Abstract
Tocilizumab (TCZ) is an interleukin-6 (IL-6) inhibitor used for the treatment of rheumatoid arthritis (RA). It was developed in 2008, and its effectiveness is supported by evidence from all over the world based on its first decade of use. Although the overall efficacy and safety profiles of TCZ are similar to those of tumor necrosis factor (TNF) inhibitors, TCZ displays certain differences. The most notable advantage of TCZ is its usefulness as a monotherapy. Additionally, TCZ is favorable in the improvement of systemic inflammatory symptoms such as anemia and fatigue. The low immunogenicity of TCZ contributes favorably to long-term drug retention. Due to frequent relapse after TCZ cessation, TCZ use should be tapered beyond remission. During TCZ therapy, C-reactive protein (CRP) is unable to recognize disease activity and the severity of infection. The most common adverse events (AEs) are infection and abnormalities in laboratory findings including dyslipidemia, neutropenia, thrombocytopenia, and abnormality of liver enzymes. TCZ obscures the symptoms of infection. Therefore, stealth infections without obvious CRP elevation can sometimes cause severe damage to patients. Lower intestinal perforation is an uncommon but serious AE in TCZ therapy. Further clinical investigations will continue to refine the IL-6 inhibitory strategy.
Introduction
Interleukin-6 (IL-6) is a major pro-inflammatory cytokine with pleiotropic functions [Citation1]. Rheumatoid arthritis (RA) is a chronic autoimmune disorder, and IL-6 is a key player of immune activation and inflammation in RA. Therefore, IL-6 inhibition is a compelling strategy to control RA. Recently, several IL-6 inhibitors have become available for clinical use. Here we overview the recent advances in the IL-6 inhibition strategy for RA treatment.
Pathogenic role of IL-6 in RA
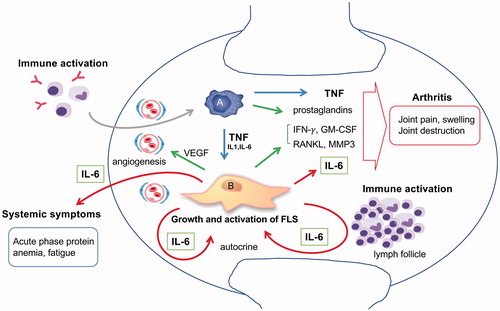
Increased levels of IL-6 have been detected in the sera and synovial fluid (SF) of RA patients [Citation2]. Relatively higher IL-6 concentrations in SF indicated that the source of IL-6 was inflamed synovium caused by RA. Normal synovium consists of the intimal lining layer and the synovial sub-lining. In RA, thickening of the intimal lining layer, lymphocytic infiltration, formation of lymphoid follicles, and increased blood vessels can be observed in the synovium’s so-called pannus formation [Citation3]. The normal intimal lining is comprised of bone marrow-derived macrophages known as type A synoviocytes and resident fibroblast-like type B synoviocytes (FLSs). Type A synoviocytes mainly produce tumor necrosis factor (TNF), and FLSs mainly produce IL-6 [Citation4,Citation5]. The growth and activation of FLSs is autocrinally stimulated by TNF and IL-6. Such tumor-like FLS proliferations generate thickening of the intimal lining layer. Activated FLS also produces many bioactive substances including MMP, RANKL, VEGF, GM-CSF, and IFN. These, in turn, generate arthritic symptoms including joint pain, swelling, bone erosion, and cartilage destruction. IL-6 is released systemically and generates systemic symptoms including fatigue, anemia, and acute phase reactions. IL-6 also induces immune activation [Citation6] and triggers the vicious circle of escalating RA disease activity. summarizes the role of IL-6 in RA pathogenesis.
Figure 1 The pathogenic role of IL-6 in RA synovitis. There are the two main cellular components in the synovium. Type A synoviocytes are bone marrow derived macrophage-like cells. Type B synoviocytes are residential fibroblast like cells also known as FLS. In the rheumatoid synovium, FLS mainly contributes to arthritis by producing IL-6, RANKL, MMP3, GM-CSF, IFN-γ, and VEGF. TNF and IL-6 stimulate FLS autocrinally and induce tumor-like proliferation of FLS. Local arthritic symptoms are generated by the collaboration of IL-6 with TNF- and FLS-derived cytokines. Systemic symptoms are caused by IL-6. IL-6 also contributes to immune activation and leads to synovitis, resulting in a vicious circle.
