Abstract
AA amyloidosis may develop in patients with active chronic inflammation. Serum amyloid A (SAA), the precursor of the AA protein, is strongly amplified in the liver under the stimulation of inflammation-associated cytokines, such as IL-6, TNF, and IL-1. Sustained inflammation, aging, and polymorphisms in the SAA1.3 genotype are dependent risk factors for the formation of AA amyloidosis. The most rational treatment strategy for AA amyloidosis is to inhibit the production of SAA. Treatments for AA amyloidosis involving biologics have recently been emphasized. TNF inhibitors and abatacept reduce SAA levels; however, complete normalization is not always achieved. IL-6 inhibitors may normalize SAA levels in most patients in whom a sufficient concentration of medication is maintained in the blood. Therefore, treatments with IL-6 inhibitors represent an excellent therapeutic strategy for AA amyloidosis and have been verified by recent studies.
Introduction
Among the complications of chronic inflammatory diseases, amyloid A (AA) amyloidosis is one of the most severe because of its poor prognosis. AA amyloidosis commonly affects the kidneys and gastrointestinal tract, and is characterized by various clinical symptoms, such as progressive proteinuria as well as renal dysfunction and failure. Control of the underlying disease, i.e. the suppression of serum amyloid A (SAA) levels, is the most critical step in the treatment of AA amyloidosis. Immunosuppressants, such as methotrexate, azathioprine, cyclophosphamide, and moderate doses of prednisolone, have previously been used to accomplish this. However, the satisfactory suppression of SAA levels cannot be achieved in some active cases, and the functions of the affected organs deteriorate. The prognosis of patients in the advanced stages of AA amyloidosis is generally poor. The major causes of death are renal failure and infection. Some retrospective studies and case reports demonstrated that anti-tumor necrosis factor (TNF) therapies were useful against AA amyloidosis [Citation1–5]. Although treatments with anti-TNF agents reduce acute-phase reactants, such as C-reactive protein (CRP) and SAA, in chronic inflammatory diseases, the complete normalization of these acute-phase proteins is low [Citation5].
On the other hand, several case reports [Citation6–11], a retrospective comparative study [Citation12], and the first nationwide survey of patients with AA amyloidosis in Japan [Citation13] showed that the anti-human interleukin-6 (IL-6) receptor monoclonal antibody, tocilizumab (TCZ), effectively suppressed SAA levels and improved the clinical symptoms of AA amyloidosis with the marked lasting regression of AA protein deposits. Therefore, treatments with IL-6 inhibitors have potential as an important therapeutic strategy for AA amyloidosis.
Pathogenesis of AA amyloidosis
AA amyloidosis resulting from the deposition of the AA protein in the extracellular matrices of various organs may lead to multiple organ dysfunctions, and the prognosis of patients with advanced AA amyloidosis is generally poor.
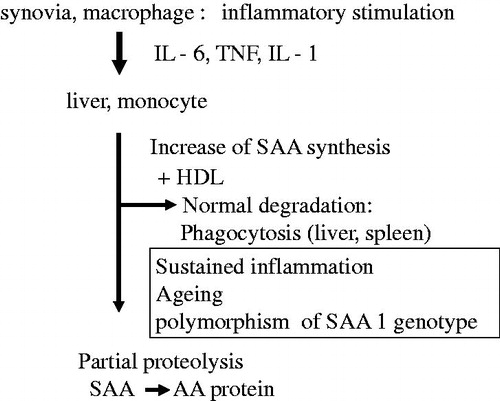
Although chronic infections, such as tuberculosis, were previously listed as the principal cause of AA amyloidosis, many cases of AA amyloidosis at present are closely associated with chronic inflammatory diseases, such as rheumatoid arthritis (RA) [Citation13]. SAA, the precursor of the AA protein, is strongly amplified in the liver under the stimulation of inflammation-associated cytokines, such as IL-6, TNF, and IL-1 [Citation14].
Prolonged elevations in SAA are the major inciting factor for AA amyloidosis developing in chronic inflammatory diseases. The extensive and persistent production of SAA under chronic active inflammatory conditions is a critical prerequisite for AA amyloid fibrillogenesis, and approximately 5–7% of Japanese RA patients have AA amyloid deposits in their gastroduodenal mucosa, as shown by endoscopic biopsy [Citation15].
