Abstract
Objective: To evaluate the long-term safety and efficacy of subcutaneous tocilizumab (TCZ-SC) monotherapy administered weekly (qw) in patients with rheumatoid arthritis who had an inadequate response to TCZ-SC every other week (q2w).
Methods: Patients who completed 12 weeks of double-blind treatment with either TCZ-SC q2w monotherapy or TCZ-SC qw monotherapy were switched to or continued to receive open-label treatment with TCZ-SC qw monotherapy for 40 weeks. Safety and efficacy were assessed. Subgroup analyses of Disease Activity Score based on 28 joints using erythrocyte sedimentation rate (DAS28-ESR) were performed at 12 weeks.
Results: The incidence of adverse events was 464.4/100 patient-years (PY). The incidence of infection was 121.3/100 PY. The safety profile of TCZ-SC qw monotherapy was consistent with that of prior studies of TCZ. No additional safety concerns were observed. Improvement from baseline in DAS28-ESR was maintained at week 52 in patients who continued TCZ-SC qw and improved in patients who switched from TCZ-SC q2w to qw. At week 12, the efficacy of TCZ-SC qw monotherapy was greater than that of TCZ-SC q2w monotherapy irrespective of weight and BMI subgroups.
Conclusion: The long-term weekly dosing of TCZ-SC monotherapy was well tolerated and efficacy was maintained over 52 weeks.
Introduction
Tocilizumab (TCZ) is a humanized monoclonal antibody that targets the interleukin-6 receptor and is approved for the treatment of rheumatoid arthritis (RA), administered as either intravenous (IV) infusion or subcutaneous (SC) injection [Citation1–10]. The efficacy and safety of TCZ-SC in combination with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) was demonstrated in the global phase III clinical trials SUMMACTA and BREVACTA [Citation9,Citation10]. SUMMACTA demonstrated the non-inferiority of TCZ-SC 162 mg weekly (qw) to TCZ-IV 8 mg/kg every 4 weeks (q4w); BREVACTA demonstrated the superiority of TCZ-SC 162 mg every other week (q2w) to placebo. Long-term extensions of both SUMMACTA and BREVACTA have confirmed that the efficacy and safety of TCZ-SC is maintained over 2 years [Citation11,Citation12].
The phase III MUSASHI study demonstrated the non-inferiority of TCZ-SC 162 mg q2w monotherapy to TCZ-IV 8 mg/kg q4w monotherapy in Japanese patients with RA over 24 weeks [Citation8]. In the open-label extension period of MUSAHI, efficacy was adequately maintained in most patients who switched from TCZ‐IV q4w monotherapy to TCZ‐SC q2w monotherapy [Citation13]. In addition, during the open-label period, patients who had an inadequate response to TCZ-SC q2w could shorten their dosing interval to receive TCZ-SC qw, which resulted in improved efficacy [Citation14].
To confirm the efficacy and safety of TCZ-SC qw monotherapy, a randomized, double-blind, parallel-group, 12-week phase III study (SHINOBI) was conducted to compare the safety and efficacy of TCZ-SC qw monotherapy with that of TCZ-SC q2w monotherapy in Japanese patients with RA who had an inadequate response to TCZ-SC q2w [Citation15]. The study met its primary endpoint by demonstrating the superiority of TCZ-SC qw to TCZ-SC q2w for adjusted mean change in Disease Activity Score based on 28 joints using erythrocyte sedimentation rate (DAS28-ESR) from baseline to week 12; the adjusted difference in the change in DAS28-ESR between the TCZ-SC qw and TCZ-SC q2w groups was −1.21 (95% CI, −2.13 to −0.30; p = .0108).
