcc.2011.02.018.
- Messer S, Ardehali A, Tsui S. Normothermic donor heart perfusion: current clinical experience and the future. Transpl Int. 2015;28(6):634–642. doi: 10.1111/tri.12361.
- Goldsmith KA, Demiris N, Gooi JH, et al. Life-years gained by reducing donor heart ischemic times. Transplantation. 2009;87(2):243–248. doi: 10.1097/TP.0b013e318190007d.
- Hassanein WH, Zellos L, Tyrrell TA, et al. Continuous perfusion of donor hearts in the beating state extends preservation time and improves recovery of function. J Thorac Cardiovasc Surg. 1998;116(5):821–830. doi: 10.1016/S0022-5223(98)00452-8.
- Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): A prospective, open-label, multicentre, randomised non-inferiority trial. Lancet. 2015;385(9987):2577–2584. doi: 10.1016/S0140-6736(15)60261-6.
- Chan JL, Kobashigawa JA, Reich HJ, et al. Intermediate outcomes with ex-vivo allograft perfusion for heart transplantation. J Heart Lung Transplant. 2017;36(3):258–263. doi: 10.1016/j.healun.2016.08.015.
- Langmuur SJJ, Amesz JH, Veen KM, et al. Normothermic ex situ heart perfusion with the organ care system for cardiac transplantation: a meta-analysis. Transplantation. Published online 2022;106(9):1745–1753. www.transplantjournal.com doi: 10.1097/TP.0000000000004167.
- Khush KK, Potena L, Cherikh WS, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: 37th adult heart transplantation report—2020; focus on deceased donor characteristics. J Heart Lung Transplant. 2020;39(10):1003–1015. doi: 10.1016/j.healun.2020.07.010.
- Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019.
- Koponen T, Karttunen J, Musialowicz T, et al. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth. 2019;122(4):428–436. doi: 10.1016/j.bja.2018.12.019.
- Sponga S, Benedetti G, de Manna ND, et al. Heart transplant outcomes in patients with mechanical circulatory support: cold storage versus normothermic perfusion organ preservation. Interact Cardiovasc Thorac Surg. 2021;32(3):476–482. doi: 10.1093/icvts/ivaa280.
- García Sáez D, Zych B, Mohite PN, et al. LVAD bridging to heart transplantation With ex vivo allograft preservation shows significantly improved: outcomes: a new standard of care? J Heart Lung Transplantation. 2015;34(4):S95. doi: 10.1016/j.healun.2015.01.252.
- Kaliyev R, Lesbekov T, Bekbossynov S, et al. Heart transplantation of patients with ventricular assist devices: impact of normothermic ex-vivo preservation using organ care system compared with cold storage. J Cardiothorac Surg. 2020;15(1):323. doi: 10.1186/s13019-020-01367-w.
- Khush KK. Donor selection in the modern era. Ann Cardiothorac Surg. 2018;7(1):126–134. doi: 10.21037/acs.2017.09.09.
- del Rizzo DF, Menkis AH, Pflugfelder PW, et al. The role of donor age and ischemic time on survival following orthotopic heart transplantation. J Heart Lung Transplant. 1999;18(4):310–319. doi: 10.1016/s1053-2498(98)00059-x.
- Wittwer T, Wahlers T. Marginal donor grafts in heart transplantation: lessons learned from 25 years of experience. Transpl Int. 2008;21(2):113–125. doi: 10.1111/j.1432-2277.2007.00603.x.
- Reich HJ, Kobashigawa JA, Aintablian T, et al. Effects of older donor age and cold ischemic time: on long-term outcomes of heart transplantation. Tex Heart Inst J. 2018;45(1):17–22. doi: 10.14503/THIJ-16-6178.
- Jawitz OK, Devore AD, Patel CB, et al. EXPANDing the donor Pool: quantifying the potential impact of a portable organ-care system for expanded criteria heart donation. J Card Fail. 2021;27(12):1462–1465. doi: 10.1016/j.cardfail.2021.07.018.
- Hamed A, Tsui S, Huber J, et al. Serum lactate Is a highly sensitive and specific predictor of post cardiac transplant outcomes using the organ care system. J Heart Lung Transplant. 2009;28(2):S71. doi: 10.1016/j.healun.2008.11.025.
- Chew HC, Iyer A, Connellan M, et al. Outcomes of donation after circulatory death heart transplantation in Australia. J Am Coll Cardiol. 2019;73(12):1447–1459. doi: 10.1016/j.jacc.2018.12.067.
- Kaliyev R, Lesbekov T, Bekbossynov S, et al. Comparison of custodiol vs warm blood cardioplegia and conditioning of donor hearts during transportation with the organ care system. J Card Surg. 2019;34(10):969–975. doi: 10.1111/jocs.14162.
- White CW, Ambrose E, Müller A, et al. Assessment of donor heart viability during ex vivo heart perfusion. Can J Physiol Pharmacol. 2015;93(10):893–901. doi: 10.1139/cjpp-2014-0474.
- Ribeiro R, Alvarez J, Gellner B, et al. Contractility versus metabolic cardiac assessment during ex situ heart perfusion: a pre-clinical transplant study. J Heart Lung Transplant. 2019;38(4):S240. doi: 10.1016/j.healun.2019.01.592.
- García Sáez D, Zych B, Sabashnikov A, et al. Evaluation of the organ care system in heart transplantation with an adverse donor/recipient profile. Ann Thorac Surg. 2014;98(6):2099–2106. doi: 10.1016/j.athoracsur.2014.06.098.
- Mehta V, Taylor M, Hasan J, et al. Establishing a heart transplant programme using donation after circulatory-determined death donors: a United Kingdom based single-centre experience. Interact Cardiovasc Thorac Surg. 2019;29(3):422–429. doi: 10.1093/icvts/ivz121.
- Ontario Health. Portable normothermic cardiac perfusion system in donation after cardiocirculatory death: a health technology assessment. Ont Health Technol Assess Ser. 2020;20(3):1–90.
- Bishawi M, Roan JN, Milano CA, et al. A normothermic ex vivo organ perfusion delivery method for cardiac transplantation gene therapy. Sci Rep. 2019;9(1):8029. doi: 10.1038/s41598-019-43737-y.
- Nilsson J, Jernryd V, Qin G, et al. A nonrandomized open-label phase 2 trial of nonischemic heart preservation for human heart transplantation. Nat Commun. 2020;11(1):2976. doi: 10.1038/s41467-020-16782-9.

