Abstract
Objectives
Patients presenting with symptoms suggestive of acute coronary syndrome (ACS) contribute to a high workload and overcrowding in the Emergency Department (ED). Accelerated diagnostic protocols for non-ST-elevation myocardial infarction have proved challenging to implement. One obstacle is the turnaround time for analyzing high-sensitivity cardiac troponin (hs-cTn). In the WESTCOR-POC study (Clinical Trials number NCT05354804) we aim to evaluate safety and efficiency of a 0/1 h hs-cTn algorithm utilizing a hs-cTnI point of care (POC) instrument in comparison to central laboratory hs-cTnT measurements.
Design
This is a prospective single-center randomized clinical trial aiming to include 1500 patients admitted to the ED with symptoms suggestive of ACS. Patients will receive standard investigations following the European Society of Cardiology 0/1h protocols for centralized hs-cTnT measurements or the intervention using a 0/1h POC hs-cTnI algorithm. Primary end-points are 1) Safety; death, myocardial infarction or acute revascularization within 30 days 2) Efficiency; length of stay in the ED, 3) Cost- effectiveness; total episode cost, 4) Patient satisfaction, 5) Patient symptom burden and 6) Patients quality of life. Secondary outcomes are 12-months death, myocardial infarction or acute revascularization, percentage discharged after 3 and 6 h, total length of hospital stay and all costs related to hospital contact within 12 months.
Conclusion
Results from this study may facilitate implementation of POC hs-cTn testing assays and accelerated diagnostic protocols in EDs, and may serve as a valuable resource for guiding future investigations for the use of POC high sensitivity troponin assays in outpatient clinics and prehospital settings.
Introduction
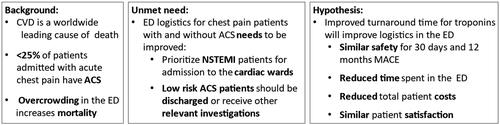
Acute ischemic coronary artery disease is a common cause of morbidity and mortality worldwide. Patients with chest pain are frequently referred to the Emergency Department (ED) [Citation1,Citation2]. Less than 25% of referred patients are finally diagnosed with acute coronary syndrome (ACS) [Citation3,Citation4] and large proportion of chest pain patients could be discharged from the ED without any treatment if the clinical suspicion of ACS could be refuted [Citation5,Citation6]. Overcrowding in the ED should be avoided as being associated with increased mortality, costs and length of stay [Citation7,Citation8].
The European Society of Cardiology (ESC) recommends that accelerated diagnostic protocols using high-sensitivity cardiac troponin (hs-cTn) should be used for rule-out and rule-in of non-ST-elevation myocardial infarction (NSTEMI) in the ED [Citation9]. Despite this recommendation these algorithms have proved difficult to implement into routine clinical practice, and are currently used by a limited number of hospitals [Citation10,Citation11]. There are a large number of international studies investigating different 0/1 h algorithms, but only a few randomized controlled trials (RCT) have been performed [Citation12,Citation13]. One possible obstacle that might reduce the utility of accelerated algorithms is the turnaround time (TAT) for hs-cTn troponin measurements. Until recently, hs-cTn assays have only been available on large centralized laboratory platforms with a typical TAT of 60 min. Consequently, the patients may need to stay in the ED for up to 2–3 h until a final risk prediction regarding NSTEMI can be made [Citation9]. Large numbers of patients waiting for test results may increase the overcrowding that accelerated diagnostic protocols were aiming to prevent. The cost-effectiveness of accelerated protocols are less frequently reported.
In 2021, a hs-cTn point of care (POC) instrument from Siemens Healthineers providing bedside results with a turnaround time of 8 min, and with sufficient analytical quality to be used in a 0/1 h algorithm received CE marking [Citation14]. Utilizing this instrument in an accelerated diagnostic protocol represents a potential game changer in the management of patients with chest pain, which could significantly improve patient flow in the ED.