Following the 12-week double-blind period of the SHINOBI study, all patients received open-label TCZ-SC qw monotherapy for 40 weeks. Here we present the results of the 40-week open-label extension (OLE) to evaluate the long-term safety and efficacy of TCZ-SC qw monotherapy in Japanese patients with RA who had an inadequate response to TCZ-SC q2w. In addition, the efficacy of TCZ-SC qw versus TCZ-SC q2w was examined across different subgroups of patients in the 12-week double-blind period.
Materials and methods
Study design
This was a 52-week, randomized, parallel-group phase III trial with a double-blind period of 12 weeks followed by a 40-week OLE in Japanese patients with RA (JapicCTI-142505). At the start of the double-blind period, patients were randomized 1:1 to receive either TCZ-SC 162 mg qw or TCZ-SC 162 mg q2w as monotherapy. After 12 weeks, all patients received open-label TCZ-SC 162 mg qw monotherapy for 40 weeks.
The eligibility criteria for participation have been described [Citation15]. Briefly, patients ≥20 years of age with RA for ≥6 months (1987 American College of Rheumatology [ACR] criteria) who had received TCZ-SC ≥4 times with the q2w regimen prior to enrollment and had an inadequate response were included. Inadequate response was defined as patients having a DAS28-ESR >3.2, a tender joint count (TJC) using 68 joints ≥4, a swollen joint count (SJC) using 66 joints ≥4 and a C-reactive protein (CRP) level ≥0.3 mg/dL. The study protocol was reviewed by the appropriate institutional review boards, and the study was performed in accordance with the ethical standards of the current version of the Declaration of Helsinki. All patients provided written informed consent to participate in the study.
Safety and immunogenicity assessment
Adverse events (AEs) were classified using the Medical Dictionary for Regulatory Activities (MedDRA), version 17.0. Laboratory parameters (e.g. white blood cells, platelets, lymphocytes, neutrophils, liver function tests, and lipid levels) were measured. The anti-TCZ antibody screening assay was performed as previously described using a bridging enzyme-linked immunosorbent assay with an additional competitive displacement step as the confirmation assay [Citation16].
Efficacy assessment
Disease activity was evaluated by DAS28-ESR; Clinical Disease Activity Index (CDAI); DAS28-ESR and CDAI response; 20%, 50%, and 70% improvement response criteria (ACR20/50/70); tender and swollen joint counts; patient and physician global assessment; patient pain; the Japanese Health Assessment Questionnaire [Citation17]; C-reactive protein levels and ESR.
Statistical analyses
Safety was evaluated for all patients who received ≥1 dose of TCZ-SC during the double-blind period; AEs were included in the analyses if they occurred during both the double-blind period and open-label period in both groups. The incidence rates of AEs were also evaluated for all patients who received ≥1 dose of TCZ-SC qw, including AEs occurring during the double-blind period in the TCZ-SC qw group and during the open-label period in both groups.
Change in DAS28-ESR from baseline at 12 weeks in the double-blind period was analyzed by previously defined subgroups with a last observation carried forward imputation. Each endpoint for the 52-week analyses was summarized descriptively without imputation for missing values.
All endpoints and analysis methods were pre-specified in the protocol and/or the statistical analysis plan. Since the superiority was reported previously [Citation15], statistical comparisons were not performed in this report in accordance with the American Statistical Association’s statement on p values [Citation18].
Pharmacokinetics
Serum TCZ concentration was measured as previously described [Citation8].
Results
Patient disposition and baseline characteristics
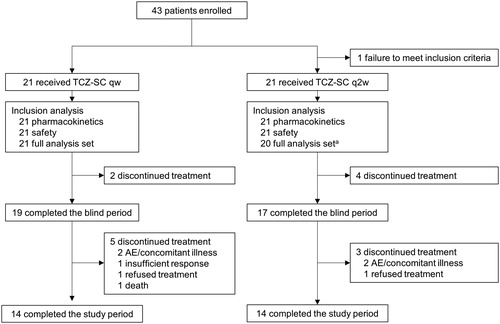
Of the 42 patients who received TCZ-SC in the 12-week double-blind period, 36 completed the blind period and continued in the OLE (). Of these 36 patients, 17 had received TCZ-SC q2w (TCZ-SC q2w/qw group) and 19 had received TCZ-SC qw (TCZ-SC qw/qw group) in the double-blind period. Fourteen patients in each group completed the OLE. Five patients in the TCZ-SC qw/qw group discontinued: 2 due to AE/concomitant illness and 1 each due to insufficient response, refusal of treatment, and death. Three patients in the TCZ-SC q2w/qw group discontinued treatment: 2 due to AE/concomitant illness and 1 due to refusal of treatment.
