Abstract
Objectives: To investigate the safety, effectiveness, and risk-benefit balance of intravenous abatacept (ABA) in non-elderly (<65 years: NEG) and elderly (≥65 years: EG) rheumatoid arthritis patients.
Methods: This sub-analysis of an all-cases postmarketing surveillance in Japan assessed safety in all enrolled patients and effectiveness in those with Disease Activity Score 28 based on C-reactive protein (DAS28-CRP) measurements at ≥2 time points including baseline. Risk-benefit was evaluated based on infections and DAS28-CRP improvement >1.2.
Results: The NEG and EG of the safety analysis set comprised 2,170 and 1,712 patients, respectively; corresponding 6-month ABA retention rates were 80.2% and 77.1%. The NEG had fewer adverse drug reactions (14.5% vs. 17.2%, p = .021) and infections (4.8% vs. 7.2%, p = .002) than the EG. DAS28-CRP changed similarly between groups. The proportion of patients with low-risk/high-benefit and high-risk/low-benefit were 33.1% and 6.9% (NEG) and 29.7% and 9.0% (EG). Low-risk/high-benefit patients were younger, had shorter disease duration and fewer comorbidities, and were with less use of oral glucocorticoid and prior biologics, more use of methotrexate and higher DAS28-CRP than high-risk/low-benefit patients at baseline.
Conclusion: ABA was well tolerated and similarly efficacious in the EG and NEG. Identification of factors related to low-risk/high-benefit may aid appropriate patient selection.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disorder in which chronic inflammation affects the synovial membrane of the joints, causing erosion of the cartilage and bone. Such chronic inflammation can lead to joint destruction and deformity, disability, and poor quality of life [Citation1,Citation2]. In recent years, there has been an increased interest in therapies targeted to elderly RA patients because in many countries, including Japan, the aging and super-aging populations are rapidly growing. The prevalence of elderly people (≥65 years) in the general Japanese population is estimated to have reached 27.2% in 2016, which is the highest percentage among developed countries [Citation3].
Previous studies have reported the safety and effectiveness of biologic disease-modifying antirheumatic drugs (bDMARDs) directed against tumor necrosis factor (TNF) and interleukin (IL)-6 receptor for the treatment of RA; caution has been raised regarding their use in elderly patients [Citation4,Citation5]. These reports suggested that the risk-benefit balance should be carefully considered, particularly for elderly patients who will receive these medications [Citation6–8]. Abatacept (ABA) is a fusion protein that has an extracellular domain for the human CTLA4 and Fc portion of human IgG1. ABA selectively inhibits T-cell activation by binding to CD80/86 and modulating its interaction with CD28 [Citation9].
An all-cases post-marketing surveillance (PMS) was required as a condition for the regulatory approval of intravenous ABA for the treatment of RA in Japan. The PMS of ABA was undertaken by Bristol-Myers Squibb K.K., under the guidance of the Japan College of Rheumatology. This PMS previously reported safety (n = 3,882) and effectiveness (n = 3,016) analyses after 6 months of treatment [Citation10]. However, given the growth of Japan’s elderly population and previous reports of the efficacy, safety, and risk-benefit balance of TNF antagonists and IL-6 receptor blockers, a sub-analysis of the efficacy, safety, and risk-benefit balance among the non-elderly and elderly patients would be of value.
Materials and methods
Study design and patients
The detailed methodology of this PMS study has been previously reported [Citation10]. Briefly, all patients with RA who received intravenous ABA after its approval in Japan (between September 2010 and June 2011) were registered at 772 sites for inclusion in the PMS study. ABA was administered according to the label for Japan as an intravenous infusion based on body weight: <60 kg, 500 mg; 60–100 kg, 750 mg; and >100 kg, 1,000 mg. ABA was subsequently administered at Weeks 0, 2, and 4. Patients were followed up once every 4 weeks during the 24-week treatment and 4-week follow-up periods [Citation11]. Data from all patients who received at least one dose of ABA were included in the safety evaluation. A total of 3,882 patients (excluding 103 patients who had received ABA in phase II or III clinical trials) were analyzed in the safety analysis set. In this study, patients who had Disease Activity Score 28 based on C-reactive protein (DAS28-CRP) data at one or more time points, in addition to the baseline data (n = 2,544), were included in the effectiveness analysis set II. Patients in both the safety analysis set and effectiveness analysis set II were categorized by age (<65 years: non-elderly group [NEG], ≥65 years: elderly group [EG]). The NEG and EG consisted of 2,170 and 1,712 patients, respectively. In the effectiveness analysis set II, the NEG and EG consisted of 1,461 and 1,083 patients, respectively.