SAA is genetically polymorphic with four loci (SAA1, SAA2, SAA3, and SAA4) located on chromosome 11. The SAA gene products SAA1 and SAA2 are both elevated with acute inflammation and are, thus, named acute-phase SAA. More than 90% of the precursor proteins of the AA protein are derived from SAA1 [Citation16]. SAA1 has several allelic variants, in which the exon 3 polymorphism generates three common isoforms in the Japanese population: SAA1.1: 52Valine/57Alanine, SAA1.3: 52Alanine/57Alanine, and SAA1.5: 52Alanine/57Valine. This polymorphism has been suggested to contribute to the susceptibility of the Japanese population to RA-associated AA amyloidosis [Citation17–20]. SAA1.3 may be a risk factor for AA amyloidosis, while SAA1.1 acts as a defense. However, SAA1.1 has been identified as a risk factor in Caucasians [Citation21], suggesting a racial difference in the contribution of the exon 3 polymorphism to the pathogenesis of AA amyloidosis in RA.
In animal model studies, elevated SAA was detected in aging mice [Citation22], and organ extracts from aged mice exhibited amyloid-enhancing factor activity that accelerated experimental SAA production [Citation23]. We examined the contribution of aging to the induction of AA amyloidosis in RA in our large cohort (388 adult-onset RA patients with AA amyloidosis) including 144 patients who been analyzed for the SAA1 polymorphism. We identified aging as a possible independent risk factor for the formation of AA amyloidosis complicating RA [Citation24]. shows the pathogenetic cascade of AA amyloidosis in rheumatic diseases.
Treatment of AA amyloidosis
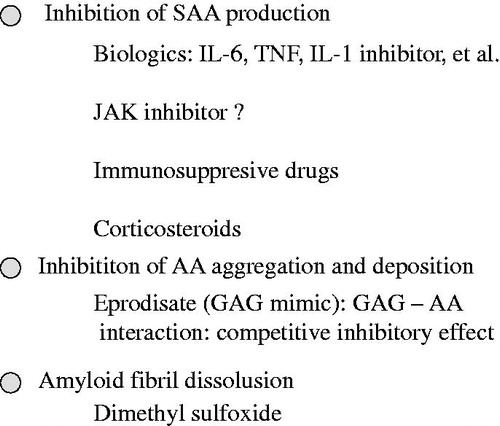
The most rational treatment for AA amyloidosis is to inhibit the production of SAA, the precursor of the AA protein. As evidence for this, Gillmore et al. used serum amyloid P (SAP) scintigraphy to evaluate the levels of amyloid deposits in the organs of patients with AA amyloidosis associated with inflammatory diseases, such as RA. They reported that when SAA concentrations in the blood were controlled at less than 10 mg/L, the levels of amyloid deposits in the organs decreased and the 10-year survival rate was good at approximately 90%, whereas in patients with SAA levels of 10 mg/L or higher, the 10-year survival rate was approximately 40%. The maintenance of SAA concentrations within the normal range correlated with reduced amyloid deposition and an improved prognosis [Citation25]. The same group investigated 374 patients with AA amyloidosis and performed a more detailed analysis of the very strong correlation between decreases in SAA concentrations and an improved survival prognosis. SAP scintigraphy revealed that the disappearance of the AA protein was significantly greater in the low concentration group than in the high concentration group. The control of SAA concentrations was stressed as the most important parameter in the treatment of amyloidosis [Citation26]. Therefore, it is very important to inhibit the activity of the underlying disease itself as much as possible. Rheumatic diseases are the most common underlying diseases of AA amyloidosis, and in the treatment of rheumatic disease patients, biologics exhibit higher efficacy than conventional treatments and may be used in current routine clinical practices.
In the treatment of AA amyloidosis from a different standpoint, the organic solvent dimethyl sulfoxide (DMSO) is sometimes administered in an attempt to eliminate the AA protein by increasing the degree of solubility of the amyloid protein deposited in tissues. Although not proven in controlled comparative studies, several case reports and case series reports suggesting its usefulness have been published [Citation27].