Figure 1. Patient disposition. AE: adverse event; TCZ-SC: subcutaneous tocilizumab; qw: weekly; q2w: every other week. a One patient was excluded due to having no evaluable measurements after study drug administration.
Baseline demographics, clinical characteristics, and history of treatment at baseline have been described previously [Citation15].
Safety
A total of 90.5% of patients experienced ≥1 AE during the 52 weeks of TCZ-SC monotherapy. The incidence of AEs was 464.4 per 100 patient-years (PY) (52 weeks, 28.9 PY, n = 38) with TCZ-SC qw administration (except the TCZ-SC q2w administration during the blind period). The most common AE by MedDRA system organ class was infections and infestations (54.8% [36 events in 23 patients]; and Supplemental Table 1), with nasopharyngitis being the most frequent. With TCZ-SC qw administration (except the TCZ-SC q2w administration during the blind period), the incidence of infections and infestations was 121.3 per 100 PY (52 weeks, 28.9 PY, n = 38). Skin and subcutaneous tissue disorders was the second most common AE (33.3% [15 events in 14 patients]), followed by gastrointestinal disorders (23.8% [14 events in 10 patients]) and investigations (21.4% [20 events in nine patients]). A total of 12 SAEs occurred in 8 patients (19.0%) in both groups (), including those occurring in two patients during the double-blind period as reported previously [Citation15]. Specifically, there was one event each of lymphoma, colon cancer, lung neoplasm malignant, septic shock, pneumonia, cellulitis, bile duct stone, cholecystitis, disseminated intravascular coagulation, aortic rupture, coronary artery stenosis, and epilepsy. Of these SAEs, a causal relationship to TCZ was ruled out for colon cancer, bile duct stone, cholecystitis, coronary artery stenosis, and epilepsy. Six patients experienced an SAE in the OLE period; SAEs resulted in discontinuation in three patients and one SAE resulted in death due to aortic rupture. This patient who died was female and 76 years old. She had abdominal pain approximately 28 weeks after treatment initiation and died the same day. A causal relationship to TCZ could not be ruled out. However, CT scan after her death revealed severe periaortic calcification; she also had concurrent diabetes and hyperlipidemia and was on oral steroid medication (10 mg/d); therefore, it is highly likely that the event was due to concurrent disease.
Table 1. Summary of adverse events (AEs) and serious adverse events (SAEs)Table Footnotea from baseline to week 52 by body system.
Laboratory parameters
No grade 3 or 4 laboratory abnormalities occurred for white blood cells, platelets, lymphocytes, neutrophils, liver function tests (aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, and bilirubin), or cholesterol. Three patients experienced a grade 3 increase in triglycerides; however, no cardiovascular and cerebrovascular events were reported. No additional findings were observed in the laboratory data.
Immunogenicity
During TCZ-SC qw administration, the proportion of patients who tested positive for anti-TCZ antibodies in the screening and confirmation assays was 0%.
