Abstract
Infected or mycotic aortic aneurysms (MAAs) are a rare type of aneurysms. Due to the high risk of rupture, MAAs are life-threatening conditions. Early diagnosis and treatment are necessary, yet MAAs are usually found coincidentally. We report 10 patients with MAAs in whom macroscopically, similar coined-sized lesions of the inner aortic wall were seen in all cases. When a coin-sized lesion in the inner aortic wall is seen during open surgical repair of an aortic aneurysm, the surgeon should consider an infectious cause. Microbiological tissue samples should be collected, and additional targeted antibiotic therapy should be started.
Background
Infected or mycotic aortic aneurysms (MAAs) are a rare type of aneurysms, constituting only 0.6–1.0% of all aortic aneurysms in Western countries [Citation1,Citation2]. The term ‘mycotic’ was introduced to describe infected aortic aneurysms with a fungus like appearance (not to be confused with a fungal infection), which is defined as an abrupt discontinuation of the aortic wall with the formation of a saccular aneurysm [Citation3]. MAAs have a wide range of clinical manifestations, such as fever, malaise, and pain, but rarely present without any symptoms [Citation1]. Early diagnosis and treatment are necessary due to the high risk of rupture, yet MAAs are usually found coincidentally. Saccular aneurysms with rapid expansion or periaortic soft tissue infiltration are often a sign of an infected aneurysm [Citation4]. Macroscopic characteristics observed during surgery are non-specific, as diagnostic criteria are limited [Citation2]. This case series presents a unique macroscopic appearance for MAAs that should prompt a surgeon to take cultures and start antibiotic therapy improving patients’ outcomes.
Materials and methods
We retrospectively reviewed 10 patients who underwent open surgical repair for MAAs between 2015 and 2021 in our center.
Results
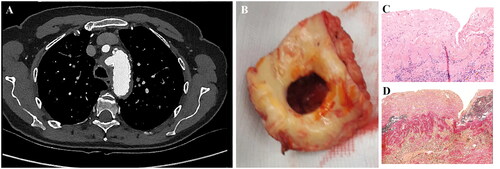
The patients had a mean age of 65.8 ± 7.8 years. Fever (70%) and pain (40%) were mostly observed in our cases and only one was asymptomatic. It was a coincidental finding on computed tomographic angiography (CTA) in all our cases (see ), with a MAA characteristic rapid increase in diameter in three. Additional [18F]FDG-PET/CT showed increased [18F]FDG uptake at the site of the MAA and disseminated areas, but little to no activity in non-infected aneurysms.
Figure 1. Illustrations of pre-operative imaging findings with corresponding macroscopic aspect of mycotic aortic aneurysms (‘typical’ coin-sized lesions of the inner aortic wall) and representative histopathological images of a mycotic aorta specimen. (A) Transverse CTA image with saccular aneurysm (aortic arch level) and (B) shows the resected part with typical coin-sized hole in the aortic wall. (C) H&E-stained section shows transmural acute inflammation, in addition to atherosclerotic changes; and (D) Elastica van Gieson staining demonstrates the medial disruption. CTA: computed tomography angiography.
Open surgery was performed in all our patients including two urgent operations due to hemodynamic instability and acute tamponade, respectively. A thoracophrenolaparotomy with left-left bypass was performed in patients with a descending aortic aneurysm (for more details see ). Four patients with an ascending aortic aneurysm had extracorporeal circulation with deep hypothermic circulatory arrest and antegrade bilateral cerebral perfusion. We showed zero surgery related mortality and none of the patients had a recurrent MAA after a median follow-up of four years.
Table 1. Patient characteristics.
Perioperatively, all patients had typical coin-sized hole like structure on the inside of the aorta at the level of the infected aneurysm with a paper-thin wall of the (false) aneurysm (). Histopathological samples showed an interruption of the medial layer (). Tissue cultures were positive for Staphylococcus aureus (n = 4), Salmonella (n = 2), Streptococcus agalactiae (n = 1), Cutibacterium acnes (n = 1), and Coxiella burnetii (polymerase chain reaction on tissue, no culture) (n = 1).