Eprodisate, a mimetic of glycosaminoglycan that serves as the extracellular scaffold for amyloid deposits in tissues, is expected to exert therapeutic effects because it prevents the accumulation of the AA protein. A multicenter randomized double-blind placebo-controlled study was conducted mainly in Europe and the United States. The risk of a decline in renal function was lowered by 42%; however, no significant differences were found in the risk of decreases in progression to end-stage renal failure and mortality [Citation28]. Applications were filed with the FDA as an orphan drug, but were not approved, and an additional international study is now in progress – International Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study of the Efficacy and Safety of Eprodisate (KIACTA™) in Preventing Renal Function Decline in Patients with AA Amyloidosis. shows the treatment strategy for AA amyloidosis.
Treatment of AA amyloidosis with biologics
Anti-TNF therapy
Previous studies indicated that anti-TNF therapy is effective for patients with rheumatic diseases complicated by AA amyloidosis [Citation1–5]. If the underlying disease of AA amyloidosis is inflammatory bowel disease (mainly Crohn’s disease) or spondyloarthritis, anti-TNF therapy may play a pivotal role [Citation13].
Anti-IL-1 therapy
Colchicine is the standard treatment in familial Mediterranean fever (FMF) patients. Anti-IL-1 therapies (Canakinumab or Anakinra) may be used to successfully treat colchicine-resistant FMF patients with amyloidosis [Citation29].
Abatacept
There is only one case report to suggest that abatacept may be effective and safe in patients with AA amyloidosis secondary to RA by immunological modulation mechanisms according to regulatory T cells and inflammatory cytokines [Citation30].
JAK inhibitors
The efficacy of JAK inhibitors in the treatment of patients with AA amyloidosis has not yet been reported.
However, these drugs are potentially useful for AA amyloidosis and, thus, reports of effective cases will be published soon.
Anti-IL-6 therapy
IL-6 and the mode of action of anti-IL-6R medication
IL-6 has a unique receptor system: although the IL-6 receptor (IL-6R) specifically binds to IL-6, it is not directly involved in signal transduction. IL-6 forms a complex by binding to IL-6R on the cell membrane, which then combines with gp130, which also resides on the cell membrane, forming a homodimer and initiating intracellular signal transduction. Furthermore, IL-6R exists in a soluble form. After forming a complex with IL-6, soluble IL-6R (sIL-6R) may also combine with gp130 on the cell membrane and enable signaling. sIL-6R is present in the blood of healthy individuals at a concentration of several tens of ng/mL, and if cells express gp130, IL-6 signaling may occur, even in the absence of IL-6R expression on the cell membrane. Reflecting this characteristic, the effects of IL-6 are diverse, and it is considered to play an extensive regulatory role, with involvement in immune responses, inflammation, bone metabolism, hematopoiesis, and the neuroendocrine system.
TCZ is a recombinant monoclonal antibody that has been humanized by the complementarity-determining region (CDR) grafting of a murine anti-human IL-6R antibody onto human IgG1. TCZ inhibits the induction of IL-6-mediated biological activity in cells that have expressed membrane-bound IL-6R and gp130 molecules and inhibits the induction of biological activity mediated by IL-6/sIL-6R complex formation in cells that express gp130 alone. Furthermore, since TCZ has the capacity to dissociate IL-6/IL-6R complexes that have already formed, it exerts extremely effective blocking effects on IL-6 signal transduction.
Pivotal role of IL-6 in SAA synthesis
The signal transduction and transcription mechanisms of TNF-alpha, IL-1-beta, and IL-6 in the production of SAA have become apparent. In a study using HepG2 cells, a cell line derived from hepatocytes, the weak expression of SAA1 and SAA2 mRNA was induced by a stimulation with IL-6 alone, whereas almost no expression was induced by the stimulation with IL-1-beta or TNF-alpha alone. However, the synergistic induction of expression was observed by a co-stimulation with IL-6 and IL-1-beta or with IL-6 and TNF-alpha [Citation31]. The activation of STAT3 by an IL-6 stimulation is essential in the production of SAA, and it is clear that SAA expression is strengthened by the supplementation of NF-kappaBp65 activity by a stimulation with TNF-alpha or IL-1-beta [Citation32]. Clinically, TNF inhibitors reduce SAA levels; however, complete normalization is rare. TCZ may normalize SAA levels in most patients in whom a sufficient concentration of TCZ is maintained in the blood.