As an animal arthritis model, SKG mice spontaneously develop T cell-mediated chronic autoimmune arthritis because of a mutation in ZAP-70, which is a key signal transduction molecule in T cells. In SKG mice, an IL-6 deficiency completely inhibits arthritis [Citation7]. In collagen-induced arthritis (CIA), which is one of the most commonly used models of RA, blockades of both TNF and IL-6 inhibit arthritis [Citation8,Citation9]. By contrast, a blockade of TNF but not of IL-6 inhibited collagen antibody-induced arthritis, which eliminates the immune reaction of anticollagen antibody development [Citation10]. These findings indicate that IL-6 is a particularly important autoimmune factor in RA.
Efficacy of the IL-6 inhibitor in RA
A humanized IL-6R antibody, tocilizumab (TCZ), was developed by collaborative research between the Chugai Pharmaceutical Company and Osaka University in Japan [Citation11]. After seven major randomized clinical trials (RCTs), an intravenous formulation of TCZ (TCZ-IV) was approved for RA treatment in Japan in 2008, in Europe in 2009, and in the USA in 2010 [Citation12]. Recently, a subcutaneous formulation of TCZ (TCZ-SC) was developed in consideration of patients’ preferences. After a non-inferiority study of TCZ-SC and TCZ-IV [Citation13], TCZ-SC was approved in Japan and the USA in 2013 and in Europe in 2014.
As shown in , TCZ displays a favorable efficacy in many RA patients, including disease-modifying anti-rheumatic drug (DMARD) naïve patients and patients with an inadequate response to conventional synthetic DMARDs (csDMARDs), methotrexate (MTX), or TNF inhibitors (TNFi) [Citation12]. The overall efficacy including clinical responses and the radiographic damage progression of TCZ was comparable to that of other biological DMARDs (bDMARDs) such as TNFi [Citation14,Citation15]. The favorable efficacy of TCZ was also confirmed in real world clinical practice [Citation16].
Table 1. The efficacy of TCZ in clinical study.
IL-6 particularly affects psychosomatic functioning, sleep-related symptoms, and fatigue [Citation17,Citation18]; therefore, TCZ improves sleep quality and fatigue [Citation19,Citation20]. The symptoms of sleep disturbance and fatigue are significantly correlated with diminished patient quality of life. Moreover, TCZ improved patient-reported outcomes according to the modified Health Assessment Questionnaire (mHAQ) including reduced morning stiffness and favorable EQ-5D responses [Citation21,Citation22]; it also ameliorated work impairment [Citation23].
The amelioration of iron metabolism by IL-6 contributes to anemia associated with chronic inflammation. IL-6 induces the production of hepcidin, which is a liver-made peptide, proposed to be a central regulator of intestinal iron absorption and iron recycling by macrophages [Citation18]. IL-6 also decreases transferrin, which is the primary iron transporter that delivers iron to the bone marrow for erythropoiesis [Citation24]. Therefore, TCZ inhibits anemia associated with chronic inflammation [Citation25]. In addition, TCZ improves insulin resistance [Citation26] and may reduce HbA1c levels in diabetic patients with RA [Citation27]. Given that IL-6 induces the serum amyloid A (SAA) protein [Citation28], longstanding IL-6 production can evoke amyloid A amyloidosis, which is a serious renal complication in RA patients [Citation29]. TCZ inhibits SAA production and eliminates amyloid deposition in amyloid A amyloidosis [Citation30].
After TCZ therapy, inflammatory markers such as C-reactive protein (CRP) rapidly normalize. However, clinical symptoms improve gradually. Normal CRP is not directly correlated with clinical improvement of arthritic symptoms during TCZ therapy. The discordance between the improvement of inflammatory markers and clinical symptoms causes some confusion. Notably, normal CRP is only a marker of reaching a sufficient concentration of TCZ to inhibit IL-6 function; it is not a disease activity marker [Citation31]. Therefore, inflammatory marker-containing composite measures, such as disease activity score-28 joints (DAS-28) or the simple disease activity index, may be unreliable for disease monitoring. A composite measure that does not contain any inflammatory markers such as the clinical disease activity index may be reliable for TCZ efficacy monitoring.