Kaplan–Meier survival analysis
Reasons for drug discontinuation were obtained from case report forms and classified as adverse events, surgery, or death; insufficient effectiveness; remission; and others. Patients with more than one reason were classified by giving priority in the following order: adverse events, surgery, or death > insufficient effectiveness > remission > other. Retention rates based on reasons for discontinuation were estimated by Kaplan–Meier survival analysis and compared using the log-rank test.
Adverse drug reactions, serious adverse drug reactions, infections, and serious infections
Definitions of adverse drug reactions (ADRs), serious ADRs, infections, and serious infections have been described previously [Citation10]. Briefly, ADRs and infections were considered noxious and unintended responses for which a causal relationship with the study drug could not be ruled out. Serious ADRs or serious infections were any ADR or serious infection that led to death, was life-threatening, or caused hospitalization, prolongation of hospitalization, disability, or permanent injury during the observation period (24-week treatment and 4-week follow-up periods) [Citation12]. Incidence rates were analyzed at Week 24 of treatment.
Disease activities
In the effectiveness analysis set II, disease activity was evaluated using the DAS28-CRP, which takes into account the number of tender and swollen joints, general health status (patient visual analog scale [mm], 0–100), and CRP (mg/dL) [Citation13], at the start of ABA treatment (baseline) and at Weeks 4, 12, and 24 of treatment. DAS28-CRP was divided into four categories as follows: remission (DAS28-CRP ≤2.6), low disease activity (DAS28-CRP >2.6–≤3.2), moderate disease activity (DAS28-CRP >3.2–≤5.1), and high disease activity (DAS28-CRP >5.1). Percent changes in the distribution of patients in each disease activity category from baseline to Week 24 in the NEG and EG were analyzed. Changes in DAS28-CRP in the NEG and EG, in patients stratified by disease activity category at baseline, and in patients who were treated with or without concomitant methotrexate (MTX) at baseline were analyzed.
Probabilities of risk and benefit
Among the patients in the effectiveness analysis set II, 2,345 patients (1,362 patients from the NEG and 983 patients from the EG) who had available data for rheumatoid factors or anti-cyclic citrullinated peptide antibody at baseline in addition to their baseline demographics were included in the risk-benefit analysis set. The probabilities of both developing infections (risk) and improving DAS28-CRP >1.2 (benefit) were analyzed on each patient in the risk-benefit analysis set, based on the variables associated with the probabilities in a previously reported logistic model analysis [Citation10]. The variables for risk were sex, age, history of respiratory disease or respiratory comorbidities, liver disorders, history of allergy, prior use of bDMARDs, and concomitant use of oral corticosteroids. The variables for benefit were sex, age, disease severity, prior use of bDMARDs, rheumatoid factors or anti-cyclic citrullinated peptide antibody, and DAS28-CRP at baseline.
Statistical analysis
The incidence rate of ADRs and infections in the safety analysis set were determined using descriptive statistics. In the effectiveness analysis set II, disease activity was analyzed using the last observation carried forward (LOCF) method to impute data for withdrawals. Data are shown as median and interquartile range or mean ± standard deviation. Differences in the proportion of patients with remission and low disease activity between the NEG and EG at Week 24 were determined using the Wilcoxon two-sample test. The χ2 test for categorical variables and the Wilcoxon two-sample test or Student t-test for continuous variables, depending on their distribution, were used for comparisons between groups. Paired t-tests were used to compare changes in DAS28-CRP scores from baseline to Week 24. Statistical significance was defined as p ≤ .05 (two-tailed tests). The reported p values are nominal without adjusting for multiplicity. Data and statistical analyses were conducted using SAS V9.2 (SAS Institute Inc., Cary, NC).