AA amyloidosis treatment by anti-IL-6 receptor therapy
TCZ has been approved in Japan for Castleman’s disease, RA, polyarticular juvenile idiopathic arthritis (JIA), systemic JIA, Takayasu arteritis, and Giant cell arteritis. TCZ is currently the most effective agent against AA amyloidosis through a mechanism of action based on SAA reductions, as described above. Most patients undergoing anti-IL-6 therapy are those who maintain TCZ blood concentrations, and SAA normalization is possible. Sarilumab, a human anti-IL-6R antibody, was recently approved for the treatment of RA, and is expected to be similarly effective for AA amyloidosis. Before TCZ approval in 2008, we provided the compassionate use of TCZ to a patient with life-threatening JIA complicated by rapidly progressing AA amyloidosis for which many drugs were ineffective. SAA was completely normalized by the early administration of TCZ, digestive tract symptoms (intractable diarrhea) disappeared, renal symptoms (proteinuria and renal dysfunction) normalized, and the AA protein markedly disappeared from tissues. The clinical course and usefulness of TCZ in this case have been reported [Citation6] ( and ). Many studies were subsequently presented at meetings and case reports have recently suggested the wide-ranging usefulness of TCZ in treating rheumatic diseases complicated by AA amyloidosis [Citation7–11].
Figure 3. Results of endoscopic examinations before and after IL-6 inhibitor therapy. (a) Before tocilizumab therapy, the appearance of the mucosa in the antrum of the stomach was coarse with reddish and edematous changes. (b) Before tocilizumab therapy was initiated, the appearance of the mucosa in the second portion of the duodenum was coarse, nodular, irregular, edematous, and reddish. (c, d) After 18 months of tocilizumab therapy, no abnormality was observed in the antrum of the stomach or in the second potion of the duodenum (quoted from Okuda et al. [Citation6]).
![Figure 3. Results of endoscopic examinations before and after IL-6 inhibitor therapy. (a) Before tocilizumab therapy, the appearance of the mucosa in the antrum of the stomach was coarse with reddish and edematous changes. (b) Before tocilizumab therapy was initiated, the appearance of the mucosa in the second portion of the duodenum was coarse, nodular, irregular, edematous, and reddish. (c, d) After 18 months of tocilizumab therapy, no abnormality was observed in the antrum of the stomach or in the second potion of the duodenum (quoted from Okuda et al. [Citation6]).](/cms/asset/14eeca83-3067-4a5c-9add-4d05b70d3d58/imor_a_1515145_f0003_c.jpg)
Figure 4. Results of gastrointestinal biopsy before and after IL-6 inhibitor therapy. (a) Massive amyloid A protein deposits were observed in the duodenal mucosa and submucosa before the start of tocilizumab therapy. (b) The marked regression of amyloid A protein deposits was observed in the duodenal mucosa and submucosa after the tocilizumab treatment (Congo red stained; 200× magnification) (quoted from Okuda et al. [Citation6]).