At the start of TCZ administration, serum IL-6 levels increase temporally. Therefore, the cessation of TCZ sometimes induces a disease flare. The so-called “Bathtub theory” illustrates the mechanism [Citation12,Citation31]. Given that TCZ does not inhibit IL-6 production directly, interfering with IL-6 binding to its receptor causes unbound IL-6 to accumulate in the serum. After the abrogation of immune activation, IL-6 serum levels gradually decrease. Thus, decreased CRP levels are not directly correlated with decreased IL-6 levels in TCZ therapy.
The efficacy of TCZ is dose dependent. The standard dose of TCZ for RA treatment is 8 mg/kg every 4 weeks (q4w). However, massive and continuous IL-6 producing diseases such as Castleman’s disease or systemic juvenile idiopathic arthritis require TCZ-IV every 2 weeks (q2w). In RA patients who have high levels of IL-6 (high body weight or high disease activity), the efficacy of TCZ-SC q2w is sometimes insufficient, so reducing the interval between TCZ administrations, such as once a week, is preferable for disease control [Citation32]. By contrast, a prolonged dose interval of TCZ-IV or TCZ-SC can sustain remission in RA patients who have low levels of IL-6 production [Citation33,Citation34].
The low necessity of MTX for maximizing the efficacy of TCZ is notable. Monotherapy with etanercept and adalimumab did not show superiority to MTX in the TEMPO and PREMIRE studies, which suggests the essential role of MTX in TNFi for RA treatment [Citation35,Citation36]. By contrast, TCZ monotherapy was superior to MTX in the AMBITION, SAMURAI, and SATORI studies [Citation37–39]. Overall efficacy of TCZ was summarized in [Citation37–47]. In the ACT-RAY study, no clinically relevant superiority of the TCZ combination with csDMARDs over TCZ monotherapy for established RA patients was observed [Citation48]. In the SURPRISE study, TCZ combination therapy with csDMARDs suppressed inflammation more rapidly than TCZ monotherapy, but the protocols became comparable at week 52 [Citation49]. In the FUNCTION study, TCZ was similarly effective as monotherapy and in combination with MTX for the treatment of early (≤2-year disease duration) MTX-naïve RA [Citation50]. In the U-ACT-Early study, TCZ with or without MTX was similarly more effective for sustained remission without an increased safety risk compared to MTX for the treatment of newly diagnosed csDMARD-naïve early RA [Citation51]. These studies suggest that MTX is not essential for the antirheumatic effect of TCZ. Recently, a head-to-head comparative study of TNFi monotherapy and TCZ monotherapy was performed. The ADACTA study demonstrated that TCZ monotherapy was superior to adalimumab monotherapy [Citation52]. However, the radiographic progression was inhibited to a greater degree in TCZ combination therapy with csDMARDs compared to TCZ monotherapy [Citation49,Citation53]. The combination of csDMARDs may have an additive effect with TCZ. Based on these accumulated findings, the 2016 update of The European League Against Rheumatism recommendations for the management of RA stated that “IL-6 pathway inhibitors may have some advantage in patients who cannot use csDMARDs as comedication” [Citation54].
The antigenicity of TCZ is low [Citation55]. Therefore, the overall drug survival of TCZ is favorable [Citation56,Citation57]. The drug survival of TCZ may be longer than that of TNFi [Citation58]. Termination primarily occurs because of adverse events (AEs) rather than a lack of efficacy. The sustainability of TCZ-free remission was examined. In the DREAM study, 13.4% of patients maintained low disease activity for one year after TCZ (monotherapy) cessation [Citation59]. In the ACT-RAY study, 8.6% of the add-on arm group (MTX + TCZ) and 3.1% of the switch arm group (TCZ without MTX) maintained remission at 52 weeks after TCZ cessation [Citation60]. In the SURPRISE study, sustained remission rates of 24% for the add-on arm group (MTX + TCZ) and 14% for the switch arm group (TCZ without MTX) were reported one year after TCZ cessation [Citation61]. These findings indicate that long-term TCZ-free remission is difficult to maintain. Although most patients eventually experienced flares, the restart of TCZ led to rapid improvement in disease activity [Citation60,Citation62]. By contrast, the dose reduction of TCZ [Citation26,Citation27] and the cessation of comedications such as corticosteroids or MTX displayed success in sustaining remission in RA patients [Citation63,Citation64]. The strategy after achieving remission remains unclear, but tapering TCZ may sustain remission to a greater degree in patients who achieved remission with TCZ therapy.
