Abstract
Objectives: To determine the safety and effectiveness of and identify associated factors in long-term adalimumab (ADA) treatment of Japanese patients with rheumatoid arthritis (RA).
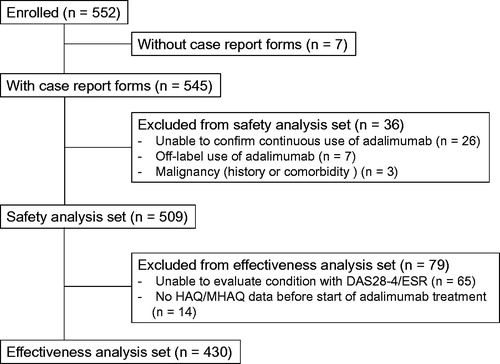
Methods: Of 7740 patients participating in the all-case postmarketing surveillance study, 552 were enrolled in the present study and observed for 3 years. The safety and effectiveness of ADA were analyzed in 509 and 430 patients, respectively.
Results: Adverse drug reactions (ADRs) were reported in 34.2% of patients (23.3/100 person-years [PYs]); serious ADRs (SADRs) were reported in 10.6% (5.9/100 PYs). The most common ADRs and SADRs were infection (16.5%) and serious infection (6.1%), respectively. Seven patients (1.4%) developed malignancies. Multivariate analysis revealed that the risk factors for SADRs were age ≥65 years and respiratory disorder at baseline. The proportion of patients who achieved remission (28-joint count Disease Activity Score based on four erythrocyte sedimentation rates <2.6) increased from 3.3% at baseline to 49.2% at 36 months. Significant predictors of failure to achieve remission were female sex, age ≥65 years, blood disorders and advanced structural change at baseline.
Conclusions: Overall, no unknown safety issues were noted during the 3-year treatment with ADA in Japanese patients with RA.
Introduction
Biological disease-modifying antirheumatic drugs (bDMARDs), namely tumor necrosis factor (TNF) inhibitor, interleukin-6 (IL-6) receptor inhibitor and T-cell costimulatory signal inhibitor, were developed for the treatment of rheumatoid arthritis (RA) and other systemic autoimmune diseases. Seven bDMARDs are currently approved in Japan for the treatment of RA and these drugs have different characteristics [Citation1]. The results of postmarketing surveillance (PMS) that examined the safety and effectiveness of infliximab [Citation2], etanercept [Citation3–5], adalimumab (ADA) [Citation6], tocilizumab (TCZ) [Citation7,Citation8] and abatacept [Citation9] have been reported.
The Japanese College of Rheumatology recommends the use of bDMARDs for patients with an inadequate response to methotrexate (MTX) or other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which is an adaptation of the European League Against Rheumatism (EULAR) recommendations with some modifications [Citation10]. TNF inhibitors are bDMARDs that suppress the response to the pro-inflammatory cytokine TNF, which mediates inflammatory synovitis, osteoclastogenesis and degradation of the articular matrix in patients with RA [Citation11]. These drugs not only improve the signs and symptoms of RA and physical function but also strongly inhibit the progression of joint destruction in these patients.
ADA has been approved in Japan for the treatment of patients with RA since 2008. ADA is a fully human anti-TNF monoclonal antibody that reduces the inflammatory response in patients with RA by binding specifically to TNF, thereby blocking its interaction with TNF receptors on the surfaces of cells. A Cochrane systematic review of data from >2300 patients with RA enrolled in six clinical trials has shown that ADA slows the progression of structural joint damage and had a consistent safety profile [Citation12]. The ARMADA study has shown the safety and effectiveness of ADA with the concomitant use of MTX [Citation13,Citation14]. In the PREMIER trial [Citation15,Citation16] and the HOPEFUL-1 study [Citation17], results indicated that the concomitant use of ADA and MTX was more beneficial than MTX monotherapy in patients with early RA (<2 or 3 years since diagnosis).
We previously reported the safety and effectiveness of ADA in the all-case PMS of 7740 Japanese patients with RA followed for up to 28 weeks. This study indicated that ADA, especially with the concomitant use of MTX, suppressed disease activity with no unexpected adverse drug reactions (ADRs) [Citation6]. In the present study, designed as follow-up to the aforementioned all-case PMS and mandated by the Japanese Ministry of Health, Labour and Welfare, we intended to determine the long-term (up to 3 years) incidence of ADRs, including infections, serious infections, and malignancies, in the clinical setting, and to identify factors associated with the safety and effectiveness of ADA.