Mean duration of antibiotic therapy was 228 ± 221 days (range 6 weeks to 26 months). The duration was primarily based on postoperative laboratory results and imaging findings. In general, S. aureus infections were treated for six weeks.
Discussion
To the best of our knowledge, these specific lesions were not earlier described as ‘coin-sized lesions’. This typical aspect differs from a more diffusely widened wall in non-infected aneurysms, which makes this a distinct characteristic for MAAs, irrespective of the pathogenic species. The coin-sized lesion could thus aid to the diagnostic process. Early diagnosis of MAAs is crucial because misrecognizing or delayed treatment could lead to deterioration with poor outcome due to sepsis, rupture, and infection of the new prosthesis. However, this could be challenging due to non-specific, or even absence of symptoms. Hemoptysis and tamponade are alarming but rare symptoms that require immediate surgery [Citation5,Citation6].
MAAs are frequently coincidental findings on CTA. Imaging for MAAs is important to define the location and number of aneurysms and evaluate disseminated disease, with CTA being the preferred imaging modality. Radiological characteristics are an irregular arterial wall, periaortic edema, and/or soft-tissue mass, with the latter seen in almost half of all cases [Citation1,Citation4]. The role of [18F]FDG-PET/CT is promising, but the evidence is limited to few case reports [Citation7,Citation8].
The suspicion should be even higher when treating patients with positive (blood) cultures for the most common micro-organisms to cause MAAs which include staphylococci, streptococci, and Salmonella species [Citation1,Citation9]. These pathogens also constituted for 70% in our series.
Therapeutic options for MAAs include intravenous antibiotics, endovascular repair ((T)EVAR), and open surgery. In Sweden, most patients with a thoracic MAA are treated with TEVAR, with survival rates of 92% and 71% at one month and five years follow-up, respectively [Citation10]. Significantly fewer perioperative complications and longer median survival were observed in the endovascular group versus open surgery and medical therapy alone [Citation11]. However, the endovascular approach was associated with a significantly higher recurrence of infection. Due to the embedded infection, more reinterventions were needed, and patients required prolonged or life-long antibiotic therapy, which resulted in higher total hospital costs [Citation12]. As such, the guidelines suggest using endovascular options or antibiotics as monotherapy, only as bridge to open surgery in hemodynamically unstable patients or patients for whom open surgery would be unsuitable [Citation13–15]. Open surgery not only enables wide debridement of necrotic tissue and resection of the infected native aorta, lowering the risk of reinfection, but also allows collection of tissue cultures leading to a clear microbiological diagnosis with targeted antibiotic therapy.
Follow-up was performed by imaging (CTA or [18F]FDG-PET/CT) and monitoring at the outpatient clinic of the infectious disease department. The duration of antibiotic therapy remains debated. A hazard ratio of 0.36 for late mortality was observed with six months of antibiotic therapy postoperatively (p = .005) [Citation16]. However, endovascularly treated patients benefit more from prolonged antibiotic therapy. International guidelines advise antibiotics for a minimum of 6 weeks up to 6 months [Citation14]. Some cases necessitate life-long antibiotics due to uncertainty over whether the infection is fully controlled or still residual. Patient centered care with customized treatment which is made in a multidisciplinary team including surgeons (vascular and thoracic) and infectious diseases specialists is needed. Additional surgery was performed in one patient with a new (mycotic) aneurysm, emphasizing close follow-up after surgical intervention [Citation13].
The difficult entity limited our study. In terms of literature, the little that is available is based on small case series and case reports, and these have tended to focus on symptoms, pathogens, and treatment.
Conclusions
When a coin-sized lesion in the inner aortic wall is seen during open surgical repair of an aortic aneurysm, the surgeon should consider an infectious cause.