![Figure 4. Results of gastrointestinal biopsy before and after IL-6 inhibitor therapy. (a) Massive amyloid A protein deposits were observed in the duodenal mucosa and submucosa before the start of tocilizumab therapy. (b) The marked regression of amyloid A protein deposits was observed in the duodenal mucosa and submucosa after the tocilizumab treatment (Congo red stained; 200× magnification) (quoted from Okuda et al. [Citation6]).](/cms/asset/2bc89ed2-e46b-459f-9dde-21dd1dac40de/imor_a_1515145_f0004_c.jpg)
Anti-cytokine therapy is reportedly useful in the treatment of AA amyloidosis complicating rheumatic diseases; however, no studies have directly compared the utility of TNF inhibition to that of IL-6 inhibition. We retrospectively compared the clinical utility of TCZ and TNF inhibitors [Citation12]. Patients were divided into a TCZ group (22 patients) and TNF inhibitor group (32 patients). The main parameters compared were the treatment retention rate, SAA profile, renal function profile, and clinical disease activity index. Five-year retention rates were 90.4% (TCZ group) and 34.3% (TNF inhibitor group) (p = .0154, the Log-rank test). Median SAA decreased from 219.2 μg/mL at treatment initiation to 5.0 μg/mL in the last observation (TCZ) and from 143.6 to 38.1 μg/mL (TNF inhibitor) (p = .0194) (). The estimated glomerular filtration rate improved in 72.7% (TCZ) and 34.4% (TNF inhibitor) of patients (p = .0062) (). The rate of remission and low disease activity in the last observation were 72.7% (TCZ) and 40.6% (TNF inhibitor) (p = .0201).
Figure 5. Changes in SAA values at treatment initiation and in the last observation. Tocilizumab suppressed SAA values significantly more than the TNF inhibitor (p = .0194, Wilcoxon’s rank-sum test) (quoted from Okuda et al. [Citation12]).
![Figure 5. Changes in SAA values at treatment initiation and in the last observation. Tocilizumab suppressed SAA values significantly more than the TNF inhibitor (p = .0194, Wilcoxon’s rank-sum test) (quoted from Okuda et al. [Citation12]).](/cms/asset/df6d62e9-a333-4357-866a-92dad87463ca/imor_a_1515145_f0005_b.jpg)
Figure 6. Changes in the estimated glomerular filtration rate (eGFR). Improvements in eGFR were observed in 16 patients (72.7%) in the tocilizumab group and 11 patients (34.4%) in the TNF inhibitor group. Although the tocilizumab treatment was initiated at a more advanced stage of renal dysfunction, significant improvements in renal function were obtained (p = .0062, Wilcoxon’s rank-sum test) (quoted from Okuda et al. [Citation12]).
![Figure 6. Changes in the estimated glomerular filtration rate (eGFR). Improvements in eGFR were observed in 16 patients (72.7%) in the tocilizumab group and 11 patients (34.4%) in the TNF inhibitor group. Although the tocilizumab treatment was initiated at a more advanced stage of renal dysfunction, significant improvements in renal function were obtained (p = .0062, Wilcoxon’s rank-sum test) (quoted from Okuda et al. [Citation12]).](/cms/asset/1097b66b-cf24-4f7f-8d24-79efa27363b0/imor_a_1515145_f0006_b.jpg)
A nationwide survey on AA amyloidosis had not yet been conducted in Japan. Therefore, on behalf of the Amyloidosis Research Committee, Intractable Disease Division of the Japanese Ministry of Health and Welfare, we conducted a survey on this disease in Japan [Citation13]. A total of 199 patients with AA amyloidosis were included in the present study. Biologics were administered to 97 patients (48.7%) to treat AA amyloidosis (). TCZ was administered to 66 patients, and good responses were obtained in 63 (95.5%). The underlying diseases of patients administered TCZ were RA (51 patients), Castleman’s disease (6), other rheumatic diseases (4), renal cell carcinoma (2), uncharacterized inflammatory disorders (2), and FMF (1). Good responses were obtained in all patients, except for those with RA. Anti-TNF inhibitors (etanercept: 15, infliximab: 8, adalimumab: 2, golimumab: 2) were administered to 27 patients, and good responses were noted in 20 (74.1%). The underlying diseases of patients administered TNF inhibitors were RA (21 patients), other rheumatic diseases (3), and Crohn’s disease (3). The efficacy of TCZ was significantly superior to that of TNF inhibitors (χ2 = 7.273, p = .007). Abatacept was administered to four patients (RA), and good responses were observed in three (75%). TCZ was the most frequently administered biologic for the various underlying diseases, including RA, and was found to be the most effective by a nationwide survey on AA amyloidosis in Japan.
Table 1. Biologic treatment and efficacy in AA amyloidosis.
