Abstract
Objective: The multibranched off-the-shelf Zenith® t-Branch (Cook Medical, Bloomington, IN) device is commonly chosen for endovascular repair of thoracoabdominal aortic aneurysms. The aim of this study was to report early and mid-term outcomes in all patients treated with the t-Branch in Norway; Design and Methods: A retrospective multicenter study with Norwegian centers performing complex endovascular aortic repair was undertaken. T-Branch patients from 2014 to 2020 were included. All postoperative computed tomography angiography images were reviewed, and demographic, anatomical, perioperative and follow-up data were analyzed; Results: Seventy patients were treated in a single-step (n = 55) or staged (n = 15) procedure. Symptomatic presentation was seen in 20 patients, six of which had a contained rupture. Technical success was 87% (n = 59), with failures caused by unsuccessful bridging of target vessels (n = 4), target vessel bleeding (n = 3), persisting type 1c endoleak (n = 1) and t-Branch malrotation (n = 1). 30-day mortality was 9% (n = 6) and was associated with high BMI (p = .038). The spinal cord ischemia rate was 21% (n = 15) and was associated with type II aneurysms (OR 5.4, 95% CI 1.1–26.7, p = .04), smoking (OR 6.0, 95% CI 1.3–27.6, p = .02) and intraoperative blood loss (OR 1.1, 95% CI 1.0–1.3, p = .01). Survival at one, two and three years was 84 ± 4%, 70 ± 6% and 67 ± 6%, respectively. Freedom from aortic-related reinterventions at one, two and three years was 80 ± 5%, 65 ± 7% and 50 ± 8%, respectively; Conclusion: The study showed low early mortality (9%) and satisfactory mid-term survival. Technical success was achieved in acceptable 87% of procedures. The rate of spinal cord ischemia was high, occurring in 21% of patients.
HIGHLIGHTS
This paper provides a national experience of all TAAA patients treated with the multibranched t-Branch stent graft in Norway in a multi-center study. As we aimed at including all Norwegian patients operated with the device, the paper adds real-world data on t-Branch outcomes from four regional smaller-volume vascular centers.
The paper provides technical and clinical mid-term results with several patients being followed up for >3 years.
Technical success was achieved in 87% of procedures.
The 30-day mortality rate was 9% and survival at one, two and three years was 85 ± 4%, 70 ± 6% and 67 ± 6%, respectively.
Spinal cord ischemia was associated with Crawford type II aneurysms, smoking and intraoperative blood loss.
1. Introduction
During the last two decades, endovascular treatment for thoracoabdominal aortic aneurysms (TAAAs) has become increasingly available with fenestrated and branched endovascular aortic repair (f/b-EVAR). Initially, the endovascular approach was mainly considered in patients unfit for open surgical repair, as addressed in 2017 by the European Society for Vascular Surgery’s guidelines for descending thoracic aortic disease [Citation1]. With enhanced experience and improved preoperative planning and postoperative care, f/b-EVAR is increasingly used as first line treatment of anatomically suitable TAAAs in patients without connective tissue disorders or aortic infections [Citation2]. Several f/b-EVAR endograft systems are available, with devices falling into the two main categories of patient specific custom-made devices (CMDs) and off-the-shelf stent grafts. Of the latter, the Zenith® t-Branch (Cook Medical, Bloomington, IN) is most used and researched. With a four-branch design for reno-visceral vessel repair, the t-Branch is anatomically suitable in at least 50% of TAAAs [Citation3,Citation4].
Research describing t-Branch outcomes overall report high technical success rates, varying from 64% for acute TAAAs to 100% in all-elective patients in a recent meta-analysis, with most studies reporting technical success at 90–95% [Citation5]. In a publication reporting >500 t-Branch patients, technical success was achieved in 97% of procedures [Citation6]. As baseline characteristics, inclusion criteria and elective vs. emergency-ratio varies between published materials, 30-day mortality differs, and is reported at 12% in the largest consecutive material and pooled at a rate of 6% in a systematic review [Citation6,Citation7]. This is comparable to that of open repair surgery, which in large series has been reported between 7–17% [Citation8].