Aims of this study
We aim to compare the safety of the currently recommended 0/1 h protocol using centralized hs-cTnT testing with a 0/1 h protocol utilizing POC hs-cTnI measurements. We will further evaluate the efficiency, cost-effectiveness and patient satisfaction of the two approaches. We hypothesize that the POC algorithm will have similar safety and better efficiency compared to currently recommend accelerated diagnostic protocols utilising central laboratory testing, see ().
Methods
Study design, study setting and inclusion of patients
The WESTCOR-POC study is a single center prospective randomized clinical trial, aiming to include 1500 patients admitted to the ED with symptoms suggestive of ACS. Key features of the study are presented in . The study will be conducted at Haukeland University Hospital in Bergen, Norway. This is a large academic hospital serving as a local hospital for approximately 470,000 persons, and functions as a referral hospital for approximately 1,000,000 inhabitants. In Norway, approximately 50% of chest pain patients have been investigated with electrocardiogram (ECG) and clinical gestalt in primary care before they present to the ED, while the rest are directed to the ED after contacting the emergency call center being operated by specially trained nurses [Citation18]. Patients who are suspected to have ACS based on this prescreening will be assessed by the study nurses upon arrival. Inclusion will be made if presenting symptoms are suggestive of ACS including; non-traumatic chest pain/discomfort not induced by other obvious non-cardiac disease, radiating pain (back/jaw/neck/arms), epigastric pain suggestive of ACS (due to risk factors or additional symptoms indicating ACS rather than gastrointestinal conditions), chest discomfort combined with nausea, dyspnea or arrhythmia. Patients will receive either standard care following the ESC 0/1h protocol with centralized hs-cTnT measurements or the intervention using a novel 0/1h POC hs-cTnI algorithm. Eligible patients (see ) will be enrolled consecutively within the designated time period 07:00–22:00 and randomized with simple randomization in concealed envelopes in a 1:1 fashion. Oral information and consent will be obtained before inclusion and full study information and written consent will be obtained in the ED when the clinical situation is stable. There will be no blinding of the study groups after inclusion.
Figure 2. Overview of the study design. ESC: European Society of Cardiology, POC: Point of care, NPR: Norwegian Patient Register, CDR: Norwegian Cause of Death Register, S: Seattle Angina Score 7, RAND 12: 12-Item Short Form Health Survey, 2014 PasOpp: Patient experience in Norwegian hospitals (2014).Questionnaires have been validated [Citation15–17].
![Figure 2. Overview of the study design. ESC: European Society of Cardiology, POC: Point of care, NPR: Norwegian Patient Register, CDR: Norwegian Cause of Death Register, S: Seattle Angina Score 7, RAND 12: 12-Item Short Form Health Survey, 2014 PasOpp: Patient experience in Norwegian hospitals (2014).Questionnaires have been validated [Citation15–17].](/cms/asset/1bb84091-25aa-4c04-8a99-a4992d6bf339/icdv_a_2272585_f0002_c.jpg)
The study will be conducted in accordance with the Helsinki declaration and is approved by the Regional Ethics Committee for Medical and Health Research (REC) (approval ID number 285544) and registered at ClinicalTrials.gov (NCT05354804). Recruitment started in March 2022 with an aim to conclude data collection by the January 2024. By mid-August 2023 a total of 1150 patients have been enrolled.
Study outcome
The primary and secondary outcomes of the study are shown in and include safety, efficiency, patient satisfaction and costs. Data will be collected from patient medical records, a 30 days follow-up phone interview, the Norwegian Patient Register (including all hospital contacts) and the Norwegian Cause of Death Register.