Conclusion
IL-6 inhibitors exhibit an excellent ability to suppress SAA levels and improve the clinical symptoms of AA amyloidosis with the marked lasting regression of AA protein deposits. Therefore, treatments with IL-6 inhibitors have potential as an important therapeutic strategy for AA amyloidosis.
Conflict of interest
None.
Additional information
Funding
References
- Elkayam O, Hawkins PN, Lachmann H, Yaron M, Caspi D. Rapid and complete resolution of proteinuria due to renal amyloidosis in a patient with rheumatoid arthritis treated with infliximab. Arthritis Rheum. 2002;46(10):2571–3.
- Gottenberg JE, Merle-Vincent F, Bentaberry F, Allanore Y, Berenbaum F, Fautrel B. Anti-tumor necrosis α therapy in fifteen patients with AA amyloidosis secondary to inflammatory arthritides: a follow up report of tolerability and efficacy. Arthritis Rheum. 2003;48(7):2019–24.
- Kuroda T, Wada Y, Kobayashi D, Murakami S, Sakai T, Hirose S. Effective anti-TNF-α therapy can induce rapid resolution and sustained decrease of gastroduodenal mucosal amyloid deposits in reactive amyloidosis associated with rheumatoid arthritis. J Rheumatol. 2009;36(11):2409–15.
- Nakamura T, Higashi S, Tomoda K, Tsukano M, Shono M. Etanercept can induce resolution of renal deterioration in patients with amyloid A amyloidosis secondary to rheumatoid arthritis. Clin Rheumatol. 2010;29(12):1395–401.
- Fernández-Nebro A, Olivé A, Castro MC, Varela AH, Riera E, Irigoyen MV, et al. Long-term TNF-alpha blockade in patients with amyloid A amyloidosis complicating rheumatic diseases. Am J Med. 2010;123(5):454–61.
- Okuda Y, Takasugi K. Successful use of a humanized anti-interleukin-6 receptor antibody, tocilizumab, to treat amyloid A amyloidosis complicating juvenile idiopathic arthritis. Arthritis Rheum. 2006;54(9):2997–3000.
- Sato H, Sakai T, Sugaya T, Otaki Y, Aoki K, Ishii K, et al. Tocilizumab dramatically ameliorated life-threatening diarrhea due to secondary amyloidosis associated with rheumatoid arthritis. Clin Rheumatol. 2009;28(9):1113–16.
- Magro-Checa C, Navas-Parejo Casado A, Borrego-Garcia E, Raya-Alvarez E, Rosales-Alexander JL, Salvatierra J, et al. Successful use of tocilizumab in a patient with nephrotic syndrome due to a rapidly progressing AA amyloidosis secondary to latent tuberculosis. Amyloid. 2011;18(4):235–9.
- Inoue D, Arima H, Kawanami C, Takiuchi Y, Nagano S, Kimura T, et al. Excellent therapeutic effect of tocilizumab on intestinal amyloid A deposition secondary to active rheumatoid arthritis. Clin Rheumatol. 2010;29(10):1195–7.
- Nishida S, Hagihara K, Shima Y, Kawai M, Kuwahara Y, Arimitsu J, et al. Rapid improvement of AA amyloidosis with humanised anti-interleukin 6 receptor antibody treatment. Ann Rheum Dis. 2009;68(7):1235–6.
- Kishida D, Okuda Y, Onishi M, Takebayashi M, Matoba K, Jouyama K, et al. Successful tocilizumab treatment in a patient with adult-onset Still’s disease complicated by chronic active hepatitis B and amyloid A amyloidosis. Mod Rheumatol. 2011;21(2):215–18.
- Okuda Y, Ohnishi M, Matoba K, Jouyama K, Yamada A, Sawada N, et al. Comparison of the clinical utility of tocilizumab and anti-TNF therapy in AA amyloidosis complicating rheumatic diseases. Mod Rheumatol. 2014;24:137–43.
- Okuda Y, Yamada T, Ueda M, Ando Y. First nationwide survey of 199 patients with amyloid A amyloidosis in Japan. Internal Med. 2018. doi:10.2169/internalmedicine.1099-18.
- Yamada T. Serum amyloid A (SAA): a concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med. 1999;37:381–8.