The t-Branch has been commercially available in Europe since late 2012 and several studies investigating t-Branch outcomes originate from highly experienced centers with large patient cohorts [Citation6,Citation9,Citation10]. The aim of this study was to assess early- and mid-term t-Branch outcomes in a nationwide study, drawing on experiences from four university hospital vascular centers.
2. Methods and materials
2.1. Design
A search in the Norwegian Vascular Surgery Registry (NORKAR), which coverage is national, identified 70 patients operated with the Zenith® t-Branch for TAAAs before January 2021 at four vascular centers in Norway (St. Olavs University Hospital, Trondheim [n = 28], Haukeland University Hospital, Bergen [n = 20], Oslo University hospital, Oslo [n = 12] and University Hospital of North Norway, Tromsø [n = 10]). The first patient was operated in 2014. All patients were included and followed up for at least six months. Patients eligible for t-Branch repair were within the manufacturer-given Instruction for Use (IFU) manual and turned down for open repair upon local evaluation, elsewise the indication for t-Branch repair was not predefined and not coordinated between institutions. Data were extracted locally from electronical medical records. Preoperative computed tomography angiography (CTA) images were used to retrospectively define aneurysm extents according to the Crawford classification, measure aortic diameters and determine clock positions of the renal arteries [Citation11]. After a semiautomatic vessel segmentation of the first follow-up CTA examination, a centerline analysis was used to measure length of aorta covered by stent graft defined from the brachiocephalic trunk to the internal iliac artery. Diameters were determined at follow-up CTA images using double oblique multiplanar reformations, and aneurysm shrinkage or expansion was identified by a diameter change of ≥5mm.
The Norwegian Directorate of Health gave dispensation from obtaining written patient consents (ref. 18/39142-2) and the Norwegian Centre for Research Data acted as data protection officer (project number 279787).
2.2. Surgical procedure
Percutaneous or surgical accesses were obtained bilaterally at the common femoral arteries and at the subclavian or axillary artery upon indication. Deployment of t-Branch followed the Instruction for Use Manual as is described extensively elsewhere [Citation12]. Haukeland University Hospital performed two-step staging with temporary aneurysm sac perfusion (TASP) via unstented branches upon individual indication (n = 14) after seeing high rates of SCI in patients operated early in the period, detailed in a dedicated conference abstract [Citation13]. The other institutions performed the operations single-staged.
Mean intra-arterial pressure (MAP) >80mmHg was aimed for in all procedures. The protocol of spinal catheters and cerebrospinal fluid (CSF) drainage was developed throughout the study period. Upon study end, in all institutions, high risk patients (type II aneurysms, previous thoracic endograft, occluded internal iliac artery) received spinal catheters preoperatively, while some institutions routinely used spinal catheters on all patients. Cerebrospinal fluid (CSF) was drained perioperatively to ≤10mmHg and reduced further in patients with neurological leg symptoms. The spinal catheter was terminated 36-72h postoperatively at no signs of SCI. A hemoglobin level <10 g/l prompted blood transfusion. The patients were typically controlled with a CTA scan prior to discharge or at one month postoperatively. Thereafter, patients were followed up clinically and with CTA imaging at six and twelve months and thereafter annually. Standard anti-thrombotic treatment was acetylsalicylic acid and low-molecular-weight heparin until mobilization and thereafter acetylsalicylic acid in monotherapy.