Efficacy
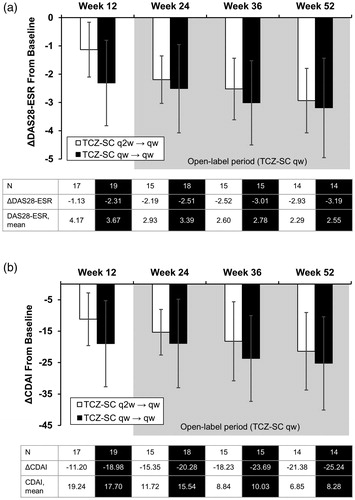
During the open-label period (when all patients received TCZ-SC qw), patients in the TCZ-SC q2w/qw group experienced improvements in DAS28-ESR 12 weeks after dose interval shortening. The mean DAS28-ESR was maintained in the TCZ-SC qw/qw group during the open-label period (). As previously reported, from baseline to week 12, patients receiving TCZ-SC qw had greater improvement in DAS28-ESR than those receiving TCZ-SC q2w [Citation15]; however, mean (SD) change in DAS28-ESR from baseline to week 52 was similar between the TCZ-SC q2w/qw and TCZ-SC qw/qw groups (−2.93 [1.14] and −3.19 [1.76], respectively). Similarly, mean CDAI scores and mean change from baseline in CDAI at week 52 improved in the TCZ-SC q2w/qw group (). Efficacy was maintained in the TCZ-SC qw/qw group in the open-label period. Mean (SD) change in CDAI from baseline to week 52 was similar between the TCZ-SC q2w and TCZ-SC qw/qw groups (−21.38 [12.33] and −25.24 [14.84], respectively). Overall, the efficacy of TCZ-SC q2w/qw mostly reached that of TCZ-SC qw/qw.
Figure 2. Mean change in DAS28-ESR and CDAI from baseline to week 52. (a) Mean change in DAS28-ESR from baseline to week 52. (b) Mean change in CDAI from baseline to week 52. CDAI: Clinical Disease Activity Index; DAS28-ESR: Disease Activity Score based on 28 joints using erythrocyte sedimentation rate; TCZ-SC: subcutaneous tocilizumab; qw: weekly; q2w: every other week. Error bars represent the standard deviation.
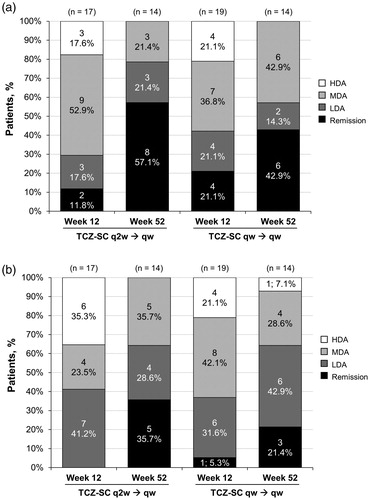
The proportions of patients achieving DAS28-ESR low disease activity (LDA) or remission was improved or maintained from week 12 to week 52 in both the TCZ-SC q2w/qw and TCZ-SC qw/qw groups. In the TCZ-SC q2w/qw group, three patients (21.4%) achieved LDA (DAS28-ESR ≥2.6 to ≤3.2) and eight patients (57.1%) achieved remission (DAS28-ESR <2.6) at week 52. In the TCZ-SC qw/qw group, two patients (14.3%) achieved DAS28-ESR LDA and six patients (42.9%) achieved DAS28-ESR remission at week 52 ( and Supplemental Table 2). Similar improvement from week 12 to week 52 was observed in the proportions of patients achieving CDAI LDA or remission. In the TCZ-SC q2w/qw group, four patients (28.6%) achieved LDA (CDAI >2.8 to ≤10), and five patients (35.7%) achieved remission (CDAI ≤2.8) at week 52. In the TCZ-SC qw/qw group, six patients (42.9%) and three patients (21.4%) achieved CDAI LDA and remission, respectively, at week 52 ( and Supplemental Table 2).