Results
Patient disposition and baseline characteristics
The age distribution of the patients in the safety analysis set is shown in Supplementary Figure S1. ABA was most frequently prescribed to patients between 60 and <70 years of age (1,332 patients, 34.3%), followed by patients between 70 and <80 years of age (929 patients, 23.9%), and patients between 50 and <60 years of age (822 patients, 21.2%). Of 1,332 patients in the group between 60 and <70 years of age, 701 patients were between 60 and <65, and 631 patients were between 65 and <70. The patient characteristics of the safety analysis set and effectiveness analysis set II are summarized in . No significant differences in baseline characteristics were observed between the safety analysis set and effectiveness analysis set II (data not shown). In the safety analysis set, the mean age and median disease duration at baseline were 52.8 years and 7.8 years in the NEG and 72.2 years and 9.7 years in the EG, respectively.
Table 1. Demographic and baseline clinical characteristics.
The proportions of low-grade patients, in both their Steinbrocker stage and Steinbrocker class [Citation14], were higher in the NEG than in the EG, indicating the presence of more advanced disease in the EG compared with the NEG. Greater proportions of patients in the EG had past medical history and comorbidities (36.6% and 78.6%, respectively) compared with the NEG (23.1% and 62.3%, respectively). Greater proportions of patients were treated with concomitant MTX in the NEG (73.3%) than in the EG (57.5%), whereas the proportions of patients treated with oral glucocorticoid were similar between groups (NEG, 60.9%; EG, 65.8%). The proportions of patients with prior use of bDMARDs (NEG, 71.8%; EG, 66.8%) and baseline DAS28-CRP (NEG, 4.4 ± 1.3; EG, 4.6 ± 1.2) were similar between groups.
Reasons for treatment discontinuation and Kaplan–Meier analysis
The reasons for treatment discontinuation in the safety analysis set are summarized in Supplementary Table S1. Treatments were discontinued in 719 of 3,882 patients (18.5%) overall, and 367 of 2,170 patients (16.9%) in the NEG and 352 of 1,712 patients (20.6%) in the EG. Common reasons for discontinuation (number of discontinued patients [percentages relative to the number of discontinued patients]) were as follows: adverse events, surgery, or death (NEG, 80 [21.8%]; EG, 127 [36.1%]); insufficient effectiveness (NEG, 211 [57.5%]; EG, 155 [44.0%]); remission (NEG, 6 [1.6%]; EG, 1 [0.3%]); and ‘other’ (NEG, 70 [19.1%]; EG, 69 [19.6%]). Included in the ‘other’ reasons for treatment discontinuation were transfer to other hospitals (NEG, 32 [8.7%]; EG, 32 [9.1%]) and patient request or financial reasons (NEG, 28 [7.6%]; EG, 27 [7.7%]).
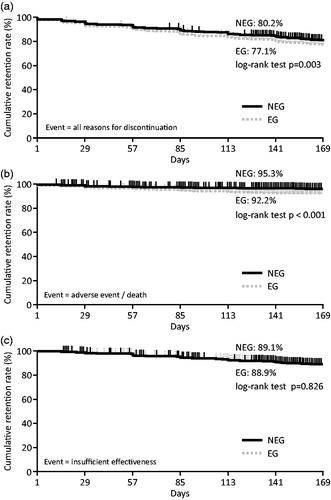
The ABA retention rate in the NEG and EG was analyzed by Kaplan–Meier survival analysis (). The ABA retention rates, based on the dropout events for any reason up to Week 24 in the NEG and EG, were 80.2% and 77.1% (p = .003), respectively (). When analyzing only dropout events caused by adverse events and death, the retention rates up to Week 24 in the NEG and EG were 95.3% and 92.2% (p < .001), respectively (). When analyzing only dropout events caused by insufficient effectiveness up to Week 24, retention rates were almost identical between groups (NEG, 89.1%, and EG, 88.9%; p = .826) ().
Figure 1. Kaplan–Meier survival analysis in the safety analysis set. Retention rates of NEG and EG in the safety analysis set (n = 3,882) were analyzed by Kaplan–Meier analysis. (a) Retention rates based on discontinuations for any reason. (b) Retention rates based on discontinuations caused by adverse events, surgery, or death. (c) Retention rates based on discontinuations caused by insufficient effectiveness. NEG: non-elderly group; EG: elderly group.