2.3. Endpoints and definitions
The primary endpoint was patient survival. Secondary endpoints were technical success, SCI, clinical success, target vessel stability, and freedom from aortic-related reintervention. Technical success was defined on an intent-to-treat basis and required successful introduction and deployment of the endograft and successful catheterization bridging of all target vessels in absence of surgical conversion or mortality, persistent and type I or III endoleak, branch occlusion, or graft limb obstruction, as proposed by Oderich in the SVS reporting standards et al. [Citation14]. Clinical success was defined according to the reporting standards as technical success with absence of procedure or aorta related death, persistent type I or III endoleak, sac expansion, device migration, aneurysm rupture, conversion to open repair and disabling permanent clinical sequalae (paraplegia, stroke or dialysis) [Citation14]. Target vessel stability was also reported in accordance with the reporting standard as any death or rupture related to branch complication or any secondary intervention indicated to treat a branch-related complication [Citation14]. SCI was classified into paraplegia (0 = no movement; 1 = minimal motion; 2 = motion, but not against resistance/gravity) or paraparesis (3 = motion against resistance/gravity; 4 = ability to stand/walk with assistance) [Citation15]. Intraoperative blood loss was determined from perioperative suction and compresses and was measured in 0.1 liter as a continuous variable.
2.4. Statistics
Continuous data are given as mean (±SD) when normally distributed and as median (interquartile range, IQR) when not. Categoric data are presented as percentages (counts). Time-to-event analyses were performed with Kaplan-Meier statistics. The Student’s T-test and the Mann Whitney U test was used for comparing independent continuous variables and the Fisher’s exact test for categorical variables. A one-way ANOVA was used for comparing length of stent graft covered aorta between Crawford classes. Binary logistic regression was used to identify an association between spinal cord ischemia and Crawford type II aneurysms, smoking, staging and intraoperative blood loss. P-values ≤0.05 were considered statistically significant and analyses were performed using SPSS Statistics version 29.0.0.1 (SPSS Inc., Chicago, Illinois).
3. Results
3.1. Demographics and TAAA morphology
Seventy patients treated with t-Branch between January 2014 and December 2020 were identified. Mean age was 71 ± 6 years, and 54% (n = 38) were male. Patient demographic and comorbidities are further described in . A combination of urgent and elective patients was treated, with 29% (n = 20) of patients presenting with symptomatic aneurysms and operated within 72 h of admission. Mean aneurysm diameter was 69 ± 11 mm, and Crawford type II aneurysms were most common, present in 39% (n = 27) of patients. Four patients were operated with carotid-subclavian bypass prior to the t-Branch procedure to achieve a good proximal sealing. Aneurysm morphology, presentation and etiology is further detailed in .
Table 1. Demographics and comorbiditiesTable Footnote*.
Table 2. Aneurysm morphologyTable Footnote*.
3.2. Perioperative outcomes
Staging with TASP was performed in 21% (n = 15) of procedures (excluding carotid-subclavian bypass, n = 4), one of which was unintentional due to primary failure of stenting the right renal artery. Three of the staged procedures were performed on symptomatic patients, but none with contained rupture. Median time between stages was five weeks (IQR 1–8). In 67% (n = 10) of staged procedures, all directional branches were left open until procedure stage 2, whereas in 33% (n = 5) of procedures, all but one target vessel were bridged to the t-Branch in the first stage. Two patients died between stages, one from aneurysm rupture, the other from subdural hematoma. Technical success was 87% (59/68, between-stage deaths excluded) and was negatively associated with previous endovascular surgery (p = .008). Technical failures were due to unsuccessful target vessel bridging (n = 4), target vessel bleeding (n = 3), type 1c endoleak (n = 1) and t-Branch malrotation (n = 1). Mean operation time was 402 ± 127 min and did not differ significantly between the first and second half of operations (411 vs. 392 min, p = .38). Median intraoperative blood loss was 0.45 L (IQR 0.25–0.82).
3.3. Stent graft
A total of 240 target vessels were repaired, with four vessels being bridged in 64% (n = 45) of patients, three vessels in 24% (n = 17) of patients, two vessels in 6% (n = 4) of patients and one vessel in 1% (n = 1) of patients. In 4% (n = 3) of patients zero vessels were repaired due either to between-stage mortality (n = 2) or intraoperative conversion to open repair (n = 1). An overview of covered bridging stents is shown in . Mean length of stent graft covered aorta was 437 ± 87mm, yielding on average 79 ± 14% aortic coverage. Crawford type II aneurysms were subject to 13 ± 3% greater aortic coverage relative to other Crawford classes (p<.001, 95% CI 7–20).