Intervention
Standard care arm
Patients randomized to standard care will be sampled at admission and after 1 h. An additional 3-h sample will be obtained according to the ESC 0/1 h algorithm (9). hs-cTnT and standard laboratory tests will be measured in the central hospital laboratory using Cobas e801 from Roche Diagnostics. ECG and HEART-score will be obtained in all patients. Other clinical investigations will be ordered by the attending physician as deemed clinically appropriate. If NSTE-ACS is unlikely (based on the hs-cTnT 0/1 h algorithm and HEART-score < 4), patients will be investigated according to the ED flow chart for non-coronary acute chest pain, in order to identify differential diagnoses. Patients will be admitted or discharged based on the clinical judgement of the attending physician.
Intervention arm
Patients randomized to the intervention arm will be treated similar as the standard arm with the exception that the admission and 1 h sample will be measured with a hs-TnI POC testing instrument from Siemens Healthineers in the ED and allocated to low risk or intermediate/high risk based on prespecified concentrations (see below). In admitted patients, further troponin measurements will be obtained as clinically indicated (including a 3 h sample as specified in the ESC guideline [Citation9]), and measured in the central laboratory using hs-cTnT. The admission- hs-cTnT measurements may be made available upon request after admission, if they are needed for comparison.
Troponin assays and cutoffs
The standard arm will use a hs-cTnT assay from Roche Diagnostics with a limit of detection (LOD) of 3 ng/L, 99th percentile 14 ng/L, and a 10% analytical within-series coefficient of variation (CVA) at 4.5 ng/L [Citation19]. The intervention arm will analyze hs-cTnI by the Atellica VTli, a POC instrument from Siemens Healhtineers with a LOD of 1.6 ng/L, 99th percentile of 23 ng/L, and a 10% CVA at 8.9 ng/L [Citation19].
An admission and 1 h sample will be obtained in all patients. This approach is a pragmatic local adaption, suggested by the ESC, when turn-around time for cTnT typically are > 60 min [Citation9]. Also, the current local cardiological practice are non-accustomed to a single sample rule-out approach (0/3 h algorithm is the standard care outside the trial).
The 0/1 h rule-out cut offs for hs-cTnT will be as recommended by the ESC [Citation9] whilst the Siemens Atellica VTli cutoffs will be based on data from the SEIGE and SAMIE study (suggesting a direct admission sample rule-out cut off of 4 ng/L [Citation20]) and correlation with other hs-cTn assays (Siemens Atellica, Abbott Anility and hs-cTnT from the Roche Diagnostics), See Supplemental Table 1. Evidence supports slightly higher baseline rule-out concentrations when two samples (and a 1 h delta) are obtained, compared to single sample rule-out [Citation21]. Unpublished reports from other centers indicated that a concentration of 4 ng/L would be able to rule-out a much lower percentage of patients than what was reported by Apple et al. [Citation20], indicating a shift in the lower concentrations measured by the assay. This situation sometimes occurs due to lot variations in hs-cTn assays [Citation22–24] but differences between cohorts measured may also lead to differences in cutoffs suggested. The current study therefore settled on a slightly higher baseline rule-out concentration of hs-cTnI <6 ng/L together with a 1 h Δ <3 ng/L.
Safety measures
hs-cTnT will be measured in the central laboratory for all patients. In the intervention group, hs-cTnT results will be concealed from the clinician and assessed by study nurses for discharged patients. Clinicians will be alerted only if baseline cTnT is ≥12 ng/L or if1-h delta is ≥3ng/L.
After enrolling 100 patients, a safety committee including cardiologists and emergency physicians reviewed all cases where clinicians had been notified of cTnT values not meeting low-risk criteria. No adverse events were detected, however the prevalence of NSTEMIs was low and it was decided to continue this safety measure throughout the study.
Adjudication
The index diagnosis will be adjudicated by two independent cardiologists (disagreements will be solved by a third adjudicator) based on all available clinical data including laboratory data, ECG, echocardiography and other imaging findings that are available up to 30 days after inclusion. Hs-cTnT will be used for adjudication in all patients (standard and intervention arm) and the unstable angina and NSTEMI diagnoses will be based on the Fourth Universal Definition of Myocardial Infarction [Citation25].
