Abstract
Objectives: Several biological disease–modifying antirheumatic drugs (bDMARDs) are currently available for the treatment of rheumatoid arthritis (RA). Increasing evidence indicates that second-line bDMARDs are effective for inadequate responders to first-line bDMARDs. However, all previous studies investigated the use of tumor necrosis factor inhibitors (TNFi) as a first-line bDMARD, while investigated the efficacy of second-line bDMARDs after the use of tocilizumab (TCZ), a non-TNFi, as a first-line bDMARD. Thus, we investigated the efficacy of golimumab (GLM) as a second-line bDMARD after treatment with TCZ as a first-line bDMARD.
Methods: The final study population consisted of 26 patients (inadequate responders to TCZ; TCZ group) with moderate or high disease activity (DAS28-ESR ≥3.2) at week 24 of treatment with TCZ as a first-line bDMARD or whose DAS28-ESR score worsened after starting TCZ treatment. These patients could be followed for another 52 weeks or more after the subsequent switch to GLM treatment. For comparison, 19 patients treated with TNFi as a first-line bDMARD and inadequate response to TNFi (TNFi group) were included.
Results: The DAS28-ESR score at week 52 after the start of treatment with GLM improved significantly compared with baseline in the TCZ and TNFi groups. However, the TCZ group showed significantly better improvement. Patients in both groups had significantly improved treatment outcomes according to European League Against Rheumatism response criteria, but there was no statistically significant difference among them. The retention rate at week 52 after the start of treatment with GLM was significantly higher in the TCZ group than in the TNFi group (81% vs. 68%, respectively). In addition, no difference was found in the progression of bone destruction determined by the change in van der Heijde modified total Sharp scoring system scores between groups.
Conclusions: GLM was an effective therapeutic option for inadequate responders to TCZ.
Introduction
Biological disease–modifying antirheumatic drugs (bDMARDs) have been used to treat rheumatoid arthritis (RA) for 10 years or more, and various effects, such as clinical remission, preventing articular destruction, and improved quality of life, have been obtained that could not be achieved by conventional synthetic DMARDs (csDMARDs). The several currently available bDMARDs can be roughly classified into tumor necrosis factor inhibitors (TNFis) and non-TNFis according to target molecule. The latter includes tocilizumab (TCZ), an inhibitor of interlukin-6 (IL-6i), abatacept (ABT), a bDMARD-targeting CD80/CD86 on T cells, and rituximab (RTX), a bDMARD-targeting CD20 on B cells. However, treatment can be initiated using any of the bDMARDs [Citation1,Citation2], although some RA treatment guidelines partially limit the use of RTX as a first-line bDMARD [Citation1]. Previous studies have reported the effect of non-TNFis in patients with an inadequate response (IR) to a TNFi as a first-line bDMARD [Citation3–5], because, historically, the development of bDMARDs started from TNFis, while non-TNFis were developed later. It has become increasingly apparent that switching from a TNFi as a first-line bDMARD to a non-TNFi with a different mechanism of action from that of TNFis has superior efficacy to switching from one TNFi to another [Citation3,Citation4]. TCZ, an IL-6i, has proven efficancy for patients with an IR to a TNFi [Citation6,Citation7].
On the other hand, TCZ is a very useful first-line bDMARD [Citation8–10]. Patients with high serum platelet counts (≥400,000/μL) produced by bone marrow megakaryocytes upon IL-6 stimulation were recently reported to have responded well to TC [Citation11], suggesting that it could be used as a first-line bDMARD in patients with a high platelet count in the future. However, regarding the efficacy of a second-line bDMARD with a different mechanism of action from that of the failed first-line bDMARD, the case has been demonstrated for switching from a TNFi used as a first-line bDMARD [Citation3,Citation4] as mentioned earlier, but the use of TNFi as a second-line bDMARD after an IR to IL-6i as a first-line bDMARD has not yet been proven. Because TCZ is more potent than TNFis [Citation12], physicians who are unaware of the measures to be taken when patients show an IR to TCZ would hesitate to select TCZ as a first-line bDMARD. Golimumab (GLM) is a bDMARD (TNFi), the efficacy of which as a second-line bDMARD has been demonstrated in patients with an IR compared to another TNFi [Citation13], the dose of which was increased during a study. Thus, we conducted an efficacy analysis in patients with an IR to TCZ who were prescribed GLM as a second-line bDMARD.