Figure 3. DAS28-ESR and CDAI response at weeks 12 and 52. (a) DAS28-ESRa response at weeks 12 and 52. (b) CDAIb response at weeks 12 and 52. CDAI: Clinical Disease Activity Index; DAS28-ESR: Disease Activity Score based on 28 joints using erythrocyte sedimentation rate; HDA: high disease activity; LDA: low disease activity; MDA: moderate disease activity; TCZ-SC: subcutaneous tocilizumab; qw: weekly; q2w: every other week. a Remission was defined as DAS28-ESR <2.6; LDA as DAS28-ESR ≥2.6 to ≤3.2; MDA as DAS28-ESR >3.2 to ≤5.1; and HDA as DAS28-ESR >5.1. bRemission was defined as CDAI ≤2.8; LDA as CDAI >2.8 to ≤10; MDA as CDAI >10 to ≤22; and HDA as CDAI >22.
A subgroup analysis of the mean change in DAS28-ESR during the double-blind period (baseline to week 12) was performed (). In each subgroup, the TCZ-SC qw group showed greater improvement than the TCZ-SC q2w group in terms of the mean change in DAS28-ESR. The trend was more evident in patients with high body weight, body mass index (BMI), baseline DAS28-ESR, or baseline CRP.
Table 2. Mean change in DAS28-ESR from baseline to week 12 by subgroup.
Discussion
This OLE period of the SHINOBI study evaluated the long-term safety and efficacy of TCZ-SC 162 mg qw monotherapy in Japanese patients with RA. The results of the double-blind period of the SHINOBI study demonstrated that shortening the dosing interval of TCZ-SC q2w to qw was a tolerable and effective treatment option for patients with RA who had an inadequate response to TCZ-SC q2w monotherapy. The OLE period of the SHINOBI study confirmed that the tolerability and efficacy of TCZ-SC qw monotherapy was maintained over an additional 40 weeks of TCZ-SC qw treatment.
Although the background of the patient population is different, the incidence of AEs (464.4 per 100 PY [52 weeks, 28.9 PY, n = 38]) and the AE safety profile of TCZ-SC qw in this study were similar to those observed in previous clinical trials of TCZ-SC q2w and TCZ-IV q4w, and also those observed over 108 weeks of TCZ treatment in the MUSASHI study (498.3 per 100 PY [108 weeks, 639.0 PY, n = 346]) [Citation14]. The incidence of AEs was also comparable to that observed with pooled TCZ-IV monotherapy studies (465.1 per 100 PY [2188 PY, n = 601]) [Citation19]. Infection, a known risk factor with TCZ treatment, was the most frequently reported AE. The incidence of infection (121.3 per 100 PY [52 weeks, 28.9 PY, n = 38]) during treatment with TCZ-SC qw monotherapy in the SHINOBI study was not increased compared with that of TCZ-SC q2w and TCZ-IV q4w monotherapy (138.3 per 100 PY [108 weeks, 639.0 PY, n = 346]) in the MUSASHI study, and was comparable to the incidence of infections and infestations observed with pooled TCZ-IV monotherapy studies (129.2 per 100 PY [2188 PY, n = 601]) [Citation19]. The incidence of infection remained consistent throughout the study (Supplemental Table 1). However, in the long-term extensions of SUMMACTA and BREVACTA, the incidences of AEs with TCZ-SC q2w and TCZ-SC qw in combination with csDMARDs were 415.9 per 100 PY (97 weeks, 1013 PY, n = 631) and 332.8 per 100 PY (96 weeks, 616.0 PY, n = 437), respectively [Citation11, Citation12]. In ACT-SURE, a multinational phase IIIb study of TCZ-IV, the incidence of AEs in patients who had previously received tumor necrosis factor (TNF) inhibitors (653.6 per 100 PY [24 weeks, 132.4 PY, n = 298]) was higher than in patients who were TNF-inhibitor naïve (551.1 per 100 PY [24 weeks, 452.1 PY, n = 976]) [Citation20]. In TOZURA, a multinational phase IV study of TCZ-SC qw, the incidences of AEs with TCZ monotherapy and in combination with csDMARDs were 622.1 per 100 PY (24 weeks, 175.7 PY, n = 353) and 622.5 per 100 PY (24 weeks, 767.6 PY, n = 1451), respectively [Citation21]. The incidences of AEs were similar between patients receiving TCZ-IV q4w, TCZ-SC q2w and TCZ-SC qw with no additional safety concerns. In the SHINOBI study, the incidence of SAE (19.0%) appeared to be high for some reason. This may have been due to not only the limited number of patients in this study but also the patient population, in which all patients had an inadequate response to TCZ-SC q2w and also included patients with an inadequate response to other biologic DMARDs. As this clinical study had criteria for careful monitoring by the investigators during TCZ dosing, careful monitoring of patients with attention to infections will be important during dosing of TCZ in clinical practice.