Incidence of ADRs and infections
The incidences of ADRs, serious ADRs, infections, and serious infections in the safety analysis set were analyzed in the NEG and EG. The incidence rate of 100 patient-years was calculated based on 1886.2 patient-years (Supplementary Figure S2). The incidence of ADRs was significantly lower in the NEG than the EG (14.5% [45.1 occurrences/100 patient years] vs 17.2% [50.2 occurrences/100 patient years]; p = .021). Similarly, incidences of serious ADRs and overall infections were also lower in the NEG than the EG (1.8% [5.4 occurrences/100 patient years] vs. 3.4% [7.7 occurrences/100 patient years]; p = .002) and (4.8% [12.2 occurrences/100 patient years] vs. 7.2% [16.6 occurrences/100 patient years]; p = .002), respectively. However, the incidence of serious infections was not significantly different (p = .163) between the NEG (0.8% [1.9 occurrences/100 patient years]) and the EG (1.3% [3.1 occurrences/100 patient years]).
Disease activity over time in the NEG and EG
The improvement of DAS28-CRP over time in the NEG and EG in the effectiveness analysis set II is shown in . The proportions of patients with low disease activity or remission in both the NEG and EG increased over 24 weeks (from 16.9% in the NEG and 11.4% in the EG at baseline to 54.1% in the NEG and 51.2% in the EG at Week 24). No significant intergroup difference was observed for the percentage of patients with low disease activity or remission at Week 24 (p = .148) (). The DAS28-CRP scores of patients in the effectiveness analysis set II decreased similarly in the NEG and EG (from 4.38 ± 1.26 in the NEG and 4.57 ± 1.19 in the EG at baseline to 3.20 ± 1.33 in the NEG and 3.33 ± 1.33 in the EG at Week 24) ().
Figure 2. Changes of DAS28-CRP in the NEG and EG over 24 weeks. Changes in DAS28-CRP scores in the NEG and EG in the effectiveness analysis set II were analyzed. Disease activity was categorized into remission (DAS28-CRP ≤2.6), low disease activity (DAS28-CRP >2.6–≤3.2), moderate disease activity (DAS28-CRP >3.2–≤5.1), and high disease activity (DAS28-CRP >5.1). Changes in DAS28-CRP (left panel) and ΔDAS28-CRP (right panel) at the indicated time point are shown in b, c, and d. (a) Percent changes in the distribution of patients in each disease activity category are shown. Statistical significance of remission and low disease activity between the NEG and EG at Week 24 was determined using the Wilcoxon two-sample test. (b) Changes in total patients. (c) Changes in patients stratified by disease activity category at baseline. (d) Changes in patients who were treated with or without concomitant MTX at baseline. DAS28-CRP, Disease Activity Score 28 based on C-reactive protein; MTX: methotrexate; NEG: non-elderly group; EG: elderly group.
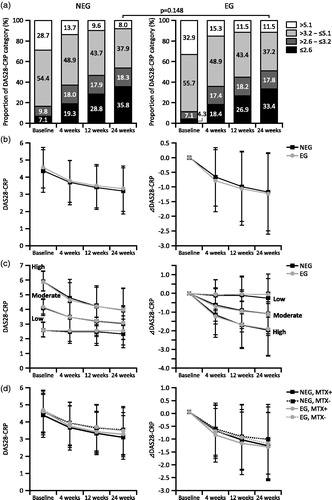
Patients in the effectiveness analysis set II were categorized by their baseline disease activities into the high disease activity group (NEG, 419 patients; EG, 356 patients), moderate disease activity group (NEG, 795; EG, 603), and low disease activity group (NEG, 247; EG, 124). DAS28-CRP decreased similarly in the NEG and EG of each category (). DAS28-CRP decreased the most from baseline to Week 24 in the high disease activity group (from 5.93 ± 0.67 in the NEG and 5.90 ± 0.66 in the EG at baseline to 3.97 ± 1.48 in the NEG and 3.99 ± 1.43 in the EG at Week 24), followed by the moderate disease activity group (from 4.12 ± 0.54 in the NEG and 4.19 ± 0.52 in the EG at baseline to 3.05 ± 1.11 in the NEG and 3.10 ± 1.15 in the EG at Week 24) and the low disease activity group (from 2.61 ± 0.47 in the NEG and 2.61 ± 0.50 in the EG at baseline to 2.35 ± 0.97 in the NEG and 2.56 ± 0.99 in the EG at Week 24). The magnitude of improvement in DAS28-CRP was found to be more dependent on the baseline disease activity and less dependent on age.