Table 3. Covered stents implanted in target vessels.
3.4. 30-day outcomes
30-day mortality, including between-stage mortality, was 9% (6/70), with death causes being multiorgan failure (n = 3), subdural hematoma (n = 1), complications to bowel ischemia (n = 1) and between-stage rupture (n = 1). No statistical difference was seen in 30-day mortality between elective (3/50) and urgent (2/20) cases (p = .619). Major postoperative complications occurred in 15 (21%) patients, detailed in . After 30 days the SCI rate was 21% (n = 15) among which 10% (n = 7) had paraplegia, detailed further in . SCI was not statistically different between staged (n = 3) and non-staged procedures (n = 12) (p = .58) and was not overrepresented in acute (n = 3) relative to elective cases (n = 12) (p = .58). SCI incidence trended lower in the second half of patients operated (32% vs. 14%, p = .09). After logistic regression, SCI was significantly associated with type II aneurysms (OR 5.4, 95% CI 1.1–26.7, p = .04), smoking (OR 6.0, 95% CI 1.3–27.6, p = .02) and intraoperative blood loss per 0.1 L (OR 1.1, 95% CI 1.0–1.3, p = .01).
Table 4. Major postoperative adverse events.
Table 5. 30-Day spinal cord ischemia.
Type 1 and 3 endoleaks occurred in 6% (n = 4) and 3% (n = 2) of patients postoperatively, and all but one type 1c endoleak resolved spontaneously within 30 days. Patients were discharged after median 7 (5–13) days.
3.5. Follow-up
Median follow-up time was 20 (range 1 – 72) months. Two patients were subject to aortic-related death (autopsy verified aortic rupture) during follow-up. Estimated overall survival (±SE) at 12, 24, 36 months was 84 ± 4%, 70 ± 6% and 67 ± 6%, respectively, displayed with Kaplan-Meier curve in . 26 patients underwent a total of 31 aorta-related reinterventions, details of which are given in , and a Kaplan-Meier curve with freedom from aorta-related reintervention is shown in . At 6, 12, 24 and 36 months an estimated 94 ± 2%, 89 ± 2%, 87 ± 2% and 87 ± 2% of target vessels were stable, respectively. Estimated primary clinical success at 6, 12, 24 and 36 months was 63 ± 6%, 53 ± 6%, 43 ± 7% and 31 ± 8%, respectively. The most common reason for failure to achieve clinical success was new-onset type 1 or 3 endoleaks (n = 12) followed by aneurysm sac expansion (n = 7), further detailed in . At 20 months postoperatively, one patient was converted to open repair with device explantation due to continuous aneurysm growth on the basis of type 2 endoleaks resistant to repeated endovascular reinterventions. Freedom from aneurysm growth at 12, 24 and 36 months follow-up was estimated to 84 ± 5%, 75 ± 7% and 70 ± 8%, respectively. Aneurysm growth was significantly associated with both type 1 or 3 endoleak (p = .004) and type 2 endoleak occurrence during follow-up (p = .007).
Figure 1. Survival postoperatively analyzed with Kaplan-Meier statistics. Curves are truncated at 36 months post-EVAR. EVAR: endovascular aortic repair.
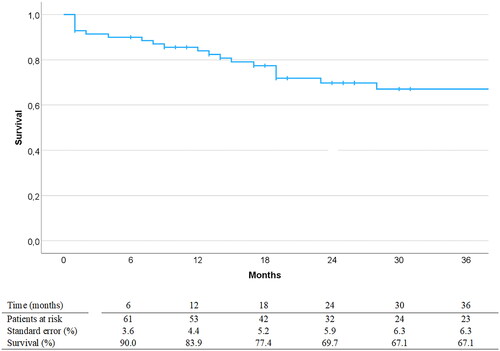
Figure 2. Freedom from aorta-related reintervention postoperatively analyzed with Kaplan-Meier statistics. Curves are truncated at 36 months post-EVAR. EVAR: endovascular aortic repair.