Methods
Study design
In this multicenter, single-cohort, observational study, patients were followed for 3 years after the start of treatment to assess the long-term safety and effectiveness of ADA in Japanese patients with RA. The primary endpoint was the occurrence of ADRs, including malignancy and infection. Risk factors for the development of ADRs, serious ADRs (SADRs) and serious infections during long-term treatment of RA with ADA, as well as predictive factors for remission using the 28-joint count Disease Activity Score based on 4 erythrocyte sedimentation rates (DAS28-4/ESR), were secondary endpoints. All ADRs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 18.0. We evaluated effectiveness using the modified Health Assessment Questionnaire (MHAQ).
The study protocol was submitted to and approved by the Pharmaceuticals and Medical Devices Agency in Japan before the initiation of the all-case PMS. This study was conducted in registered medical institutions in compliance with Good Post-marketing Study Practice in Japan, and is registered at the ClinicalTrials.gov registry (NCT01163318).
Patients
The target number of patients for registration was set at 600 (the rationale for the determination of the target sample size is described in the Statistical Analysis section). From November 2009 to December 2010, we enrolled the first 552 patients who completed the previous all-case PMS for 6 months and met the following conditions: (1) completion of the Health Assessment Questionnaire (HAQ) or MHAQ at baseline of the all-case PMS; (2) had been prescribed ADA in accordance with the Japanese package inserts for the treatment of RA (40 mg administered subcutaneously every other week, increased to 80 mg in case of insufficient efficacy) at the start of the present long-term study; (3) were using ADA continuously for 6 months during the all-case PMS; (4) were free of malignancy or history of malignancy at baseline of the all-case PMS; and (5) had evaluable disease activity as assessed using the DAS28. The exclusion criteria were contraindications to the use of ADA [Citation18], including serious infection, tuberculosis, history of hypersensitivity to any ingredient of ADA, demyelinating disease or any history of demyelinating disease, and congestive heart failure.
Data were available for 545 patients. Of these patients, 36 were excluded since they did not meet the inclusion criteria, including seven patients who had off-label use of ADA, 26 who did not receive continuous ADA administration, and three who had a medical history or comorbidity of malignancy. Consequently, 509 patients were included in the safety analysis set. Of these patients, 430 were included in the effectiveness analysis set; 65 patients who could not be assessed for DAS28-4/ESR and 14 patients who did not have HAQ/MHAQ scores prior to the initiation of ADA treatment were excluded from the analysis ().
Collection of data
Information on safety and effectiveness was collected every 6 months using an electronic data capture system or paper case report forms. The data obtained during the 3 years after the initiation of ADA treatment, including the first 6-month period of the all-case PMS, were analyzed. Whenever ADA treatment was discontinued during the observation period, the investigator filled out a questionnaire form once every 6 months until 3 years after the initiation of ADA therapy to report the presence/absence of malignancy, tuberculosis and death. For these three adverse events, observation was stopped after 3 years of treatment with ADA, or on the date a patient died, was lost to follow-up or met the exclusion criteria, whichever came first. For other safety and effectiveness data, observation was stopped at the discontinuation of ADA, after 3 years of treatment with ADA, or on the date a patient died, was lost to follow-up, or met the exclusion criteria, whichever came first.
Statistical analysis
Based on the 1% incidence of malignancy in patients enrolled in preapproval clinical studies in Japan, a target sample size of 300 patients was set to give a 95% probability of detecting at least 1 patient with malignancy. The target number of patients for registration was set at 600 to compensate for the number of patients who were expected to discontinue treatment or withdraw consent during the long-term observation period of this study.