Changes in DAS28-CRP scores of the NEG and EG based on concomitant MTX use (NEG, 1,079 patients; EG, 643 patients) and no MTX use (NEG, 382 patients; EG, 440 patients) at baseline were also analyzed (). DAS28-CRP decreased similarly in the NEG and EG receiving concomitant MTX (from 4.34 ± 1.25 in the NEG and 4.55 ± 1.15 in the EG at baseline to 3.09 ± 1.28 in the NEG and 3.25 ± 1.28 in the EG at Week 24). Similar decreases in DAS28-CRP were observed in the NEG and EG not receiving concomitant MTX at baseline (from 4.51 ± 1.28 in the NEG and 4.61 ± 1.25 in the EG at baseline to 3.50 ± 1.41 in the NEG and 3.44 ± 1.39 in the EG at Week 24). The magnitude of improvement in DAS28-CRP was not greatly dependent on age or concomitant use of MTX at baseline.
Risk-benefit balance
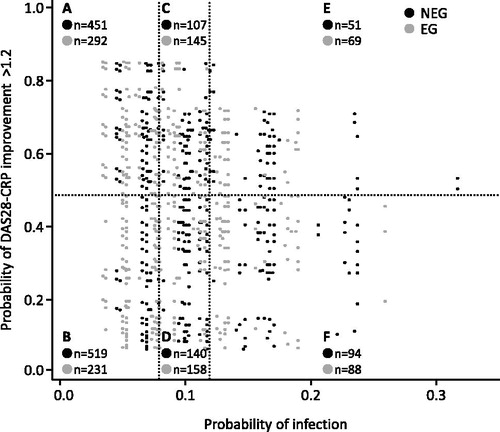
Patient disposition from enrollment to the risk-benefit analysis set is shown in Supplementary Figure S3. The probabilities of both developing infections and improving DAS28-CRP >1.2 in each patient in the risk-benefit analysis set were calculated. The risk was defined according to a 0–1 scale of the probability of developing an infection during the observation period, where 0 was no probability and 1 was the highest probability. The degrees of risk were divided into three segments: the low-risk segment (the probability of developing an infection was lower than the actual incidence of infections in the safety analysis set [7.96%]); high-risk segment (the probability was higher than 1.5-fold the percentage of the actual incidence [11.94%]); and moderate-risk segment (the probability was between that in the low- and high-risk segments). The benefit was defined according to a 0–1 scale of the probability of achieving DAS28-CRP improvement >1.2 at Week 24 (assessed using the LOCF method). The degrees of benefit were divided into two segments: the high-benefit segment (the probability of achieving a DAS28-CRP improvement >1.2 was higher than the actual score achieved in the effectiveness analysis set II [48.51%]) and the low-benefit segment (the probability was lower than that). The three risk groups and the two benefit groups were combined into six segments. Each patient of the risk-benefit analysis set was plotted on the matrix of the probability of developing an infection (x-axis) vs. the probability of improvement in DAS28-CRP >1.2 (y-axis) and belonged to one of the six segments (segments A–F, ).
Figure 3. Patient distribution in risk-benefit model analysis on the probability matrix. The probability of infection (risk) and of achievement of DAS28-CRP improvement >1.2 (benefit) of each patient included in the risk-benefit analysis set were calculated as described in the “Methods section”. Each patient was mapped on the risk-benefit matrix and belonged to one of six groups (defined in the Results section). DAS28-CRP, Disease Activity Score 28 based on C-reactive protein; NEG: non-elderly group; EG: elderly group.
The patient demographics of the risk-benefit analysis set are shown in the second column of . No apparent differences in patient characteristics were observed between the risk-benefit analysis set and safety analysis set ().
Table 2. Patient demographic and clinical characteristics for the risk-benefit model analysis (n = 2,345).
The proportion of non-elderly patients in the risk-benefit analysis set was 58.1% (95% confidence interval [CI] 56.1–60.1). Overall, 1,493 patients (NEG, 970; EG, 523) were assigned to the low-risk segments (sum of segments A and B). The proportion of non-elderly patients in the low-risk segments was 65.0% (95% CI 62.5–67.4), meaning that non-elderly patients were concentrated in the low-risk segments. Conversely, 302 patients (NEG, 145; EG, 157) were assigned to the high-risk segments (sum of segments E and F). The proportion of non-elderly patients in the high-risk segments was 48.0% (95% CI 42.3–53.8), meaning that elderly patients were concentrated in the high-risk segments. Additionally, 1,115 patients (NEG, 609; EG, 506) were assigned to the high-benefit segments (sum of segments A, C, and E), and 1,230 patients (NEG, 753; EG, 477) were assigned to the low-benefit segments (sum of segments B, D, and F). The proportion of non-elderly patients in the high-benefit and low-benefit segments were 54.6% (95% CI 51.6–57.6) and 61.2% (95% CI 58.4–64.0), respectively.