In a prior study of TCZ-SC qw (SUMMACTA) [Citation9], weekly administration of TCZ-SC began at TCZ initiation. In the BREVACTA study [Citation10, Citation12], escape therapy with TCZ-SC qw was available, and favorable tolerability and safety were reported. However, these studies were not designed to include randomized control arms of dose–interval shortening, and data were limited on efficacy improvement after dose interval shortening of TCZ-SC following an inadequate response to TCZ-SC q2w. The present study is the first report of a randomized, controlled study of dose–interval shortening of TCZ-SC. Our data confirmed that dose–interval shortening of TCZ-SC q2w to TCZ-SC qw is feasible and clinically meaningful. Subgroup analyses of the efficacy of TCZ-SC qw in the 12-week double-blind period of this study showed that the efficacy was not influenced by body weight or BMI categories. In contrast, TCZ-SC q2w was less effective in patients of higher body weight (≥70 kg) or BMI (≥25 kg/m2). In addition, the difference between qw and q2w was greater in patients with higher baseline DAS28-ESR (≥5.1) or CRP (≥1 mg/dL). These data suggest that although TCZ-SC qw appears to benefit all groups of patients with an inadequate response to TCZ-SC q2w, patients with higher body weight, higher BMI, DAS28-ESR and/or CRP may be particularly likely to benefit from TCZ-SC qw compared with TCZ-SC q2w.
Maintenance of long-term efficacy is important for the management of RA. A limitation of this study is that the design had a double-blind period in which half of the patients received TCZ-SC q2w for 12 weeks; therefore, in half of the patients who received TCZ-SC qw after week 12, the safety of TCZ-SC qw was not assessed for the entire 52 weeks. As the small sample size of this study limits the ability to draw definitive conclusions on the safety profile of TCZ-SC qw, further studies using real-world data in Japan would be one possible method to understand the overall safety of TCZ-SC qw. Data on the efficacy of extending the dose intervals after a good clinical response following TCZ-SC qw dosing remain to be determined. From the previous TCZ-SC studies, including the SHINOBI study, the clinical response to TCZ-SC was observed within 12 weeks. If patients attain good clinical response after dose–interval shortening to TCZ-SC qw, extending the dosing interval up to q2w after TCZ-SC qw administration for 12 weeks should be considered. In contrast, if clinical response with TCZ-SC qw administration is not seen within 12 weeks, continuation of the treatment plan should be carefully reconsidered [Citation22].
Conclusion
The long-term safety profile of TCZ-SC qw monotherapy in patients with RA who experienced an inadequate response to prior TCZ-SC q2w was consistent with that observed in previous clinical trials of TCZ. The sustainable efficacy of long-term administration of TCZ-SC qw indicated that shortening the TCZ-SC dosing interval up to qw might be considered as a treatment option for patients with RA.