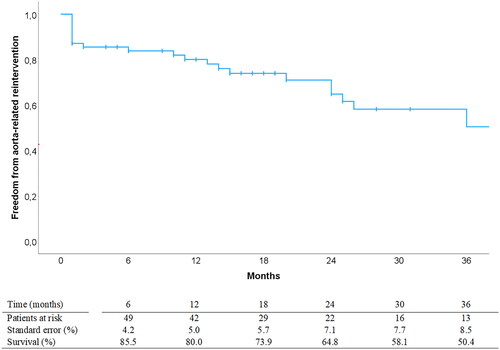
Table 6. Reinterventions during 36 months follow-up.
Table 7. Reasons for failure of primary clinical success.
4. Discussion
In this national study of patients treated with the Zenith® t-Branch EVAR device for TAAAs, we found satisfactory rates of 30-day mortality (9%) and follow-up survival. Technical success was achieved in 87% of patients, and 21% developed SCI. Reinterventions were performed on estimated one in two patients within three years. Primary clinical success at one year was just above 50%.
The present early mortality rate fares well with other studies investigating early f/b-EVAR mortality. In a meta-analysis including >2000 f/b-EVAR patients distributed among 24 studies, the pooled early mortality rate was 7.4% (95% CI, 5.9–9.1) [Citation16]. For t-Branch procedures in particular, Konstantinou et al. reported a pooled rate of 5.8% (95% CI 2.5–10.0) in a 2020 meta-analysis among seven observational t-Branch studies [Citation7]. As of 2021, a major supplement to the t-Branch literature was provided by Kölbel et al. in 2021 with early outcomes in >500 German and Polish patients, in whom the early mortality rate was 12.3% [Citation6]. Survival at 1-year varies in previous reports from 56% in all-urgent cases to 91% [Citation17–21]. Two year-survival has been reported by Katsargyris et al. and Silingardi et al. at 73% and 86%, respectively, while the present study finds a two-year survival of 70%. It is, however, difficult to draw any direct comparisons of survival data between published materials, as the premorbid level varies, different proportions of acute/elective cases are treated and the ratio between extensive and shorter TAAAs vary. Overall, considering the burden of comorbidity in our patient cohort and the relatively high proportion of large extent aneurysms (63% type II or III), we find the present survival outcomes to be satisfactory.
We report unsatisfactorily high rates of SCI, though, with SCI occurring in 21% of patients and permanent paraplegia in 10%. In early t-Branch experiences, the SCI incidence is reported as high as 33%, and several other institutions report in-hospital SCI incidence > 20% after f/b-EVAR, among which the papers by Spanos et al. and Bosiers et al. include only t-Branch patients [Citation22–27]. Despite this, lower SCI incidence is generally reported in the literature, pooled at 12% in a t-Branch meta-analysis [Citation7]. In a recent Italian multi-center study reporting >350 f/b-EVAR patients, SCI occurred in 13.4% of patients [Citation28]. Furthermore, in the abovementioned meta-analysis of f/b-EVAR studies by Rocha et al. the combined SCI incidence was 13% (95% CI 11 – 17%), with highly experienced centers reporting SCI rates as low as 5% [Citation16,Citation29].
One explanatory factor for the high SCI incidence, can be found when comparing results from the early and late period (32% vs. 14%). Though statistically insignificant (p = .09), the numbers provide support for more favorable outcomes toward the end of the study period. The trend is likely explained by an institutional learning curve that the authors Dias et al. for instance, have investigated in more detail. In their study on f/b-EVAR outcomes between 2008 and 2014, they found a tendency of decreasing SCI incidence in the latter part of their experience (39% vs 24%) [Citation24].
In the present t-Branch study, staging with the TASP technique did not reproduce the significantly lower SCI rates demonstrated by other studies, with 20% (3/15) of staged patients and 22% (12/55) of non-staged patients developing the complication. Nonetheless, staged procedures have increasingly been chosen for repair of extensive TAAAs at the other participating centers, as there is sound evidence to support a staged approach to high-risk cases. The scientific evidence was further strengthened recently with a meta-analysis by Dias-Neto et al. showing that the composite endpoint of 30-day mortality and/or permanent paraplegia was significantly reduced in staged procedures for extent type I to III TAAAs (14% vs. 6%) [Citation30].