- Okuda Y. Amyloid A amyloidosis complicating rheumatoid arthritis. Stomach Intest. 2014;49:335–43 [article in Japanese].
- Liepnieks JJ, Kluve-Beckerman B, Benson MD. Characterization of amyloid A protein in human secondary amyloidosis: the predominant deposition of serum amyloid A1. Biochim Biophys Acta. 1995;1270:81–6.
- Baba S, Masago SA, Takahashi T, Kasama T, Sugimura H, Tsugane S, et al. A novel allelic variant of serum amyloid A, SAA1γ: genomic evidence, evolution, frequency, and implication as a risk factor for reactive systemic AA-amyloidosis. Hum Mol Genet. 1995;4(6):1083–7.
- Okuda Y, Yamada T, Takasugi K, Takeda M, Nanba S, Onishi M, et al. Serum amyloid A (SAA)1, SAA2 and apolipoprotein E isotype frequencies in rheumatoid arthritis patients with AA amyloidosis. Ryumachi. 1999;39:3–10 [article in Japanese].
- Moriguchi M, Terai C, Koseki Y, Uesato M, Nakajima A, Inada S, et al. Influence of genotypes at SAA1 and SAA2 loci on the development and the length of latent period of secondary AA-amyloidosis in patients with rheumatoid arthritis. Hum Genet. 1999;105(4):360–6.
- Nakamura T, Higashi S, Tomoda K, Tsukano M, Baba S, Shono M. Significance of SAA1.3 allele genotype in Japanese patients with amyloidosis secondary to rheumatoid arthritis. Rheumatology (Oxford). 2006;45(1):43–9.
- Booth DR, Booth SE, Gillmore JD, Hawkins PN, Pepys MB. SAA1 alleles as risk factors in reactive systemic AA amyloidosis. Amyloid. 1998;5:262–5.
- Hsu HC, Zhou T, Yang PA, Herrera GA, Mountz JD. Increased acute-phase response and renal amyloidosis in aged CD2-fas-transgenic mice. J Immunol. 1997;158(12):5988–96.
- Yokota T, Ishihara T, Kawano H, Takahashi M, Fujinaga Y, Uchino F. Amyloid enhancing factor (AEF) in the aging mouse. Virchows Arch A Pathol Anat Histopathol. 1989;414(6):511–14.
- Okuda Y, Yamada T, Matsuura M, Takasugi K, Goto M. Ageing: a risk factor for amyloid A amyloidosis in rheumatoid arthritis. Amyloid. 2011;18(3):108–11.
- Gillmore JD, Lovat LB, Persey MR, Pepys MB, Hawkins PN. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet. 2001;358(9275):24–9.
- Lachmann HJ, Goodman HJB, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, Hawkins PN. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356(23):2361–71.
- Ravid M, Shapira J, Lang R, Kedar I. Prolonged dimethylsulphoxide treatment in 13 patients with systemic amyloidosis. Ann Rheum Dis. 1982;41(6):587–92.
- Dember LM, Hawkins PN, Hazenberg BPC, Gorevic PD, Merlini G, Butrimiene I, et al. Eprodisate for the treatment of renal disease in AA amyloidosis. N Engl J Med. 2007;356(23):2349–60.
- Özçakar ZB, Özdel S, Yılmaz S, Kurt-Şükür ED, Ekim M, Yalçınkaya F. Anti-IL-1 treatment in familial Mediterranean fever and related amyloidosis. Clin Rheumatol. 2016;35(2):441–6.
- Nakamura T, Kumon Y, Hirata S, Takaoka H. Abatacept may be effective and safe in patients with amyloid A amyloidosis secondary to rheumatoid arthritis. Clin Exp Rheumatol. 2014;32(4):501–8.
- Hagihara K, Nishikawa T, Isobe T, Song J, Sugamata Y, Yoshizaki K. IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem Biophys Res Commun. 2004;314(2):363–9.
- Hagihara K, Nishikawa T, Sugamata Y, Song J, Isobe T, Taga T, et al. Essential role of STAT3 in cytokine-driven NF-kappaB-mediated serum amyloid A gene expression. Genes Cells. 2005;10(11):1051–63.