The incidence of infections, as well as the achievement of DAS28-CRP improvement >1.2 in the NEG and EG of each segment are shown in . Infections occurred in 55 of 970 patients (5.7%) in the NEG and 34 of 523 (6.5%) patients in the EG in low-risk segments (sum of segments A and B), compared with 21 of 145 (14.5%) patients in the NEG and 29 of 157 (18.5%) patients in the EG in high-risk segments (sum of segments E and F). Regarding benefit, 412 of 609 patients in the NEG (67.7%) and 347 of 506 patients in the EG (68.6%) in high-benefit segments (sum of segments A, C, and E) achieved a DAS28-CRP score improvement >1.2. In low-benefit segments (sum of segments B, D, and F), 245 of 753 patients in the NEG (32.5%) and 157 of 477 patients in the EG (32.9%) achieved a DAS28-CRP improvement >1.2.
Table 3. Number of events and proportions of patients in each group in the risk-benefit model analysis (n = 2,345).
Patient characteristics of segments A (low-risk/high-benefit segment) and F (high-risk/low-benefit segment) are compared in . The parameters indicating a significantly higher percentage/value in segment F were age, disease duration, comorbidities, prior use of bDMARDs, and concomitant oral glucocorticoid use. The parameters indicating a significantly higher percentage/value in segment A were concomitant MTX use and baseline DAS28-CRP score. Similar analyses were implemented separately for the NEG and EG. All patient characteristics were significantly different between segments A and F in the NEG. In the EG, all characteristics except age, disease duration, and Steinbrocker’s class were significantly different (Supplementary Table S2).
Discussion
An all-cases PMS was a mandatory condition in Japan to obtain regulatory approval of ABA and four other bDMARDs for the treatment of RA. The results for each bDMARD have been previously reported [Citation15–19]. During the conduct of these PMSs, the age distribution of the population shifted from middle-aged to elderly patients. For instance, in the infliximab PMS that began in 2004 [Citation15], the proportion of patients aged between 50 and 59 years was the highest compared with the other age groups. In a subsequent PMS of etanercept that began in 2005, the proportions of patients aged between 50 and 59 years and those aged 60–69 years were almost equal; these proportions were higher than the remaining age groups [Citation16]. In contrast, in the adalimumab PMS that began in 2008 [Citation18], as well as the ABA PMS that began in 2010 [Citation9], the proportion of patients aged between 60 and 69 years was the highest compared with the other age groups. Thus, studies focusing on the safety and effectiveness of bDMARDs in elderly patients have been recently gaining more relevance in Japan.
In this study, the incidence rates of ADRs, serious ADRs, and infections were significantly higher in the EG compared with the NEG. These results may reflect the differences in the background demographic and clinical characteristics, such as longer disease duration, higher proportions of patients with more advanced disease stages (Steinbrocker stage or class) and a greater number of comorbidities in patients in the EG compared with the NEG. Considering that the incidence rates of ADRs and adverse events in the PMS reports of other bDMARDs ranged between 20.95% and 43.9%, and those of serious ADRs and serious adverse events ranged between 4.5% and 9.6% [Citation15–19], ABA may be considered relatively safe for both non-elderly and elderly RA patients in relation to other bDMARDs. It is noteworthy that there were no significant differences in the incidence rates of serious infection between the NEG and EG, while older age was reported as one of the significant risk factors of serious infections in PMS of TNF inhibitors and an IL-6 inhibitor in Japan [Citation16–18]. The risk of serious infection, especially in elderly patients, is one of the main issues to consider when selecting a bDMARD in a clinical setting.