Safety of the IL-6 inhibitor in RA
The overall safety outcomes are summarized in [Citation16,Citation45,Citation48,Citation65–71]. The most common AEs are infection and abnormal laboratory findings including neutropenia, thrombocytopenia, hyperlipidemia, and abnormality of liver enzymes such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The network meta-analysis of controlled trials and open-label studies demonstrated that the safety profiles of TCZ were comparable to those of TNFi [Citation72,Citation73]. However, daily clinical practice including post-marketing surveillance (PMS) in Japan suggests that the incidence of AEs during TCZ therapy appears to have increased compared to other biologics in clinical practice [Citation16,Citation74]. One possible explanation is the incidence of stealth infections, which can cause severe damage to patients because TCZ obscures the symptoms of infection [Citation75]. Overlooking early symptoms of infection such as cough, sputum, nasal discharge, or sore throat when receiving TCZ therapy in daily clinical practice is a problem. However, most patients experience the above-mentioned signs or symptoms of infection before developing a serious infection [Citation76]. Since the symptoms of infection are relatively mild during TCZ therapy, patients may postpone hospitalization; however, the increasing severity of the infection can significantly harm the patients. Patient education and accuracy of safety management in daily clinical practice is important.
Table 2. AEs of TCZ.
A previous report demonstrated that bDMARDs in RA are associated with an increased risk of opportunistic infections (OIs), such as tuberculosis (TB) and herpesvirus-related infections, particularly in long-lasting disease [Citation77]. There is no convincing evidence for the increased risk of Pneumocystis jirovecii pneumonia (PCP) or fungal infections [Citation77]. The overall rate of OIs did not differ significantly between TCZ and TNFi therapies [Citation78]. Among RA patients, the rates and adjusted hazard ratios of herpes zoster were similar among biologics, including TCZ [Citation79]. The risk of reactivation of latent TB is not increased during TCZ treatment [Citation80]. TB antigen-induced IFN-γ production is inhibited by TNFi but not by TCZ [Citation81], suggesting that TB immunity may not inhibited by TCZ. Therefore, TCZ therapy may be safer than TNFi therapy in Tb infection. The incidence of PCP was similar among biologics, including TCZ [Citation82]. The risk of reactivation of the hepatitis B virus (HBV) by TCZ is controversial [Citation83,Citation84]. Latent HBV infection, however, should be treated before TCZ therapy to prevent de novo hepatitis.
Neutropenia is frequently observed in RA patients undergoing TCZ therapy. However, neutropenia in patients taking TCZ does not appear to be associated with serious infections [Citation85]. Although IL-6 can stimulate stem cell proliferation and the formation of multilineage blast cell colonies, IL-6 knockout mice displayed normal steady-state hematologic parameters [Citation86]. TCZ does not directly affect neutrophil functions [Citation87]. By contrast, IL-6 mobilizes neutrophils into the circulating pool from the marginated pool, which includes the lymph nodes and spleen [Citation88]. Therefore, neutropenia induced by TCZ may reflect a shift of neutrophils to the marginated pool rather than myelosuppression. Although neutrophil recruitment to inflammation sites may be delayed, the overall host defense to infection is not significantly compromised by TCZ-induced neutropenia.
Thrombocytopenia is also frequently observed during TCZ therapy. Baseline platelet production is dependent on thrombopoietin (TPO), and IL-6 stimulates thrombopoiesis through TPO induction [Citation89]. Therefore, mice lacking TPO or its receptor c-Mpl are profoundly thrombocytopenic whereas IL-6 deficient mice are not thrombocytopenic [Citation90]. In normal physiology, platelets are continuously produced by megakaryocytes via an IL-6 independent process of platelet formation. Therefore, TCZ-induced thrombocytopenia was not associated with serious bleeding events in clinical trials.