Continuous variables are presented as means ± standard deviation (SD). Categorical variables are presented as percentages. A logistic regression model with a stepwise selection method was applied to identify factors potentially influencing the development of ADRs, SADRs and serious infections and the achievement of DAS28-4/ESR remission. Candidate variables for the logistic regression analyses were selected using univariate analyses from variables at baseline as follows: age (≥15 to <65, ≥65), sex (female, male), weight (<30 kg, ≥30 kg to <40 kg, ≥40 kg to <50 kg, ≥50 kg to <60 kg, ≥60 kg), duration of RA (<10 years, ≥10 years), history of allergy (yes, no, unknown), coexisting hepatic disorder (yes, no), renal disorder (yes, no), cardiovascular disorder (yes, no), respiratory disease (yes, no) or blood disorder (yes, no), previous/coexisting diabetes mellitus (yes, no), previous tuberculosis (yes, no), previous bacterial bronchitis (yes, no), previous interstitial pneumonia (yes, no), previous obstructive pulmonary disease (yes, no), previous pancytopenia (yes, no), smoking history (yes, no, unknown), concomitant MTX use (yes, no), concomitant glucocorticoid use (yes, no), prior bDMARDs therapy (yes, no), prior DMARD use (yes, no), prior glucocorticoid use (yes, no), Steinbrocker RA stage (I and II, III and IV), Steinbrocker functional class (1 and 2, 3 and 4), leukocyte count (<4000/μL, ≥4000/μL), lymphocyte count (<1000/μL, ≥1000/μL), immunoglobulin (<870 mg/dL, ≥870 mg/dL) and DAS28-4/ESR (≤5.1, >5.1).
The Kaplan–Meier method was used to estimate the treatment persistence rate throughout the observation period. The standardized mortality rate (SMR) was calculated as the ratio of observed deaths to expected deaths using data from the Ministry of Health, Labour and Welfare in 2008 as a reference. Statistical tests were two-tailed, with a significance level of p < .05. DAS28-4/ESR and MHAQ missing data were not imputed for each variable, and were calculated as observed. Analyses were carried out employing SAS version 9.1.3 or later versions (SAS Institute Inc., Cary, NC). No adjustment was made for multiple centers because of the small number of patients per center.
Results
Baseline patient characteristics and treatment persistence rate
summarizes the baseline characteristics of the safety population. The baseline characteristics of the patients in this study were similar to those of the all-case PMS [Citation6]. Of the 509 patients, 417 (81.9%) were female, the mean age of patients was 59.5 years, and the duration of RA was 10.4 years. Comorbidities were recorded for 327 patients (64.2%), including cardiovascular disease (23.0%), respiratory disorder (13.6%), renal disorder, as determined by the physician's report and/or when eGFR < 60 mL/min/1.73 m2 (12.6%), hepatic disorder (7.3%) and blood disorder (7.1%). More than half of the patients had relatively advanced disease (Steinbrocker’s stage III and IV: 26.9% and 35.0%, respectively), and almost a quarter had substantially impaired functional status (Steinbrocker’s class 3 and 4: 23.6% and 1.4%, respectively). Previous use of bDMARDs was noted in 132 patients (25.9%), which was somewhat less than that in the all-case PMS (42.1%) [Citation6]. csDMARDs were the most frequently used prior medication (97.2%), followed by glucocorticoids (57.8%).
Table 1. Baseline patient characteristics (n = 509).
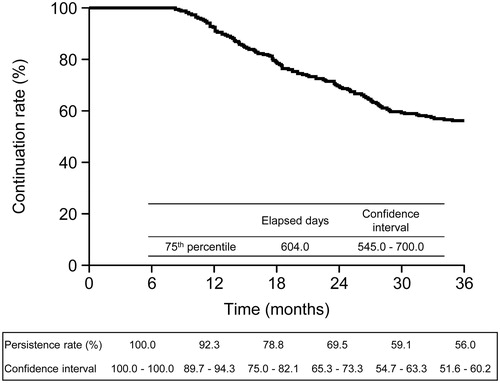
Of the 509 patients enrolled, 92.3% (95% confidence interval [CI], 89.7–94.3), 69.5% (95% CI, 65.3–73.3), and 56.0% (95% CI, 51.6–60.2) of patients continued treatment with ADA at 12, 24, and 36 months, respectively (). At the 75th percentile, 604.0 days (545.0–700.0) had elapsed. A total of 224 patients (44.0%; 224/509) discontinued treatment. Reasons included insufficient effectiveness (14.9%; 76/509), ADRs (8.1%; 41/509), loss to follow-up (7.7%; 39/509), patient decision to stop ADA (3.3%; 17/509), other reasons (8.4%; 43/509), and unknown reasons (1.6%; 8/509). Concomitant use of MTX was not associated with the treatment continuation rate of ADA in the present study (Supplementary Figure 1).