Author contributions
Conceptualization: WM. Data collection: HN, TS and PL. Writing – original draft: HN and TS. Writing – reviewing: HN, TS, PL, IK, GG and WM. Visualization: HN, TS and PL. All authors have read, reviewed and agreed to the published version of the manuscript.
Ethical approval
The authors confirm that written consent for submission and publication of the cases has been obtained from the patients or their next of kin. The ethical board of the Radboudumc approved anonymous publication of these cases of deceased patients where next of kin could not be reached by any means.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Not applicable.
Additional information
Funding
References
- Lee W-K, Mossop PJ, Little AF, et al. Infected (mycotic) aneurysms: spectrum of imaging appearances and management. Radiographics. 2008;28(7):1853–1868. doi: 10.1148/rg.287085054.
- Sörelius K, di Summa PG. On the diagnosis of mycotic aortic aneurysms. Clin Med Insights Cardiol. 2018;12:1179546818759678. doi: 10.1177/1179546818759678.
- Osler W. The Gulstonian lectures, on malignant endocarditis. Br Med J. 1885;1(1262):467–470. doi: 10.1136/bmj.1.1262.467.
- Macedo TA, Stanson AW, Oderich GS, et al. Infected aortic aneurysms: imaging findings. Radiology. 2004;231(1):250–257. doi: 10.1148/radiol.2311021700.
- Inam H, Zahid I, Khan SD, et al. Hemoptysis secondary to rupture of infected aortic aneurysm – a case report. J Cardiothorac Surg. 2019;14(1):144. doi: 10.1186/s13019-019-0959-y.
- Chen IM, Chang HH, Hsu CP, et al. Ten-year experience with surgical repair of mycotic aortic aneurysms. J Chin Med Assoc. 2005;68(6):265–271. doi: 10.1016/S1726-4901(09)70148-0.
- Husmann L, Huellner MW, Gruenig H, et al. Impact of PET/CT among patients with suspected mycotic aortic aneurysms. PLOS One. 2021;16(10):e0258702. doi: 10.1371/journal.pone.0258702.
- Husmann L, Huellner MW, Ledergerber B, et al. Diagnostic accuracy of PET/CT and contrast enhanced CT in patients with suspected infected aortic aneurysms. Eur J Vasc Endovasc Surg. 2020;59(6):972–981. doi: 10.1016/j.ejvs.2020.01.032.
- Hsu RB, Chen RJ, Wang SS, et al. Infected aortic aneurysms: clinical outcome and risk factor analysis. J Vasc Surg. 2004;40(1):30–35. doi: 10.1016/j.jvs.2004.03.020.
- Sörelius K, Wanhainen A, Wahlgren C-M, et al. Nationwide study on treatment of mycotic thoracic aortic aneurysms. Eur J Vasc Endovasc Surg. 2019;57(2):239–246. doi: 10.1016/j.ejvs.2018.08.052.
- Cullen JM, Booth AT, Mehaffey JH, et al. Clinical characteristics and longitudinal outcomes of primary mycotic aortic aneurysms. Angiology. 2019;70(10):947–951. doi: 10.1177/0003319719858784.
- Luo Y, Zhu J, Dai X, et al. Endovascular treatment of primary mycotic aortic aneurysms: a 7-year single-center experience. J Int Med Res. 2018;46(9):3903–3909. doi: 10.1177/0300060518781651.
- Wanhainen A, Verzini F, Van Herzeele I, et al. European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8–93. doi: 10.1016/j.ejvs.2018.09.020.
- Wilson WR, Bower TC, Creager MA, et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation. 2016;134(20):e412–e460. doi: 10.1161/CIR.0000000000000457.
- Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg. 2007;46(5):906–912. doi: 10.1016/j.jvs.2007.07.025.
- Sörelius K, Wanhainen A, Furebring M, et al. Nationwide study of the treatment of mycotic abdominal aortic aneurysms comparing open and endovascular repair. Circulation. 2016;134(23):1822–1832. doi: 10.1161/CIRCULATIONAHA.116.024021.