We find, like others, that Crawford type II aneurysms was an independent risk factor for SCI, as these aneurysms are subject to significantly longer stent graft coverage than other Crawford classes. The ANOVA analysis did, however, not identify any significant differences in coverage between the other Crawford classes. This might be explained by the fact that the t-Branch often requires proximal thoracic and distal infrarenal and iliac components for attachment. In that fashion, an unnecessary sacrifice of healthy aorta and segmental arteries might take place in type IV and V aneurysms compared to what potentially could have achieved with a CMD or newer off-the-shelf multibranched device. Based on this perspective, type IV and V TAAAs are now rarely treated with the t-Branch at participating centers in an elective setting, as we believe these TAAAs in general are better served with CMDs or newer off-the-shelf multibranched stent grafts covering less heathy aorta and fewer segmental arteries.
We report on the causes of failure to achieve the composite endpoint of primary clinical success, as is suggested by the 2021 SVS reporting standards on f/b-EVAR. The treatment success rate is surprisingly low, with only just above half of the patients successfully treated at twelve months follow-up. Few other experiences have systematically given accounts of this endpoint, so a meaningful comparison to the literature is difficult. New-onset type 1 and 3 endoleaks were the most common cause of treatment failure, the majority of which were treated with secondary interventions. These secondary endoleaks might not occur only due to device integrity issues resulting from the primary procedure, but can also arise due to progression of the primary aortic disease [Citation31]. It is increasingly being recognized that the diameters of the sealing zone or of untreated aortic regions, may continue to grow after the initial procedure and become prone to endoleaks. Thus, the goal is not necessarily to have zero secondary reinterventions on the basis of type 1 or 3 endoleaks. The focus ought rather to be accounting for the possibility of these events to occur when designing the main procedure.
The findings on SCI, combined with the non-impressive technical success rates and the low primary clinical success, necessitates a discussion on the efficacy of smaller-volume centers performing EVAR procedures for TAAAs. 70 patients spanning over six years distributed among four centers yields an average of <3 t-Branch procedures per institution per year. Although we currently do not have available data on the total number of complex EVAR procedures in the period, the volume is likely too low for achieving excellent results on complex EVAR, as mastering preoperative planning, procedural execution and postoperative care requires great institutional and personnel experience [Citation32]. One might thus argue that complex EVAR ought to be centralized to fewer institutions in Norway.
Along with the retrospective design, the main limitation of this study is heterogeneity of the cohort, concerning modality of treatment (elective or urgent), surgical procedure (one or two steps) and post-operative management (spinal catheter variation throughout the study period and between institutions). Also, we have no data on patients that were turned down for intervention due to comorbidities nor those who underwent open surgery in the same time frame. The strengths, on the other hand, include the national experience with the inclusion of all available t-Branch patients.
5. Conclusions
The study showed satisfactory survival outcomes with a 30-day mortality rate of 9% and follow-up survival after two and three years at 70% and 67%. Freedom from reintervention was comparable to similar studies and shrinking or stable aneurysms were found in the majority of patients. Technical success was acceptable at 87%, but SCI rates were high with 21% of patients developing the complication.
Acknowledgements
Associate Professor Turid Follestad at the Unit of Applied Clinical Research, Faculty of Medicine and Health Sciences, NTNU, for statistical support.
Disclosure statement
The authors report that there are no competing interests to declare.
Additional information
Funding
References
- Riambau, V, Böckler, D, Brunkwall, J, et al. Editor’s choice – management of descending thoracic aorta diseases: clinical practice guidelines of the european society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53(1):4–52. doi: 10.1016/j.ejvs.2016.06.005.
- Tenorio ER, Dias-Neto MF, Lima GBB, et al. Endovascular repair for thoracoabdominal aortic aneurysms: current status and future challenges. Ann Cardiothorac Surg. 2021;10(6):744–767. doi: 10.21037/acs-2021-taes-24.