Conflicts of interest
A. Ogata, personal fees: Chugai, Daiichi Sankyo, Mitsubishi-Tanabe, AYUMI, Bristol-Myers Squibb, Janssen, Asahi-Kasei Pharma, Sanofi, Novartis, GlaxoSmithKline. Y. Tanaka, grants: Chugai, Mitsubishi Tanabe, Takeda, Daiichi-Sankyo, Bristol-Myers Squibb, MSD, Astellas, AbbVie, Eisai, Ono, Taisho-Toyama; personal fees: Chugai, Mitsubishi Tanabe, Takeda, Daiichi-Sankyo, Bristol-Myers Squibb, Astellas, AbbVie, Eisai, Asahi-Kasei, YL Biologics, Sanofi, Janssen, Eli-Lilly, GlaxoSmithKline, UCB, Novarits. T. Ishii, grants: Chugai; speakers bureaus: Ono, Astellas, Pfizer, Chugai, AbbVie, Mitsubishi Tanabe, Janssen, Bristol-Myers Squibb, Teijin, Daiichi-Sankyo, Takeda. M. Kaneko, grants: Chugai; personal fees: Chugai, Eisai, Bristol-Myers Squibb, Janssen, Pfizer, UCB Japan, Asahi Kasei, Mitsubishi Tanabe, AbbVie, Ono, Astellas. H. Miwa, employee/personal fees: Chugai. S. Ohsawa, employee/personal fees: Chugai. R. Yamakawa, employee/personal fees: Chugai.
Data sharing statements
We provide qualified researchers access to individual patient level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details of Chugai’s Data Sharing Policy are available here (www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html).
Supplemental Material
Download MS Word (28.6 KB)Acknowledgements
The authors thank all investigators for their contribution to the implementation of the study. This study was supported by Mai Morioka and pharmacokinetic analysis was provided by Jun Tanaka, of Chugai Pharmaceutical Co., Ltd. Support for third-party writing assistance for this manuscript, provided by Ellen Mercado, PhD, of Health Interactions, Inc., was supported by F. Hoffmann-La Roche Ltd.
Additional information
Funding
References
- Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67(11):1516–23.
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58(10):2968–80.
- Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69(1):88–96.
- Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63(3):609–21.
- Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–97.
- Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66(9):1162–7.
- Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19(1):12–9.
- Ogata A, Tanimura K, Sugimoto T, Inoue H, Urata Y, Matsubara T, et al. Phase III study of the efficacy and safety of subcutaneous versus intravenous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(3):344–54.
- Burmester GR, Rubbert-Roth A, Cantagrel A, Hall S, Leszczynski P, Feldman D, et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis. 2014;73(1):69–74.
- Kivitz A, Olech E, Borofsky M, Zazueta BM, Navarro-Sarabia F, Radominski SC, et al. Subcutaneous tocilizumab versus placebo in combination with disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(11):1653–61.
- Burmester GR, Rubbert-Roth A, Cantagrel A, Hall S, Leszczynski P, Feldman D, et al. Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA). Ann Rheum Dis. 2016;75(1):68–74.
- Kivitz A, Olech E, Borofsky MA, Zazueta B, Navarro-Sarabia F, Radominski SC, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45(4):456–64.
- Ogata A, Atsumi T, Fukuda T, Hirabayashi Y, Inaba M, Ishiguro N, et al. Sustainable efficacy of switching from intravenous to subcutaneous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67(10):1354–62.
- Ogata A, Amano K, Dobashi H, Inoo M, Ishii T, Kasama T, et al. Longterm safety and efficacy of subcutaneous tocilizumab monotherapy: results from the 2-year open-label extension of the MUSASHI study. J Rheumatol. 2015;42(5):799–809.
- Ogata A, Tanaka Y, Ishii T, Kaneko M, Miwa H, Ohsawa S. A randomized, double-blind, parallel-group, phase III study of shortening the dosing interval of subcutaneous tocilizumab monotherapy in patients with rheumatoid arthritis and an inadequate response to subcutaneous tocilizumab every other week: results of the 12-week double-blind period. Mod Rheumatol. 2018;28(1):76–84.