Regarding disease activity improvement, age has been reported as a determining factor for some bDMARDs [Citation4,Citation6,Citation20]. In contrast, some studies have reported that ABA improves disease activity less dependently on age [Citation21,Citation22], which is consistent with the results of this study. In the present PMS, the retention rates based only on dropout events caused by insufficient effectiveness at Week 24 were similar between the NEG and EG. This finding further supports previous reports in which the effectiveness of ABA was considered less dependent on age [Citation21,Citation22]. A substantial proportion of elderly patients seem to have a low tolerance for concomitant treatment with MTX compared with non-elderly patients because of decreased renal function and other comorbidities; thus, the availability of bDMARDs that are effective without concomitant MTX administration is one important factor in the selection of treatment for elderly patients. TNF inhibitors are more efficacious when they are used concomitantly with MTX [Citation23,Citation24], while ABA is reported to improve disease activity less dependently on concomitant use of MTX [Citation11,Citation25–27]. In this study, DAS28-CRP improved similarly in patients with or without concomitant use of MTX at baseline in both the EG and NEG. These results suggest that ABA may be a suitable treatment option for elderly patients who cannot tolerate optimal dosages of concomitant MTX or for those in whom MTX is not indicated.
The comparison of patient demographic and clinical characteristics between segments A and F in the risk-benefit analysis may prove useful to identify the ideal type of patients for whom ABA treatment can deliver the most benefit. Based on the present results, the risk-benefit balance seems to be better if ABA is used as a first bDMARD in RA patients <65 years old with short disease duration who have insufficient response to MTX. ABA may also be useful for patients in relatively difficult conditions, but the risk-benefit analysis clearly showed that rheumatologists should consider early treatment with ABA for bDMARD-naive, active RA patients.
The present study has some limitations. Of 3,882 patients in the safety analysis set, 1,338 patients were excluded because of insufficient DAS28-CRP data. There was a relatively small number of patients with high probability of infection in the risk-benefit analysis. However, the present study is the first to compare the risk-benefit balance between non-elderly and elderly patients treated with bDMARDs.
In conclusion, the risk-benefit balance of ABA was somewhat better for the NEG compared with the EG. The risk-benefit balance of ABA as a first bDMARD should be investigated in clinical settings.
Conflict of interest
YY, IC, and TN are employees of Bristol-Myers Squibb K.K.
MH, NI, SI, TM, JR, ST, TT, Y. Tanaka, Y. Takasaki, HY, and TK are members of the Postmarketing Surveillance (PMS) Committee of the Japan College of Rheumatology. It is the belief of the authors that this does not constitute a conflict of interest. The authors participated in review and analysis of the PMS data in their capacity as committee members.
Supplemental Material
Download MS Power Point (55 KB)Supplemental Material
Download MS Word (29.2 KB)Acknowledgements
The authors thank all the medical institutions and physicians who participated in this PMS for their cooperation. The authors would also like to thank Toru Yada of EPS Corporation for providing statistical analysis, and Keyra Martinez Dunn, MD, and JL Croxford, PhD, of Edanz Medical Writing for providing medical writing assistance, which was funded by Bristol-Myers Squibb K.K. and Ono Pharmaceutical Co. Ltd. The PMS was funded by Bristol-Myers Sruibb K.K.
References
- Silman AJ, Rheumatoid arthritis. In: Silman AJ, Hochberg MC, editors. Epidemiology of the Rheumatic Diseases. 2nd ed. New York, NY: Oxford University Press; 2002. p. 31–71.
- Pollard L, Choy EH, Scott DL. The consequences of rheumatoid arthritis: quality of life measures in the individual patient. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S43–S52.
- Statistics Bureau. Ministry of Internal Affairs and Communications Japan. Statistical Handbook of Japan 2015. http://www.stat.go.jp/english/data/handbook/pdf/2015all.pdf (accessed 26 Feb 2018).
- Pers YM, Schaub R, Constant E, Lambert J, Godfrin-Valnet M, Fortunet C. Efficacy and safety of tocilizumab in elderly patients with rheumatoid arthritis. Joint Bone Spine. 2015;82(1):25–30.
- Cho SK, Sung YK, Kim D, Won S, Choi CB, Kim TH, et al. Drug retention and safety of TNF inhibitors in elderly patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2016;17:333.
- Radovits BJ, Kievit W, Fransen J, van de Laar MA, Jansen TL, van Riel PL, et al. Influence of age on the outcome of antitumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68(9):1470–3.
- Genevay S, Finckh A, Ciurea A, Chamot AM, Kyburz D, Gabay C. Tolerance and effectiveness of anti-tumor necrosis factor alpha therapies in elderly patients with rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;57(4):679–85.