Safety
During the 3-year observation period of the present study, 292 ADRs were reported in 174 patients (34.2%, 95% CI 30.07–38.49%; 174/509) whose data were included in the safety analysis set. Total patient exposure time was 1251.3 patient-years (PYs), such that there were 23.3 ADRs per 100 PYs. The most common ADRs were in the ‘infections and infestations’ class: 114 events (incidence rate, 6.7/100 PYs) in 84 patients (16.5%; 84/509). In total, 74 serious ADRs (SADRs; incidence rate, 5.9/100 PYs) were reported in 54 patients (10.6%, 95% CI 8.1–13.6%; 54/509). Most of these were in the ‘infections and infestations’ class: 37 events (incidence rate, 2.5/100 PYs) in 31 patients (6.1%; 31/509; ). Unlike all-case PMS, no tuberculosis or Pneumocystis pneumonia cases were reported. The incidence of ADRs and SADRs classified by MedDRA system organ class is shown in Supplementary Table 1. The incidence rates of ADRs and infection decreased over time, peaking during the initial 6-month period. The incidence rates of SADRs, which had risen up modestly to 18 months, had stabilized by the end of study. The incidence of serious infections had risen up slightly to 12 months and then stabilized thereafter (, respectively).
Figure 3. Incidence rates of ADRs, SADRs, infection and serious infection over time. (a) ADR and SADR. (b) Infection and serious infection. The incidence rate was calculated by dividing the number of patients who developed ADRs, SADRs, infection and serious infection during each period by the number of patients who were followed at the beginning of each period. ADR: adverse drug reaction; SADR: serious adverse drug reaction.
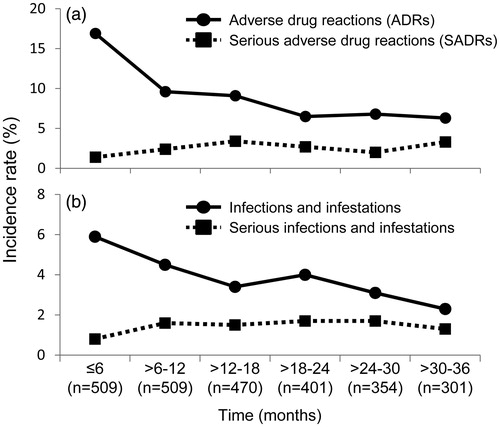
Table 2. Incidences of adverse drug reactions and serious adverse drug reactions in the safety analysis set (n = 509).
Eight malignancies (incidence rate: 0.64/100 PYs; 1.4%, 7/509) were diagnosed in seven patients; one patient had metastatic lung cancer and malignant lung neoplasm, and two patients had colon cancer. The other patients had gastric cancer, malignant lung neoplasm, prostate cancer and neuroendocrine carcinoma of the skin (). In six of the seven patients, a causal relationship between these malignancies and ADA could not be ruled out (1.2%; 6/509; ). One patient was diagnosed with metastatic lung cancer and malignant lung neoplasm >1 year after treatment discontinuation (#4 in ).
Table 3. Malignancies reported in this study.
Eight patients died during the treatment period (1251.30 PYs). As some patients were followed after the discontinuation of treatment, a total of 11 deaths occurred during the 3-year observation period. The SMR was 0.88 (95% CI, 0.44–1.56). The causes of death in five of these patients were pneumonia, peritonitis, infection from medical devices (1 patient each), and interstitial lung disease (2 patients). A causal relationship with ADA could not be ruled out in any of these cases.
The following risk factors for ADRs were identified by the stepwise multiple logistic regression analysis: hepatic disorder (odds ratio [OR], 2.12; 95% CI, 1.04–4.33), respiratory disorder (OR, 2.01; 95% CI, 1.15–3.51) and poorer functional classes at baseline (Steinbrocker’s class 3 or 4; OR, 1.71; 95% CI, 1.09–2.70). Risk factors for SADRs were age ≥65 years (OR, 4.05; 95% CI, 1.95–8.43) and respiratory disorder (OR, 2.50; 95% CI, 1.20–5.20; ). Factors associated with serious infection were age ≥65 years (OR, 10.20; 95% CI, 1.28–82.75) and respiratory disorder (OR, 4.82; 95% CI, 1.36–17.09).