- Stubenrauch K, Wessels U, Birnboeck H, Ramirez F, Jahreis A, Schleypen J. Subset analysis of patients experiencing clinical events of a potentially immunogenic nature in the pivotal clinical trials of tocilizumab for rheumatoid arthritis: evaluation of an antidrug antibody ELISA using clinical adverse event-driven immunogenicity testing. Clin Ther. 2010;32(9):1597–609.
- Matsuda Y, Singh G, Yamanaka H, Tanaka E, Urano W, Taniguchi A, et al. Validation of a Japanese version of the Stanford Health Assessment Questionnaire in 3,763 patients with rheumatoid arthritis. Arthritis Rheum. 2003;49(6):784–8.
- Wasserstein RL, Lazar NA. The ASA's statement on p-values: context, process, and purpose. Am Statistician. 2016;70(2):129–33.
- Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20(3):222–32.
- Bykerk VP, Ostor AJ, Alvaro-Gracia J, Pavelka K, Ivorra JA, Graninger W, et al. Tocilizumab in patients with active rheumatoid arthritis and inadequate responses to DMARDs and/or TNF inhibitors: a large, open-label study close to clinical practice. Ann Rheum Dis. 2012;71(12):1950–4.
- Choy E, Caporali R, Xavier R, Fautrel B, Sanmarti R, Bao M, et al. Subcutaneous tocilizumab in rheumatoid arthritis: findings from the common-framework phase 4 study programme TOZURA conducted in 22 countries. Rheumatology (Oxford). 2018;57(3):499–507.
- Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77.
Appendix
The names and institutions of the investigators of the SHINOBI study group are listed below:
Tatsuya Atsumi (Hokkaido University), Masaya Mukai (Sapporo City General Hospital), Tomonori Ishii (Tohoku University Graduate School of Medicine), Yasuhiko Munakata (Northern Capital Clinic), Yasuhiko Hirabayashi (Hikarigaoka Spellman Hospital), Koichi Amano (Saitama Medical Center, Saitama Medical University), Hayato Nagasawa (Saitama Medical Center, Saitama Medical University), Masato Moriguchi (Rabbit Clinic), Motohide Kaneko (Kaneko Internal Medicine Rheumatology Clinic), Wataru Hirose (Hirose Clinic of Rheumatology), Hisashi Yamanaka (Tokyo Women’s Medical University), Yuko Kaneko (Keio University), Ryutaro Matsumura (National Hospital Organization Chiba-East Hospital), Shigeto Tohma (National Hospital Organization Sagamihara National Hospital), Toshihisa Kojima (Nagoya University Graduate School of Medicine), Daihei Kida (National Hospital Organization Nagoya Medical Center), Teiji Kontani (Morita Hospital), Atsushi Ogata (Osaka University Graduate School of Medicine), Hitoshi Goto (Osaka City University Graduate School of Medicine), Kentaro Inui (Osaka City University Graduate School of Medicine), Makoto Hirao (Osaka University Graduate School of Medicine), Kenrin Shi (Osaka University Graduate School of Medicine), Taro Kuritani (Osaka Rheumatology Clinic), Yuji Yamanishi (Hiroshima Rheumatology Clinic), Hiroaki Dobashi (Kagawa University), Yasuaki Okuda (Dohgo Spa Hospital), Shinichi Mizuki (Matsuyama Red Cross Hospital), Yoshiya Tanaka (University of Occupational and Environmental Health), Tomoya Miyamura (National Hospital Organization Kyushu Medical Center), Hisaaki Miyahara (National Hospital Organization Kyushu Medical Center), Masakazu Kondo (Kondo Clinic for Rheumatology and Orthopaedics), Yukitaka Ueki (Sasebo Chuo Hospital), Atsushi Kawakami (Nagasaki University Graduate School of Biomedical Sciences), and Toshihiko Hidaka (Shimin-no-Mori Hospital).