- Bisdas T, Donas KP, Bosiers M, et al. Anatomical suitability of the T-branch stent-graft in patients with thoracoabdominal aortic aneurysms treated using custom-made multibranched endografts. J Endovasc Ther. 2013;20(5):672–677. doi: 10.1583/13-4400MR.1.
- Gasper WJ, Reilly LM, Rapp JH, et al. Assessing the anatomic applicability of the multibranched endovascular repair of thoracoabdominal aortic aneurysm technique. J Vasc Surg. 2013;57(6):1553–1558; discussion 1558. doi: 10.1016/j.jvs.2012.12.021.
- Bilman V, Rinaldi E, Loschi D, et al. Suitability of current off-the-shelf devices for endovascular TAAA repair: a systematic review. J Cardiovasc Surg. 2023;64(5):459–469. doi: 10.23736/S0021-9509.23.12704-2.
- Kölbel T, Spanos K, Jama K, et al. Early outcomes of the t-branch off-the-shelf multi-branched stent graft in 542 patients for elective and urgent aortic pathologies: a retrospective observational study. J Vasc Surg. 2021;74(6):1817–1824. doi: 10.1016/j.jvs.2021.05.041.
- Konstantinou N, Antonopoulos CN, Jerkku T, et al. Systematic review and meta-analysis of published studies on endovascular repair of thoracoabdominal aortic aneurysms with the t-branch off-the-shelf multibranched endograft. J Vasc Surg. 2020;72(2):716–725.e1. doi: 10.1016/j.jvs.2020.01.049.
- Moulakakis KG, Karaolanis G, Antonopoulos CN, et al. Open repair of thoracoabdominal aortic aneurysms in experienced centers. J Vasc Surg. 2018;68(2):634–645.e12. doi: 10.1016/j.jvs.2018.03.410.
- Gallitto E, Faggioli G, Melissano G, et al. Preoperative and postoperative predictors of clinical outcome of fenestrated and branched endovascular repair for complex abdominal and thoracoabdominal aortic aneurysms in an Italian multicenter registry. J Vasc Surg. 2021;74(6):1795–1806.e6. doi: 10.1016/j.jvs.2021.04.072.
- Eleshra A, Oderich GS, Spanos K, et al. Short-term outcomes of the t-branch off-the-shelf multibranched stent graft for reintervention after previous infrarenal aortic repair. J Vasc Surg. 2020;72(5):1558–1566. doi: 10.1016/j.jvs.2020.02.012.
- Safi HJ, Winnerkvist A, Miller CC, et al. Effect of extended cross-clamp time during thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 1998;66(4):1204–1209. doi: 10.1016/s0003-4975(98)00781-4.
- Tsilimparis N, Fiorucci B, Debus ES, et al. Technical aspects of implanting the t-branch off-the-shelf multibranched stent-graft for thoracoabdominal aneurysms. J Endovasc Ther. 2017;24(3):397–404. doi: 10.1177/1526602817690730.
- Halvorsen H, Aass T, Jenssen GL, et al. A simplified method of staging branched endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2019;58(6):e177–e8. doi: 10.1016/j.ejvs.2019.06.738.
- Oderich GS, Forbes TL, Chaer R, et al. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J Vasc Surg. 2021;73(1S):4S–52S. Supplement doi: 10.1016/j.jvs.2020.06.011.
- Greenberg RK, Lu Q, Roselli EE, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation. 2008;118(8):808–817. doi: 10.1161/CIRCULATIONAHA.108.769695.
- Rocha RV, Lindsay TF, Friedrich JO, et al. Systematic review of contemporary outcomes of endovascular and open thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2020;71(4):1396–1412.e12. doi: 10.1016/j.jvs.2019.06.216.
- Bosiers M, Kölbel T, Resch T, et al. Early and midterm results from a postmarket observational study of zenith t-branch thoracoabdominal endovascular graft. J Vasc Surg. 2021;74(4):1081–1089.e3. doi: 10.1016/j.jvs.2021.01.070.