Table 4. Factors associated with development of ADRs or SADRs.
Effectiveness
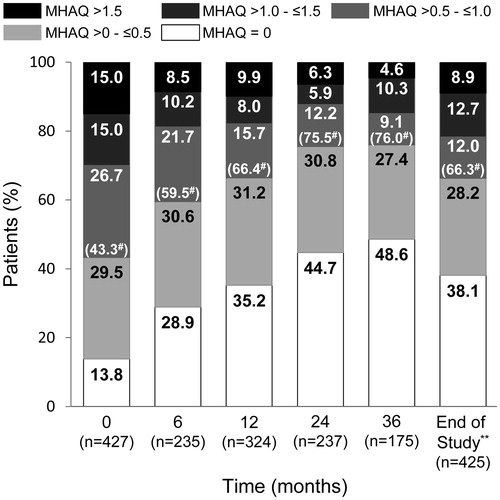
The proportion of patients who achieved remission (DAS28-4/ESR <2.6) increased over time from 3.3% at baseline to 49.2% at 36 months, while the proportion of patients with high disease activity (DAS28-4/ESR >5.1) decreased from 51.9% at baseline to 1.7% at 36 months (). At the end of the study, using the last observation carried forward (LOCF) method, 38.6% of patients (166/430) achieved DAS28-4/ESR remission and 57.2% of patients (246/430) had low disease activity (DAS28-4/ESR <3.2). Similarly, the proportion of patients with MHAQ =0 increased over time from 13.8% at baseline to 48.6% at 36 months, while the proportion of patients with MHAQ >1.5 decreased from 15.0% at baseline to 4.6% at 36 months (). At the end of the study, using the LOCF method, 66.3% of patients (282/425) achieved MHAQ remission (MHAQ score ≤0.5).
Figure 4. Changes in patient distribution in disease activity status over time. Patients were categorized into high, moderate, low disease activity or remission groups using DAS28-4/ESR scores and the last observation carried forward method. DAS28-4/ESR, 28-joint count Disease Activity Score based on four erythrocyte sedimentation rate. *p < .001 for significant linear trend by Cochran–Armitage test.
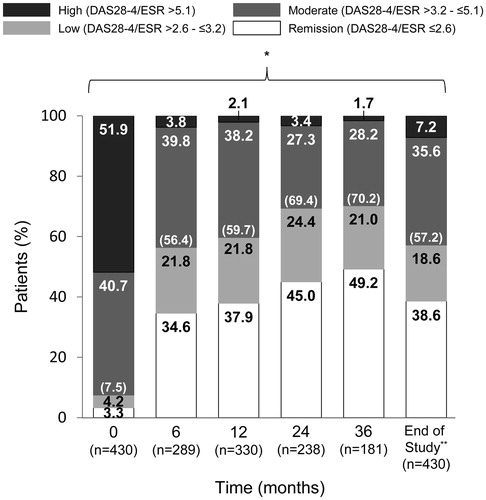
Figure 5. Changes in patient distribution in MHAQ score over time. Patients were categorized using MHAQ score and the last observation carried forward method. MHAQ: modified Health Assessment Questionnaire. *p < .001 for significant linear trend by Cochran–Armitage test.
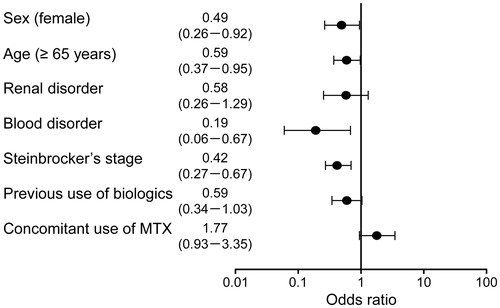
Factors associated with not achieving DAS28-4/ESR remission at the end of the study were female sex (OR, 0.49; 95% CI, 0.26–0.92), age ≥65 years (OR, 0.59; 95% CI, 0.37–0.95), blood disorder (OR, 0.19; 95% CI, 0.06–0.67) and advanced Steinbrocker’s stage at baseline (OR, 0.42; 95% CI, 0.27–0.67) ().