Statistical methods
We will present demographics and baseline characteristics as descriptive statistics. The analysis of the safety outcome for myocardial infarction (MI), death or acute revascularization within 30 days and 12 months will be analyzed using ordinary analysis of clinical sensitivity, specificity, positive and negative predictive values, area und the receiver operating curve and compared using McNamar or Delongs test as applicable. The 12 months endpoint will be compared using C-statistics, multivariable logistic or Cox proportional hazard regression analysis. Net Reclassification Index and Integrated Discrimination Index will be calculated when applicable.
Statistical analyses of the efficiency related outcome; time spent in the ED, (minutes in the ED) and proportions discharged will be calculated using Student t-test, Mann Whitney U test and Chi-square test as applicable. Kaplan-Meier curves and log-rank test for equality of survivor functions will be calculated to compare time from admission to hospital discharge in the two intervention groups.
The economic evaluation will be performed as a cost-utility analysis, comparing quality-adjusted life-years and costs among the intervention group with the control group. Quality adjusted life-years will be estimated based on combining survival with quality of life. Costs during initial stay will be calculated based on costs per patient as measured by each hospital administration system, while costs of further contacts will be based on national averages per diagnosis related group (DRG) as reported by the Norwegian directorate of health.
Sample size
Based on earlier experience [Citation23,Citation26] approximately 15% of included patients will be diagnosed with a primary endpoint (MI, death or acute revascularization within 30 days). Inclusion of 750 patients in each arm would give a CI width of approximately 5% around a proportion of 99%, meaning that we have sufficient power to detect a drop in sensitivity from 99% to < 94%.
Few RCTs compare accelerated diagnostic protocols. One earlier publication showed that implementing an accelerated diagnostic pathway would reduce length of stay in hospital for low risk patients from 10.1 (SD 4.1) hours to 6.8 (SD 3.9) hours [Citation13]. Another study found a reduction in length of stay in the ED from 5.6 (IQR 4.0–7.0) hours to 4.6 (IQR 3.4–6.4) hours [Citation12]. We estimate the standard arm to have a length of stay in the ED of 180 (SD 90) minutes whilst the POC arm stayed for 150 (SD 90) minutes. The resulting power analysis shows that 142 patients should be included in each arm (independent t test, power 0.80 and alpha 0.05). If we aim for a power of 0.90 (alpha of 0.05) 190 patients should be included in each arm. If the difference between the two groups is 15 min, corresponding numbers are 566 and 758, respectively.
Discussion
Traditionally the utility of biomarkers has been investigated using cross sectional or prospective observational studies. These kinds of studies provide less robust data and the effect of biomarkers in clinical practice may be hard to determine. Accordingly, there is a growing consensus that novel biomarkers, including troponin assays, should be investigated using robust methodology, and ideally by RCTs [Citation27,Citation28]. cTn POC testing assays achieving high sensitivity criteria have been commercially available for a few years [Citation29,Citation30]. Apart from the Atellica VTLi used in this study, there are currently two other high sensitivity POC testing instruments available, see webpage from the International Federation of Clinical Chemistry Committee of Clinical Application of Cardiac Bio-Markers (IFCC C-CB) [Citation19]. As POC testing has the ability to provide rapid results directly to the clinician, increased efficiency in the ED management of patients with symptoms suggestive of ACS is expected. The overall safety for patients with chest pain could be improved as patients with NSTEMI may be allocated to cardiac treatment earlier and early rule-out of ACS may allow for more rapid evaluation of critical differential diagnosis in those with non-cardiac diseases.
Portability and the potential for non- laboratory staff to use POC devices may enable use of hs-cTn in new arenas such as prehospital services, nursing homes or other institutions without a central laboratory or 24h laboratory staff services.