Materials and methods
Patients
The study population consisted of patients with RA who met the 1987 American College of Rheumatology (ACR) criteria [Citation14] and were presented to our clinic. Of them, patients who showed an IR to TCZ after at least 24 weeks of treatment (intravenous administration 10 cases and subcutaneous administration 16 cases) and could be followed for another 52 or more weeks after the subsequent switch to GLM treatment were enrolled. An IR to TCZ was defined as a disease activity score in 28 joints based on erythrocyte sedimentation rate (DAS28-ESR ≥3.2; i.e. moderate disease activity [MDA] or high disease activity [HAD]) [Citation15] that worsened after starting treatment with TCZ. As a concomitant oral drug, methotrexate (MTX) was used at doses of 6–12 mg/week for 24 weeks or more before a switch was made to GLM as a csDMARD in 22 patients. GLM was switched without washout interval. The MTX treatment was continued and the dose was not changed after the switch to GLM. We also included patients who were using another csDMARD, prednisolone (PSL), or a non-steroidal anti-inflammatory drug (NSAID) as concomitant drugs for at least 24 weeks before starting GLM. Among them, as a csDMARD, sulfasalazine (SASP) was used in eight patients and bucindolol (BUC) was used in five. PSL was used in four patients at doses of 1–5 mg/day, and an NSAID was used in 10 patients.
All of these concomitant drugs used before GLM treatment were also continued without a dose change. In addition, if GLM was ineffective during the study, a reassessment based on the DAS28-ESR score was performed 24 weeks after the switch to GLM, and the GLM dose was increased from 50 to 100 mg/month for patients with a DAS28-ESR score ≥3.2. If an IR to GLM or adverse reactions were still observed after the dose increase, treatment could be withdrawn at any time. The control group comprised patients with inadequate treatment outcomes after TNFi treatment for at least 24 weeks as a first-line bDMARD during the same period (DAS28-ESR score ≥3.2) who received GLM as a second-line bDMARD. Concomitant drugs used at constant doses for at least 24 weeks before the start of GLM treatment were MTX at doses of 6–12 mg/week in 26 patients, SASP in eight patients, BUC in two patients, PSL in six patients, and NSAIDs in 11 patients (including overlapping patients). All of these concomitant drugs were continued without a dose change as in the patients switched from TCZ to GLM.
Patients were eligible for the study if they met the condition of changing to a second-line bDMARD and enrolled in the study once they provided written informed consent. This study was conducted according to Declaration of Helsinki principles.
Procedure
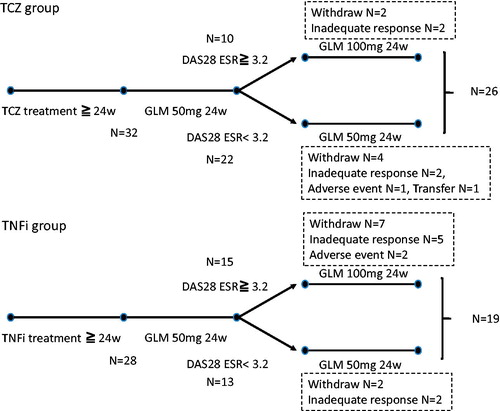
Analyses were performed separately for the TCZ and TNFi groups, which were distinguished from each other by the first-line bDMARD used before the change to GLM. An assessment was performed 24 weeks after the switch to GLM. For patients who were considered poor responders to GLM (DAS28-ESR score ≥3.2), the dose of MTX, a concomitant drug, was maintained, while the dose of GLM was increased from 50–100 mg/month. The TNF is used in the TNFi group before GLM treatment included etanercept (ETN) in 19 patients, adalimumab in seven, and infliximab in two. Because a number of patients in each group withdrew from the study (), 26 patients in the TCZ group and 19 patients in the TNFi group were ultimately included in the analysis. The details of GLM withdrawal is as follows: Inadequate response 4 case, adverse event 1 + case, hospital transfer 1 case. TNFi group is as follows: Inadequate response 7 case, adverse event 2 cases.
Figure 1. Treatment protocol. An assessment of disease activity score in 28 joints based on erythrocyte sedimentation rate performed at week 24 of golimumab (GLM) treatment. If the patient had moderate or high disease activity, the GLM dose was increased to 100 mg/month. If the patient had LDA or achieved remission, treatment with GLM 50 mg/month was continued.