Discussion
In this multicenter, single-cohort, observational study, we enrolled Japanese patients who had participated in the prior PMS for ADA [Citation6]. We assessed the safety and effectiveness of ADA as long-term (up to 3 years) treatment in patients with RA. In a previous analysis of global clinical trials enrolling >20,000 patients with various autoimmune diseases including RA and Crohn's disease, the long-term safety profile of ADA was consistent with that of other TNF inhibitors [Citation19]. Malignancy has been a concern since the introduction of TNF inhibitors for the treatment of patients with RA; however, assessing the risk of malignancy in patients treated with bDMARDs requires long-term observation. Therefore, we set our primary objective of this study as the overall incidence of ADRs, as well as those of infections and malignancy, in a clinical setting in Japan.
Infection was the most common ADR in the present study, and the second most common, after ‘skin and subcutaneous tissue disorders,’ in the prior all-case PMS [Citation6]. ADRs classified as ‘infections and infestations’ were reported in 16.5% of patients (84/509), representing a rate of 6.7/100 PYs in the present study, compared with 7.0% of patients (538/7740; 17.5/100 PYs) in the all-case PMS. Serious infection was the most common SADR in both studies; 6.1% of patients (31/509; 2.5/100 PYs) in the present study and 2.4% of patients (182/7740; 5.9/100 PYs) in the earlier study had a serious infection. These difference in the incidence of infections and serious infections per 100 PYs between the all-case PMS and this study can be explained by the eligibility criteria of this study, which enrolled patients who continued ADA for 6 months; those who did not develop infections or serious infections during the first 6 months of treatment with ADA were more likely to be enrolled in this study.
The incidence rates of ADRs and SADRs changed over time. The rates of ADRs and infection peaked during the first 6 months but decreased thereafter. Among ADRs, skin and subcutaneous tissue disorders, general disorders and administration site and gastrointestinal disorders frequently occurred early in the study. The decline in the incidence rates of these ADRs partly accounted for the overall decreased incidence of ADRs. Other possible reasons for the decreased incidence of ADRs and infection were improvements in patient conditions as a result of decreased disease activity in response to treatment and consequent decreases in the doses of corticosteroids (or even their discontinuation). The rates of SADRs and serious infection tended to increase slightly after 6 months. The 3-year follow-up results for a PMS program of TCZ were recently published [Citation20]. Overall, 5620 out of 7901 patients who were enrolled in the all-case PMS were registered and 4527 patients completed 3 years of follow-up. The mean age of patients in that study was 58.7 years and 81.5% of patients were females, similar to the demographics of our study. The rate of serious infection was 5.67/100 PYs in the first year and it was much higher compared with this study because the majority of patients who were enrolled in the all-case PMS of TCZ were followed in the 3 years PMS as well; the rate decreased to 3.25 and 2.16/100 PYs in the second and third years, respectively, and the decline could be easily observed. The overall incidence rate of serious infection in the current study (2.5/100 PYs) was as low as that observed in the third year of the TCZ study and similar to that of Japanese patients who were not exposed to bDMARDs in an observational study (2.72/100 PYs) [Citation21], which probably made it difficult to observe further declines of the incidence rates over time. Nonetheless, careful monitoring and education of patients are mandatory for the prevention and early diagnosis of serious infections during treatment with ADA.
In the present study, eight malignancies were observed in seven patients (0.64/100 PYs); however, no patients developed malignant lymphoma. In the SECURE study, the age- and sex-standardized incidence rate of malignancy in Japanese patients with RA who had received bDMARDs was 313.9/105 PYs (95% CI, 271.4–361.3), which was lower than the estimated incidence rate of malignancies in the Japanese general population (462.4/105 PYs) [Citation22]. However, the standard incidence rate ratio of malignant lymphoma in RA patients was significantly increased in the SECURE cohort [Citation22]. Furthermore, in a recent review of the adverse effects of ADA across all indications, the overall malignancy rates in ADA-treated patients (0.7/100 PYs for malignancies not including lymphoma [0.1/100 PYs] and non–melanoma skin cancer [0.2/100 PYs]) were as expected for the general population [Citation19].