Dyslipidemia is a common AE in TCZ therapy. In general population, dyslipidemia is associated with higher cardiovascular (CV) risk. However, this adverse alteration in the lipid profile by TCZ administration does not increase CV risk. Recent reports have demonstrated that the CV risk of TCZ were comparable to that of TNFi which reduce the risk for acute coronary syndrome [Citation91–93]. In RA, reductions in high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, and total cholesterol have been reported. Despite the reduction of lipids in the sera, RA patients have an increased CV risk, and the dampening of inflammation increases lipid moieties and reduces the risk of CV disease in RA patients [Citation94]. Inflammation is linked with accelerated atherosclerosis and associated with a paradoxical inversion of the relationship between CV risk and lipid levels in patients with untreated RA, recently coined the lipid paradox. IL-6 may up-regulates the expression of scavenger receptors, promotes foam cell formation, and enhances atherogenesis [Citation95,Citation96]. TCZ inhibits lipid uptake to atherosclerotic lesions, and non-uptake lipids may overflow into the serum.
Transaminase (AST and ALT) elevations induced by TCZ are frequent, but serious liver enzyme abnormalities are rare [Citation97]. Most of the transaminase elevations occurred during the first year of treatment, and the incidence of liver enzyme abnormalities increased in patients who received MTX/DMARDs. In most cases, the serious increase in transaminase levels returns to normal after TCZ termination, and this elevation rarely repeats. The abundant expression of IL-6R on hepatocytes may reflect the direct liver damage caused by TCZ. However, TCZ does not have antibody-dependent cellular cytotoxicity activity. In addition, the incidence of elevated liver enzymes by the IL-6 ligand antibody Sirukumab is similar to that of TCZ [Citation98], suggesting that the cause of liver damage is interfering with the IL-6 signal rather than direct toxicity. The main function of IL-6 in the liver is the induction of acute phase proteins such as CRP and SAA [Citation28,Citation99]. IL-6 also helps protect against liver damage and participates in regeneration of the liver [Citation100]. Therefore, TCZ may interfere with recovery from liver injuries, such as those caused by MTX.
Gastrointestinal (GI) perforation is an uncommon but serious AE in TCZ therapy. The incidence of GI perforation was significantly increased in TCZ therapy compared to all other treatments [Citation101,Citation102]. In particular, the risk of lower GI tract perforation was significantly elevated with TCZ therapy. Physicians must be aware that lower GI perforation under TCZ treatment may not present typical symptoms such as acute abdominal pain. Diverticulitis or other GI conditions and corticosteroids are risk factors. In mice, IL-6 stimulates intestinal epithelial proliferation, decreases intestinal injury, and improves barrier function following ischemia reperfusion of the small bowel [Citation103,Citation104]. IL-6 null mice exhibited impaired recovery following massive enterectomy and increased apoptosis [Citation104]. Therefore, TCZ may interfere with recovery from intestinal injuries caused by diverticulitis or other GI conditions. We should be aware that lower GI perforation may occur with only mild symptoms and without CRP elevation during TCZ therapy [Citation102].
The incidence of malignancies in patients who were treated with TCZ is comparable to that of general RA patients. It is well known that the risk of lung and lymphocyte malignancies is increased in RA patients compared to the general population [Citation105]. The incidence of all malignancies from TCZ therapy combined, excluding non-melanoma skin cancer (NMSC), was slightly increased compared to the general population, but the incidence of overall or site-specific malignancies was not increased [Citation106]. The risks of malignancy excluding NMSC in RA patients who are initiating TCZ therapy versus TNFi are comparable [Citation107]. IL-6 plays a prominent role in tumorigenesis and metastasis [Citation108]. The modulation of immune cell function by IL-6 causes dysfunction of innate and adaptive immunity against tumors [Citation109]. Therefore, the IL‐6 targeting approach may desensitize and prevent the dysfunction of innate and adaptive immunity against tumors. Additionally, an IL-6 blockade does not affect CD19 chimeric antigen receptor (CAR) T cell-driven antileukemic cytotoxicity [Citation110]. Therefore, TCZ is the mainstay pharmacologic therapy for cytokine release syndrome, which is a systemic inflammatory response caused by cytokines released by CAR T cell therapy [Citation111].