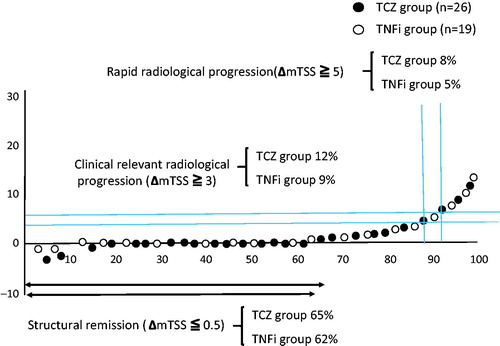
Clinical assessments were made based on DAS28-ESR score and European League Against Rheumatism (EULAR) response criteria [Citation15]. In addition, matrix metalloproteinase-3 (MMP-3) levels were measured monthly. Radiographs of the hands and feet were obtained at baseline and at week 52. Each radiograph was assessed using the van der Heijde modified total Sharp scoring system (mTSS). X-ray assessment was performed by two readers under double-blind conditions. To compare the radiological progression of articular destruction, the radiographic data were analyzed using a cumulative probability plot, and the percentages of patients who had structural remission (ΔmTSS ≤0.5), clinically relevant radiological progression (ΔmTSS ≥3) [Citation9], or rapid radiological progression (ΔmTSS ≥5) [Citation16] were examined for each group.
The bDMARD continuation in the two groups was estimated using the Kaplan–Meier method and compared using the log-rank test (Mantel–Cox test). The last observation carried forward method was used in each analysis. The results are expressed as mean ± standard deviation (SD) or percentage. All of the analysis proportions were analyzed for treatment differences with the χ2 test and continuous variables were compared using Student’s t-test. Statistically significant differences were noted at values of p < 0.05.
Results
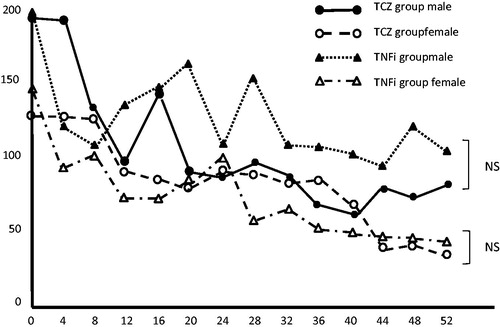
There were no statistically or clinically significant differences between the TCZ and TNFi groups in any baseline characteristic except for sex and ESR (). The female ratio at baseline is different between TCZ and TNFi groups. However, the gender ratio at baseline does not impact on our main results. The proportion of patients achieving DAS28-defined remission was 42.0% of TCZ group patients at week 52. We confirmed that there is no statistical significant difference in the proportion patients achieving DAS 28-defined remission between male and female (mean male 40.0% vs. mean female 43.8% at week 52). shows the percentages of patients who achieved remission (DAS28 ≤ 2.6) or had low disease activity (LDA; 2.6 < DAS28 < 3.2), MDA (3.2 ≤ DAS28 ≤ 5.1), or HDA (DAS28 > 5.1) as determined by DAS28-ESR score at weeks 24 and 52 of GLM treatment for the two groups. Both groups showed statistically significant improvement at week 24 of GLM treatment. This improvement tendency was remarkable from week 52 onward. Treatment efficacy against disease activity at week 52 of GLM treatment was significantly higher in the TCZ group than in the TNFi group.
Figure 2. Changes in disease activity as determined by the disease activity score in 28 joints based on erythrocyte sedimentation rate. The tocilizumab (TCZ) and tumor necrosis factor inhibitor (TNFi) groups showed significant improvement at week 24 after the start of treatment with golimumab (GLM). The TCZ group showed significantly better improvement at week 52 of GLM treatment. *p < 0.05; NS, not statistically significant.
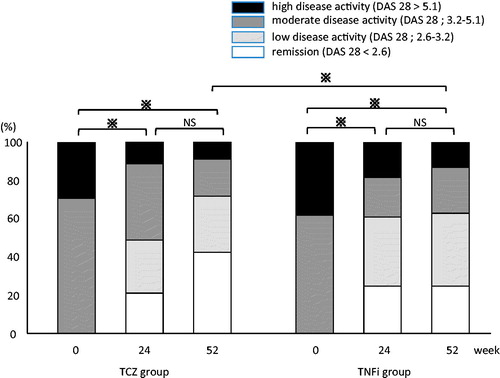
Table 1. Baseline characteristics of patients with RA who switched from TNFi or TCZ.