Eleven patients died during the observation period and the SMR was 0.88 (95% CI, 0.44–1.56). In a large, single-center, Japanese cohort of patients with RA, the SMR was between 1.46 (1.32–1.60) and 1.90 (1.75–2.07). Major causes of death included malignancies, respiratory disease, and myocardial infarction [Citation23]. The long-term PMS program of TCZ reported a SMR of 1.27 (95% CI, 1.08–1.50) [Citation20]. These data indicate that the vital prognosis of patients treated with ADA was similar to the prognosis of patients in clinical practice and patients who received TCZ.
Our secondary objective was to identify factors affecting the safety and effectiveness of ADA. Identified risk factors for ADRs in this study included hepatic disorder, respiratory disorder, and poorer physical function (Steinbrocker’s class 3 or 4). Serious ADRs were more likely in older patients and those with respiratory disorder. In the prior all-case PMS, age, pulmonary disease history or comorbidity, coexisting diabetes mellitus, concomitant MTX therapy (>8 mg/week) and concomitant glucocorticoid therapy (>5 mg/day) were identified as risk factors for infection [Citation6]. It is necessary to evaluate these risk factors before initiation of treatment with ADA and to start cautiously and continue ADA treatment under close monitoring.
Both disease activity and functional status as assessed by DAS28-4/ESR and MHAQ improved over the 3-year observation period. This finding adds to evidence supporting the effectiveness of ADA in the short-term treatment of patients with RA [Citation6,Citation12]. Patients with a lower probability of remission were female, were ≥65 years, had blood disorders and suffered from advanced structural change (Steinbrocker’s stage III or IV). Biologic-naïve and concomitant use of MTX were significantly associated with better clinical response at week 24 in the all-case PMS [Citation6], but these aspects did not reach statistical significance in this long-term PMS.
Another objective of this study was to evaluate the long-term toxicity of ADA, especially serious infections and malignancies, in Japanese patients with RA. The rate of serious infections in this study (2.5/100 PYs) was similar to or even lower than those reported in other long-term investigations on the safety profiles of ADA, such as the ARMADA study (2.03/100 PYs [Citation14]), the DE019 10-year study (4.3/100 PYs [Citation24]), and the PREMIER study (2.6/100 PYs [Citation16]). In addition, the low rates of malignancies observed in this study (0.64/100 PYs) are consistent with previous reports, including the DE019 10-year study (1.1/100 PYs, excluding nonmelanoma skin cancer [NMSC] and lymphoma [Citation24]) and the PREMIER study (0.8/100 PYs, other than NMSC, melanoma, lymphoma or leukemia [Citation16]). Thus, the present study supports and expands the known safety profile of ADA, with no additional safety signals in relation to the incidence of serious infections or malignancies.
The present study has several limitations. First, it was a single-arm, observational study and it did not have a control or comparison group. It was not possible to compare the effectiveness and safety of ADA with other treatments for RA. Second, the enrolled patients had been receiving ADA treatment continuously for 6 months during the all-case PMS. These patients had tolerated and presumably responded, at least partially, to the treatment. Third, the evaluation of effectiveness did not include radiographic assessment, as the primary objective of this study was evaluation of safety. Despite these limitations, we believe that our study provides useful information on the long-term safety and effectiveness of ADA treatment in a real-world clinical setting in Japan.
In conclusion, the safety profile of ADA for this group of Japanese patients with RA was found to be consistent with the existing profile; no new safety concerns were identified. ADA treatment resulted in significant long-term improvement in patients with RA in the present study. ADA decreased disease activity and increased functional status. However, special care should be taken when considering the long-term use of ADA in patients with risk factors for ADRs and SADRs. Physicians need to strike an appropriate balance between the benefits and risks of not only short-term ADA treatment but also long-term treatment.
Conflict of interest
This study was sponsored by AbbVie GK and Eisai Co., Ltd. The sponsors contributed to the study design, participated in the collection, analysis, and interpretation of the data, and in the writing, reviewing, and approval of the final manuscript. M. Harigai received research grants, honoraria or consultant fees from AbbVie Japan Co., Ltd., Bristol-Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Co., Santen Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Teijin Pharma, Ltd., and Pfizer Japan Inc. T. Tsuchiya, K. Kawana, and S. Kurimoto are employees of AbbVie.
Supplementary_figure_1.tif
Download TIFF Image (104.8 KB)Supplementary_table_1.docx
Download MS Word (18.2 KB)Acknowledgements
The authors thank all of the patients, investigators and support staff who participated in this study. The authors acknowledge the contribution of Mariko Miyajima in writing the manuscript (Springer Healthcare); this assistance was funded by AbbVie.