- Sugihara T, Harigai M. Targeting low disease activity in elderly-onset rheumatoid arthritis: current and future roles of biological disease-modifying antirheumatic drugs. Drugs Aging. 2016;33(2):97–107.
- Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561–9.
- Harigai M, Ishiguro N, Inokuma S, Mimori T, Ryu J, Takei S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2016;26(4):491–8.
- Takahashi N, Kojima T, Kaneko A, Kida D, Hirano Y, Fujibayashi T, et al. Longterm efficacy and safety of abatacept in patients with rheumatoid arthritis treated in routine clinical practice: effect of concomitant methotrexate after 24 weeks. J Rheumatol. 2015;42(5):786–93.
- ICH Steering Committee, ICH Harmonised Tripartite Guideline, Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting E2D. Recommended for Adoption at Step 4 of the ICH Process on 12 November 2013. https://www.pmda.go.jp/files/000156499.pdf
- Prevoo MLL, Van'T Hof MA, Kuper HH, Van Leeuwen MA, Van De Putte LBA, Van Riel PLCM. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8.
- Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc. 1949;140(8):659–62.
- Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, Yamanaka H, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67(2):189–94.
- Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, et al. Postmarketing surveillance of safety and effectiveness of etanercept in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2011;21(4):343–51.
- Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, et al. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol. 2014;41(1):15–23.
- Koike T, Harigai M, Ishiguro N, Inokuma S, Takei S, Takeuchi T, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of 7740 patients. Mod Rheumatol. 2014;24(3):390–8.
- Kanbori M, Suzuka H, Yajima T, Kishino E, Morishige R, Momohara S, et al. Postmarketing surveillance evaluating the safety and effectiveness of golimumab in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2018;28(1):66–75.
- Matsubara H, Kojima T, Kaneko A, Hirano Y, Ishikawa H, Hattori Y, et al. Long-term retention rate and risk factor for discontinuation due to insufficient efficacy and adverse events in Japanese patients with rheumatoid arthritis receiving etanercept therapy. J Rheumatol. 2014;41(8):1583–9.
- Lahaye C, Soubrier M, Mulliez A, Bardin T, Cantagrel A, Combe B, et al. Effectiveness and safety of abatacept in elderly patients with rheumatoid arthritis enrolled in the French Society of Rheumatology's ORA registry. Rheumatology (Oxford). 2016;55(5):874–82.
- Sekiguchi M, Fujii T, Matsui K, Murakami K, Morita S, Ohmura K, et al. Differences in predictive factors for sustained clinical remission with abatacept between younger and elderly patients with biologic-naive rheumatoid arthritis: results from the ABROAD study. J Rheumatol. 2016;43(11):1974–83.
- Kameda H, Kanbe K, Sato E, Ueki Y, Saito K, Nagaoka S, et al. Continuation of methotrexate resulted in better clinical and radiographic outcomes than discontinuation upon starting etanercept in patients with rheumatoid arthritis: 52-week results from the JESMR study. J Rheumatol. 2011;38(8):1585–92.
- Takeuchi T, Tanaka Y, Kaneko Y, Tanaka E, Hirata S, Kurasawa T, et al. Effectiveness and safety of adalimumab in Japanese patients with rheumatoid arthritis: retrospective analyses of data collected during the first year of adalimumab treatment in routine clinical practice (HARMONY study). Mod Rheumatol. 2012;22(3):327–38.
- Takahashi N, Kojima T, Terabe K, Kaneko A, Kida D, Hirano Y, et al. Clinical efficacy of abatacept in Japanese rheumatoid arthritis patients. Mod Rheumatol. 2013;23(5):904–12.
- Mochizuki T, Yano K, Ikari K, Hiroshima R, Takaoka H, Kawakami K, et al. The efficacy of abatacept in Japanese patients with rheumatoid arthritis: 104 weeks radiographic and clinical results in clinical practice. Mod Rheumatol. 2016;26(4):499–506.
- Nash P, Nayiager S, Genovese MC, Kivitz AJ, Oelke K, Ludivico C, et al. Immunogenicity, safety, and efficacy of abatacept administered subcutaneously with or without background methotrexate in patients with rheumatoid arthritis: results from a phase III, international, multicenter, parallel-arm, open-label study. Arthritis Care Res (Hoboken). 2013;65(5):718–28.