Challenges in enhancing ACS management in the ED
The most commonly used accelerated diagnostic protocols are those recommended by the ESC [Citation9] or suggested by the high- STEACS investigators [Citation27]. Efficiency in the ED is complex, and current time-limiting factors are likely to vary between locations. Both the turnaround time for hs-cTn embedded in the accelerated diagnostic protocols or long waiting hours prior to physician review might be time limiting steps. Another possibility is that rapid allocation to a low risk category for ACS (e.g. after measuring a very low hs-cTn) might introduce longer waiting hours for low risk patients due to re-prioritisation, reducing efficiency for the large group of low-risk patients.
Optimal efficiency of accelerated diagnostic protocols is also dependent on how ruled-out patients are managed. According to the ECS guidelines non-invasive or invasive imaging may be indicated, also in low risk patients, to identify conditions such as unstable angina [Citation9]. The High-US study showed that 42.7% of 1020 patients were admitted despite meeting 0/1h rule-out criteria for ACS. The frequency of admitted patients varied significantly between locations. Admitted patients were generally older and had more risk factors, and many received further investigations for ACS or differential diagnoses. The rates of MI/death within 12 months were similarly low between the groups [Citation28], raising the question if inpatient management was the optimal strategy.
Challenges with POC testing
Earlier studies integrating POC tests in ACS diagnostics in the ED have provided inconsistent benefits in efficiency and cost-effectiveness [Citation31–35]. Several multicenter studies have revealed variation in the impact of POC testing strategies between different locations [Citation35,Citation36], suggesting that local logistics may influence the benefits of POC tests. Attention to local settings to support POC testing, such as clear guidance for use, interpretation and management, needs to be in place before implementation. The actual benefits in routine clinical practice should be evaluated, preferably by RCTs.
Limitations
The first limitations of our study is that it is a single- center study. As for the setting and logistics at our site, the university hospital currently uses the ESC 0/3h algorithm with a hs-cTnT assay from Roche Diagnostics. The study therefore introduces a new algorithm and a new troponin assay as well as the use of POC testing in our ED. The unfamiliarity may influence the clinician behavior, especially in the early phases of this study, which could underestimate the potential gains in effectiveness.
Secondly, at the start of this trial there are no validated 0/1h algorithms cutoffs for the Atellica VTLi assay. The cutoffs chosen are focused on safety and might not be optimal for efficiency. Earlier studies have shown that shifts in assay stability may affect the efficiency of published algorithms in a substantial way [Citation22–24], and long-term data on assay stability of the Atellica VTLi has not been reported.
As the effects of incorporating POC testing are dependent on setting, the benefits might vary throughout the day. To manage enrollment even during busy hours we use study personnel for enrolment from 07:00–22:00 seven days a week. However, like many other comparable studies, we do not have enrolment during the night shift, which might affect the generalizability. POC tests are intended to be used (after proper training) by non- laboratory staff. The study personnel used for enrolment and POC testing analyzes in our study have similar background as regular ED staff but are a smaller group than what would be expected in real life use, which could positively bias our understanding of user friendliness.
Finally, the use of hs-cTnT as standard assay could also influence our results. The adjudication is based on hs-cTnT, which might underestimate the diagnostic performance of the hs-cTnI POC algorithm. Furthermore, the hs-cTnT assay is known to diagnose chronic myocardial injury more often compared to hs-cTnI [Citation37,Citation38]. This could influence safety and costs since elevated concentrations might lead to more investigations and potentially also treatment if subclinical disease is detected more often in the standard arm. This reflects true biological differences between the cTn molecules, still it should be acknowledged that our study might produce different results compared to a study comparing central laboratory hs-cTnI to POC hs-cTnI.
Possible implications of this study
To our knowledge, there is no published data from any RCTs evaluating efficiency, costs or patient satisfaction after implementing hs-cTn POC testing in combination with accelerated risk stratification algorithms. This study will inform feasibility of hs-cTnI POC test implementation in EDs of Scandinavian hospitals. Our experience from the study setting will be of high value and make it possible to implement the necessary logistics, both locally and within similar health care systems internationally. If the results from this study show positive safety results additional studies should be carried out to evaluate performance in a prehospital setting.