References
- Kaneko Y, Takeuchi T. A paradigm shift in rheumatoid arthritis over the past decade. Intern Med. 2014;53:1895–903.
- Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, Yamanaka H, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:189–94.
- Koike T, Harigai M, Inokuma S, Inoue K, Ishiguro N, Ryu J, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol. 2009;36:898–906.
- Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, et al. Postmarketing surveillance of safety and effectiveness of etanercept in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2011;21:343–51.
- Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, et al. Safety and effectiveness responses to etanercept for rheumatoid arthritis in Japan: a sub-analysis of a post-marketing surveillance study focusing on the duration of rheumatoid arthritis. Rheumatol Int. 2012;32:1511–9.
- Koike T, Harigai M, Ishiguro N, Inokuma S, Takei S, Takeuchi T, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of 7740 patients. Mod Rheumatol. 2014;24:390–8.
- Koike R, Harigai M, Atsumi T, Amano K, Kawai S, Saito K, et al. Japan College of Rheumatology 2009 guidelines for the use of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, in rheumatoid arthritis. Mod Rheumatol. 2009;19:351–7.
- Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis. 2011;70:2148–51.
- Harigai M, Ishiguro N, Inokuma S, Mimori T, Ryu J, Takei S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2016;26:491–8.
- Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509.
- Smolen JS, Redlich K, Zwerina J, Aletaha D, Steiner G, Schett G. Pro-inflammatory cytokines in rheumatoid arthritis: pathogenetic and therapeutic aspects. Clin Rev Allergy Immunol. 2005;28:239–48.
- Navarro-Sarabia F, Ariza-Ariza R, Hernandez-Cruz B, and Villanueva I. Adalimumab for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2005;20:CD005113.
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45.
- Weinblatt ME, Keystone EC, Furst DE, Kavanaugh AF, Chartash EK, and Segurado OG. Long term efficacy and safety of adalimumab plus methotrexate in patients with rheumatoid arthritis: ARMADA 4 year extended study. Ann Rheum Dis. 2006;65:753–9.
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37.
- Keystone EC, Breedveld FC, van der Heijde D, Landewé R, Florentinus S, Arulmani U, et al. Longterm effect of delaying combination therapy with tumor necrosis factor inhibitor in patients with aggressive early rheumatoid arthritis: 10-year efficacy and safety of adalimumab from the randomized controlled PREMIER trial with open-label extension. J Rheumatol. 2014;41:5–14.
- Takeuchi T, Yamanaka H, Ishiguro N, Miyasaka N, Mukai M, Matsubara T, et al. Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis. 2014;73:536–43.
- HUMIRA (adalimumab) [prescribing Information]. Pharmaceuticals and medical devices agency website (in Japanese). 2016. Available from: http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/112130_3999426G1024_2_12#page=2 [last accessed 21 March 2017].
- Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis. 2013;72:517–24.
- Yamamoto K, Goto H, Hirao K, Nakajima A, Origasa H, Tanaka K, et al. Longterm safety of tocilizumab: results from 3 years of followup postmarketing surveillance of 5573 patients with rheumatoid arthritis in Japan. J Rheumatol. 2015;42:1368–75.
- Sakai R, Komano Y, Tanaka M, Nanki T, Koike R, Nagasawa H, et al. Time-dependent increased risk for serious infection from continuous use of tumor necrosis factor antagonists over three years in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1125–34.
- Harigai M, Nanki T, Koike R, Tanaka M, Watanabe-Imai K, Komano Y, et al. Risk for malignancy in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs compared to the general population: a nationwide cohort study in Japan. Mod Rheumatol. 2016;26:642–50.
- Nakajima A, Inoue E, Tanaka E, Singh G, Sato E, Hoshi D, et al. Mortality and cause of death in Japanese patients with rheumatoid arthritis based on a large observational cohort, IORRA. Scand J Rheumatol. 2010;39:360–7.
- Keystone EC, van der Heijde D, Kavanaugh A, Kupper H, Liu S, Guérette B, et al. Clinical, functional, and radiographic benefits of longterm adalimumab plus methotrexate: final 10-year data in longstanding rheumatoid arthritis. J Rheumatol. 2013;40:1487–97.