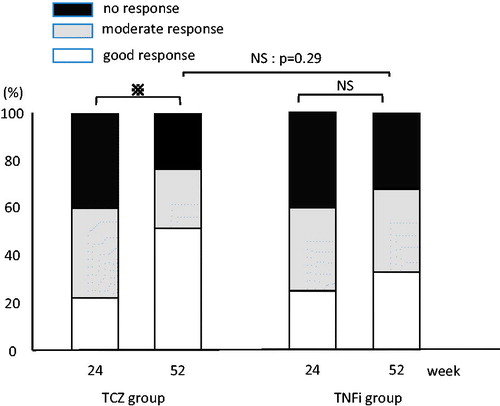
The percentage of patients with “no response” on EULAR response criteria was lower at week 52 than week 24 for the TCZ and TNFi groups. This decrease in percentage was significant for both groups, although the intergroup difference was not statistically significant ().
Figure 3. European League Against Rheumatism response criteria. Both groups showed favorable improvement rates, but no significant difference was found in improvement rate at week 52. p < 0.05; NS, not statistically significant.
Serum MMP-3 levels decreased over time after GLM treatment in the TCZ and TNFi groups (), although the intergroup difference was no statistically significant (p = 0.184).
Figure 4. Changes in the mean serum matrix metalloproteinase-3 (MMP-3) levels after the start of golimumab (GLM) treatment; MMP-3 levels decreased over time after the start of GLM treatment in the tocilizumab (TCZ) and tumor necrosis factor inhibitor (TNFi) groups, but no significant intergroup difference was noted in the MMP-3 levels at week 52. Because the reference value of MMP-3 varies by sex, the mean serum MMP-3 levels of each group were graphed separately for men and women and statistically processed. NS, not statistically significant.
There was no statistically significant difference in ΔmTSS (baseline mTSS divided by disease duration) between TCZ and TNFi groups at baseline. (mean ΔmTSS TCZ group 5.2 vs. mean ΔmTSS TNFi group 5.6, p = 0.51). The mTSS scores at week 0 and 52 were similar in the TCZ and TNFi groups (). After correction by linear extrapolation, the proportion of patients in radiographic remission (ΔmTSS ≤0.5) was 65% in the TCZ group and 62% in the TNFi group, and there was no significant difference in the median change between the two groups. Clinically relevant radiographic progression (CRRP), defined as a change in mTSS ≥3 from baseline, was 12% in the TCZ group and 9% in the TNFi group. The percentage of CRRP was not significantly different between the two groups. The percentage of rapid radiographic progression (ΔmTSS ≥5) was also not significantly different between the two groups (8% vs. 5%, respectively).
Figure 5. Cumulative probability plot of change in the van der Heijde modified total Sharp scoring system score (mTSS) from baseline to week 52. There was no significant difference in median change between the tocilizumab (TCZ) and tumor necrosis factor inhibitor (TNFi) groups.
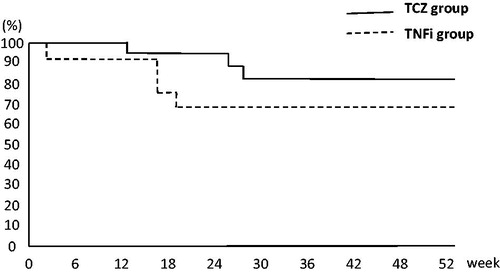
demonstrates the Kaplan–Meier curves of the retention rates among patients with RA over 52 weeks of GLM treatment. The probability of treatment retention of the TCZ group was significantly higher than that of the TNFi group (p = 0.03).
Figure 6. Kaplan–Meier curves of retention rates among patients with rheumatoid arthritis over 52 weeks of golimumab (GLM) treatment. The probability of treatment retention in the tocilizumab (TCZ) group was significantly higher than that in the tumor necrosis factor inhibitor (TNFi) group (p = 0.03).
Discussion
The bDMARD effect is known to be reduced because of the occurrence of antidrug antibodies, the development of antibodies against treatment drugs associated with long-term use [Citation17]. The investigations of the selection and effect of second-line bDMARDs have been conducted in only patients with an IR to a TNFi as a first-line bDMARD [Citation3–7,Citation13,Citation18–20]. This is the first report showing the effect of GLM in patients who received TCZ. This result may instill the confidence in the use of TCZ as a first bDMARD to physicians. Because RA treatment recommendations and guidelines do not clearly state which bDMARD should be selected as a first-line bDMARD [Citation1,Citation2], non-TNF are also likely to be selected as first-line bDMARDs for some patients in the future.
For example, the ACR guidelines recommend the use of ABT for the treatment of patients at a high risk of developing infections despite admitting to a low evidence level. In addition, the efficacy of TCZ in the treatment of RA is widely known [Citation8–10], and some reports show that its effect is superior to that of TNFis [Citation12,Citation18]. It has been clarified that platelet count is a useful indicator to determine whether TCZ should be selected as a first-line bDMARD [Citation11]; therefore, TCZ could be selected in the future as a first-line bDMARD in patients with high pretreatment platelet counts.