- Gallitto E, Faggioli G, Spath P, et al. Urgent endovascular repair of thoracoabdominal aneurysms using an off-the-shelf multibranched endograft. Eur J Cardiothorac Surg. 2022;61(5):1087–1096.
- Gallitto E, Gargiulo M, Freyrie A, et al. Off-the-shelf multibranched endograft for urgent endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2017;66(3):696–704.e5. doi: 10.1016/j.jvs.2016.12.129.
- Katsargyris A, de Marino PM, Botos B, et al. Single center experience with endovascular repair of acute thoracoabdominal aortic aneurysms. Cardiovasc Intervent Radiol. 2021;44(6):885–891. doi: 10.1007/s00270-021-02798-1.
- Silingardi R, Gennai S, Leone N, et al. Standard “off-the-shelf” multibranched thoracoabdominal endograft in urgent and elective patients with single and staged procedures in a multicenter experience. J Vasc Surg. 2018;67(4):1005–1016. doi: 10.1016/j.jvs.2017.08.068.
- Bosiers MJ, Bisdas T, Donas KP, et al. Early experience With the first commercially available Off-the-shelf multibranched endograft (t-branch) in the treatment of thoracoabdominal aortic aneurysms. J Endovasc Ther. 2013;20(6):719–725. doi: 10.1583/13-4428R.1.
- Cochennec F, Kobeiter H, Gohel MS, et al. Impact of intraoperative adverse events during branched and fenestrated aortic stent grafting on postoperative outcome. J Vasc Surg. 2014;60(3):571–578. doi: 10.1016/j.jvs.2014.02.065.
- Dias NV, Sonesson B, Kristmundsson T, et al. Short-term outcome of spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2015;49(4):403–409. doi: 10.1016/j.ejvs.2014.12.034.
- Kasprzak P, Gallis K, Cucuruz B, et al. Editor’s choice–temporary aneurysm sac perfusion as an adjunct for prevention of spinal cord ischemia after branched endovascular repair of thoracoabdominal aneurysms. Eur J Vasc Endovasc Surg. 2014;48(3):258–265. doi: 10.1016/j.ejvs.2014.05.020.
- Sobel JD, Vartanian SM, Gasper WJ, et al. Lower extremity weakness after endovascular aneurysm repair with multibranched thoracoabdominal stent grafts. J Vasc Surg. 2015;61(3):623–628. doi: 10.1016/j.jvs.2014.10.013.
- Spanos K, Kölbel T, Theodorakopoulou M, et al. Early outcomes of the t-branch off-the-shelf multibranched stent-graft in urgent thoracoabdominal aortic aneurysm repair. J Endovasc Ther. 2018;25(1):31–39. doi: 10.1177/1526602817747282.
- Rinaldi E, Melloni A, Gallitto E, et al. Spinal cord ischemia After thoracoabdominal aortic aneurysms endovascular repair: from the Italian multicenter fenestrated/branched endovascular aneurysm repair registry. J Endovasc Ther. 2023;30(2):281–288. doi: 10.1177/15266028221081074.
- Oderich GS, Ribeiro M, Reis de Souza L, et al. Endovascular repair of thoracoabdominal aortic aneurysms using fenestrated and branched endografts. J Thorac Cardiovasc Surg. 2017;153(2):S32–S41.e7. doi: 10.1016/j.jtcvs.2016.10.008.
- Dias-Neto M, Tenorio ER, Huang Y, et al. Comparison of single- and multistage strategies during fenestrated-branched endovascular aortic repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2023;77(6):1588–1597.e4. e4. doi: 10.1016/j.jvs.2022.03.485.
- Mezzetto L, D'Oria M, Lepidi S, et al. A scoping review on the incidence, risk factors, and outcomes of proximal neck dilatation after standard and complex endovascular repair for abdominal aortic aneurysms. J Clin Med. 2023;12(6):2324.
- Eagleton MJ. 10 Key lessons learned in complex EVAR. Endovasc Today. 2016;15(3):56–61.