Supplemental Material
Download MS Word (14.2 KB)Disclosure statement
K.M.A. is associate editor of Clinical Biochemistry, chair of the IFCC Committee on Clinical Application of Cardiac Biomarkers, has served on advisory boards for Roche Diagnostics, Siemens Healthineers and SpinChip, received consultant honoraria form CardiNor, lecturing honorarium from Siemens Healthineers and Snibe Diagnostics and research grants from Siemens Healthineers and Roche Diagnostics.
L.C. is the co-chair of the National Heart Foundation and Cardiac Society of Australia and New Zealand Assessment and management of ACS guidelines and has served on advisory boards for Abbott Diagnostics and Siemens Healthineers, received consultant honoraria from Abbott Diagnostics, Beckman Coulter, Radiometer Pacific and Siemens Healthineers. LCs institution has received grants from Abbott Diagnostics, Beckman Coulter, Radiometer Pacific, Roche and Siemens.
P. O. C. is an associate editor of the Journal of Applied Laboratory Medicine and on the advisory board of and has previously advised Psyros Diagnostics, Radiometer, LumiraDx and Siemens Healthineers and has received Honoria for lectures from Siemens.
F.S.A: Consultant: HyTest Ltd, AWE Medical Group; Associate Editor: Clinical Chemistry; Advisory Boards: Werfen, Siemens Healthineers, Qorvo, Abbott Vascular; Honorarium for Speaking at Industry Conferences: Siemens Healthineers, Beckman Coulter; PI on Industry Funded Grants (non-salaried) on cardiac biomarkers through Hennepin Healthcare Research Institute: Abbott Diagnostics, Abbott POC, BD, Beckman Coulter, Ortho-Clinical Diagnostics, Roche Diagnostics, Siemens Healthcare, ET Healthcare, Qorvo.
T.O has received research support from Abbott Diagnostics, ChromaDex, Novartis, and Roche Diagnostics; consulting fees and/or speaker honoraria from Abbott Diagnostics, Bayer, CardiNor, and Roche Diagnostics; and stock and stock options from CardiNor.
I.V.L.T, S.M.F.J, O.C.L, J.K, O.T.S, T.N, TW, K.V, R.O B report no disclosures.
Additional information
Funding
References
- Goodacre S, Cross E, Arnold J, et al. The health care burden of acute chest pain. Heart. 2005;91(2):229–230. doi:10.1136/hrt.2003.027599.
- Langlo NM, Orvik AB, Dale J, et al. The acute sick and injured patients: an overview of the emergency department patient population at a norwegian university hospital emergency department. Eur J Emerg Med. 2014;21(3):175–180. doi:10.1097/MEJ.0b013e3283629c18.
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–867. doi:10.1056/NEJMoa0900428.
- Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868–877. doi:10.1056/NEJMoa0903515.
- Westwood ME, Armstrong N, Worthy G, et al. Optimizing the use of High-Sensitivity troponin assays for the early rule-out of myocardial infarction in patients presenting with chest pain: a systematic review. Clin Chem. 2021;67(1):237–244. doi:10.1093/clinchem/hvaa280.
- Twerenbold R, Costabel JP, Nestelberger T, et al. Outcome of applying the ESC 0/1-hour algorithm in patients With suspected myocardial infarction. J Am Coll Cardiol. 2019;74(4):483–494. doi:10.1016/j.jacc.2019.05.046.
- Sun BC, Hsia RY, Weiss RE, et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61(6):605–611 e6. doi:10.1016/j.annemergmed.2012.10.026.
- Jones S, Moulton C, Swift S, et al. Association between delays to patient admission from the emergency department and all-cause 30-day mortality. Emerg Med J. 2022;39(3):168–173. doi:10.1136/emermed-2021-211572.
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi:10.1093/eurheartj/ehaa575.