GLM was selected as a second-line bDMARD for the following two reasons: first, GLM is an effective second-line bDMARD in patients with an IR to a TNFi [Citation13]; and second, GLM is a bDMARD, the dose of which can be increased during treatment if ineffective. In our study, because of an IR to GLM, the GLM dose was increased from 50 to 100 mg/month in 32% (10/32) of patients who were switched from TCZ to GLM.
Interestingly, the disease activity as determined by DAS28-ESR score decreased over time from HDA or MDA to MDA, LDA, or remission in both groups. However, the improvement was significantly higher in the TCZ group than in the TNFi group (). This result seems to be a mirror image of the results of previous reports [Citation3,Citation4] showing that a switch from a TNFi that failed as a first-line bDMARD to a non-TNFi with a different mechanism of action is more effective. In other words, this result may indicate the effect of switching from TCZ that failed as a first-line bDMARD to GLM, a TNFi with a different mechanism of action. In addition to these results, our investigation demonstrated that the retention rate is significantly higher in the TCZ group than in the TNFi group (81% vs. 68%, respectively; p = 0.03 at week 52).
Overall, our investigation demonstrated that GLM is a bDMARD that can be reliably effective in patients with an IR to TCZ and that the next treatment is secure if patients have an IR to TCZ used as a first-line bDMARD.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509.
- Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26.
- Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology (Oxford). 2014;53:1664–8.
- Kim HL, Lee MY, Park SY, Park SK, Byun JH, Kwon S, et al. Comparative effectiveness of cycling of tumor necrosis factor-α (TNF-α) inhibitors versus switching to non-TNF biologics in rheumatoid arthritis patients with inadequate response to TNF-α inhibitor using a Bayesian approach. Arch Pharm Res. 2014;37:662–70.
- Hirabara S, Takahashi N, Fukaya N, Miyake H, Yabe Y, Kaneka A, et al. Clinical efficacy of abatacept, tocilizumab, and etanercept in Japanese rheumatoid arthritis patients with inadequate response to anti-TNF monoclonal antibodies. Clin Rheumatol. 2014;33:1247–54.
- Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–23.
- Tanaka Y, Takeuchi T, Amano K, Saito K, Hanami K, Nawata M, et al. Effect of interleukin-6 receptor inhibitor, tocilizumab, in preventing joint destruction in patients with rheumatoid arthritis showing inadequate response to TNF inhibitors. Mod Rheumatol. 2014;24:399–404.
- Nishimoto N, Miyasaka N, Yamamoto K. Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–19.
- Kaneko Y, Atsumi T, Tanaka Y, Inoo M, Kobayashi-Haraoka H, Amano K, et al. Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study). Ann Rheum Dis. 2016;208426. doi: 10.1136/annrheumdis-2015-208426.
- Ogata A, Amano K, Dobashi H, Inoo M, Ishii T, Kasama T, et al. Long-term safety and efficacy of subcutaneous tocilizumab monotherapy: results from the 2-year open-label extension of the MUSASHI study. J Rheumatol. 2015;42:799–809.
- Matsuno H. Remarkable efficacy of tocilizumab for treating rheumatoid arthritis in patients with high platelet counts. Mod Rheumatol. 2015;25:38–42.
- Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–50.
- Smolen JS, Kay J, Matteson EL, Landewé R, Hsia EC, Xu S, et al. Insights into the efficacy of golimumab plus methotrexate in patients with active rheumatoid arthritis who discontinued prior anti-tumor necrosis factor therapy: post hoc analyses from the GO-AFTER study. Ann Rheum Dis. 2014;73:1811–18.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
- Fransen J, van Riel PL. The disease activity score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35:745–57.
- Vastesaeger N, Xu S, Aletaha D, St Clair EW, Smolen JS. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology (Oxford). 2009;48:1114–21.
- Thomas SS, Borazan N, Barroso N, Duan L, Taroumian S, Kretzmann B, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis. BioDrugs. 2015;29:241–58.
- Romão VC, Santos MJ, Polido-Pereira J, Duarte C, Nero P, Miguel C, et al. Comparative effectiveness of tocilizumab and TNF inhibitors in rheumatoid arthritis patients: data from the rheumatic diseases Portuguese register, Reuma.pt. Biomed Res Int. 2015;279890. doi: 10.1155/2015/279890.
- Chatzidionysiou K, Askling J, Eriksson J, Kristensen LE. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890–6.
- Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303–8.