- Apple FS, Collinson PO, Kavsak PA, et al. Getting cardiac troponin right: Appraisal of the 2020 European Society of Cardiology guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-Segment elevation by the International Federation of Clinical Chemistry and Laboratory Medicine Committee on Clinical Applications of Cardiac Bio-Markers. Clin Chem. 2021;67(5):730–735. doi:10.1093/clinchem/hvaa337.
- Collinson P, Suvisaari J, Aakre KM, et al. How well do laboratories adhere to recommended guidelines for cardiac biomarkers management in Europe? The CArdiac MARker guideline uptake in Europe (CAMARGUE) study of the european federation of laboratory medicine task group on cardiac markers. Clin Chem. 2021;67(8):1144–1152. doi:10.1093/clinchem/hvab066.
- Chew DP, Lambrakis K, Blyth A, et al. A randomized trial of a 1-Hour troponin T protocol in suspected acute coronary syndromes: the rapid assessment of possible acute coronary syndrome in the emergency department With High-Sensitivity troponin T study (RAPID-TnT). Circulation. 2019;140(19):1543–1556. doi:10.1161/CIRCULATIONAHA.119.042891.
- Anand A, Lee KK, Chapman AR, et al. High-Sensitivity cardiac troponin on presentation to rule Out myocardial infarction: a Stepped-Wedge cluster randomized controlled trial. Circulation. 2021;143(23):2214–2224. doi:10.1161/CIRCULATIONAHA.120.052380.
- Apple FS, Schulz K, Schmidt CW, et al. Determination of sex-specific 99th percentile upper reference limits for a point of care high sensitivity cardiac troponin I assay. Clin Chem Lab Med. 2021;59(9):1574–1578. doi:10.1515/cclm-2021-0262.
- Chan PS, Jones PG, Arnold SA, et al. Development and validation of a short version of the seattle angina questionnaire. Circ Cardiovasc Qual Outcomes. 2014;7(5):640–647. doi:10.1161/CIRCOUTCOMES.114.000967.
- Pettersen KI, Veenstra M, Guldvog B, et al. The patient experiences questionnaire: development, validity and reliability. Int J Qual Health Care. 2004;16(6):453–463. doi:10.1093/intqhc/mzh074.
- Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi:10.1097/00005650-199603000-00003.
- Blinkenberg J, Pahlavanyali S, Hetlevik O, et al. General practitioners’ and out-of-hours doctors’ role as gatekeeper in emergency admissions to somatic hospitals in Norway: registry-based observational study. BMC Health Serv Res. 2019;19(1):568. doi:10.1186/s12913-019-4419-0.
- High-Sensitivity* Cardiac Troponin I and T Assay Analytical Characteristics Designated by Manufacturer. IFCC Committee on Clinical Applications of Cardiac Bio-Markers (C-CB) v012019. Assessed 2023. https://wwwifccorg/media/477656/high-sensitivity-cardiac-troponin-i-and-t-assay-analytical-characteristics-designated-by-manufacturer-v052022.pdf
- Apple FS, Smith SW, Greenslade JH, et al. Single High-Sensitivity point-of-Care Whole-Blood cardiac troponin I measurement to rule Out acute myocardial infarction at low risk. Circulation. 2022;146(25):1918–1929. doi:10.1161/CIRCULATIONAHA.122.061148.
- Collet JP, Thiele H, Barbato E, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev Esp Cardiol. 2021;74(6):544. 2021
- Haagensen K, Collinson P, Åsberg A, et al. How does the analytical quality of the High-Sensitivity cardiac troponin T assay affect the ESC rule Out algorithm for NSTEMI? Clin Chem. 2019;65(3):494–496. doi:10.1373/clinchem.2018.298703.
- Tjora HL, Steiro OT, Langørgen J, et al. Cardiac troponin assays With improved analytical quality: a Trade-Off Between enhanced diagnostic performance and reduced Long-Term prognostic value. J Am Heart Assoc. 2020;9(23):e017465.
- Tjora HL, Steiro OT, Langørgen J, et al. Diagnostic performance of novel troponin algorithms for the Rule-Out of Non-ST-Elevation acute coronary syndrome. Clin Chem. 2022;68(2):291–302. doi:10.1093/clinchem/hvab225.
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–2264. doi:10.1016/j.jacc.2018.08.1038.
- Steiro OT, Tjora HL, Langorgen J, et al. Clinical risk scores identify more patients at risk for cardiovascular events within 30 days as compared to standard ACS risk criteria: the WESTCOR study. Eur Heart J Acute Cardiovasc Care. 2020;10(3):287–301.
- Chapman AR, Anand A, Boeddinghaus J, et al. Comparison of the efficacy and safety of early Rule-Out pathways for acute myocardial infarction. Circulation. 2017;135(17):1586–1596. doi:10.1161/CIRCULATIONAHA.116.025021.
- Nowak RM, Jacobsen G, Limkakeng A, Jr., et al. Outpatient versus observation/inpatient management of emergency department patients rapidly ruled-out for acute myocardial infarction: findings from the HIGH-US study. Am Heart J. 2021;231:6–17. doi:10.1016/j.ahj.2020.10.067.
- Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem. 2009;55(7):1303–1306. doi:10.1373/clinchem.2009.128363.
- Collinson PO, Aakre KM, Saenger A, et al. Cardiac troponin measurement At The point Of care: educational recommendations on analytical And clinical aspects by the IFCC committee on cardiac biomarkers (IFCC C-CB). Clin Chem Lab Med. 2023;61(6):989–998. doi:10.1515/cclm-2022-1270.
- Collinson PO, John C, Lynch S, et al. A prospective randomized controlled trial of point-of-care testing on the coronary care unit. Ann Clin Biochem. 2004;41(Pt 5):397–404. doi:10.1258/0004563041731547.
- Goodacre SW, Bradburn M, Cross E, et al. The randomised assessment of treatment using panel assay of cardiac markers (RATPAC) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Heart. 2011;97(3):190–196. doi:10.1136/hrt.2010.203166.
- Loten C, Attia J, Hullick C, et al. Point of care troponin decreases time in the emergency department for patients with possible acute coronary syndrome: a randomised controlled trial. Emerg Med J. 2010;27(3):194–198. doi:10.1136/emj.2008.069427.
- Renaud B, Maison P, Ngako A, et al. Impact of point-of-care testing in the emergency department evaluation and treatment of patients with suspected acute coronary syndromes. Acad Emerg Med. 2008;15(3):216–224. doi:10.1111/j.1553-2712.2008.00069.x.
- Ryan RJ, Lindsell CJ, Hollander JE, et al. A multicenter randomized controlled trial comparing Central laboratory and point-of-care cardiac marker testing strategies: the disposition impacted by serial point of care markers in acute coronary syndromes (DISPO-ACS) trial. Ann Emerg Med. 2009;53(3):321–328. doi:10.1016/j.annemergmed.2008.06.464.
- Bradburn M, Goodacre SW, Fitzgerald P, et al. Interhospital variation in the RATPAC trial (randomised assessment of treatment using panel assay of cardiac markers). Emerg Med J. 2012;29(3):233–238. doi:10.1136/emj.2010.108522.
- Vestergaard KR, Jespersen CB, Arnadottir A, et al. Prevalence and significance of troponin elevations in patients without acute coronary disease. Int J Cardiol. 2016;222:819–825. doi:10.1016/j.ijcard.2016.07.166.
- Árnadóttir Á, Vestergaard KR, Pallisgaard J, et al. High-sensitivity cardiac troponin T is superior to troponin I in the prediction of mortality in patients without acute coronary syndrome. Int J Cardiol. 2018;259:186–191. doi:10.1016/j.ijcard.2018.01.131.