IL-6 deficient mice displayed significantly delayed cutaneous wound healing compared to wild-type control animals [Citation112]. This characteristic occurred because IL-6 is a growth factor of epidermal keratinocytes [Citation113]. The American College of Rheumatology guidelines recommend five-week TCZ-IV holding and two-week TCZ-SC holding prior to surgery in patients undergoing arthroplasty; moreover, TCZ should be restarted after careful assessment of the patient’s wound status and surgical and nonsurgical site infections [Citation114]. However, no increase in complications of superficial or deep infections or delays in wound healing after joint surgery were reported in patients who ceased TCZ-IV administration two weeks prior to surgery and restarted TCZ two weeks after surgery [Citation115]. The incidence of delayed wound healing in TCZ-treated RA patients was higher in patients with foot and spinal surgeries [Citation116]. We should be aware that mild injuries often cause phlegmon without obvious symptoms during TCZ therapy. Wounds must be disinfected to prevent phlegmon development.
There is limited information on pregnancy outcomes in women exposed to TCZ. In an analysis of global pregnancy-related reports, an increased preterm birth rate was observed [Citation117]. In Japan, the spontaneous abortion rate of TCZ-exposed women is comparable to the rate in the general population [Citation118]. Both reports demonstrated no indication of a substantially increased malformation risk [Citation117,Citation118]. Although IL‐6 plays important roles in reproductive performance, embryonal development, parturition, and postnatal development, IL‐6 signal blockade with a rat antimouse IL-6 receptor antibody (MR16‐1) and IL-6-deficient mice revealed no biologically important effects on fertility, embryonic implantation, or prenatal/postnatal development [Citation119,Citation120]. However, the expression of IL-6 is reduced in the endometrium of women with recurrent miscarriage and in the fetal-placental tissue of abortion prone CBA × DBA/2 mice, suggesting that insufficient local IL-6 levels may contribute to fetal loss [Citation121]. We must be cautious regarding the administration of TCZ therapy during pregnancy.
Recent advancements in IL-6 inhibitors
A fully human monoclonal antibody against IL-6R, Sarilumab (SAR), was developed by Regeneron and Sanofi. SAR was approved in Japan, the US, and the EU in 2017. The affinity of SAR for the human IL-6R is greater than that of TCZ, and it has a prolonged half-life. The overall efficacy and safety of SAR seems to be comparable with TCZ in standard clinical doses [Citation122,Citation123]. By contrast, a fully human anti-IL-6 monoclonal antibody, Sirukumab (SIR), was withdrawn in 2017 because it increased overall mortality. The most commonly reported causes of death (n = 30) were major adverse CV events (MACE) (13/29), infection (7/29), and malignancies (4/29). Although IL-6 has a possible protective effect on cardiac ischemia reperfusion injury [Citation124,Citation125], there was no previous association with MACE with IL-6 inhibitors [Citation66].
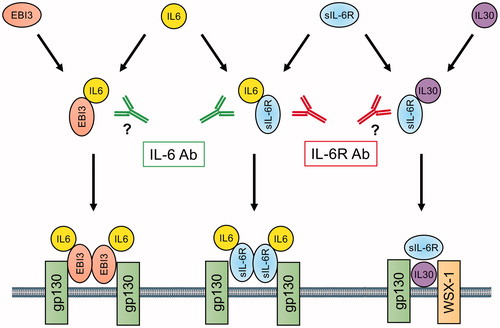
Interestingly, a recent report demonstrated that IL-6 can activate the MAPK cascade without sIL-6R in cardiomyocytes [Citation126]. If so, the IL-6 inhibitor but not the IL-6R inhibitor may reduce MAPK activation, which is associated with the cardioprotective effect of IL-6. Attritionary wound healing and dextran sodium sulfate-induced colitis were also interfered in IL-6 deficient mice but not in IL-6R deficient mice [Citation127,Citation128]. These results suggest the existence of alternative IL-6 signaling pathways that do not use IL-6R. A recent report demonstrated that the Epstein-Barr virus-induced gene 3 (EBI3), which is a subunit of the composite cytokines IL-27, IL-35, and IL-39, can mediate IL-6 trans-signaling to gp130 [Citation129]. This pathway does not use IL-6R: the IL-6 inhibitor, but the not IL-6R inhibitor, may interfere with EBI3-dependent signaling. In addition, IL-30, the p28 subunit of the heterodimeric cytokine IL-27, binds IL-6R and modulates inflammatory responses [Citation130,Citation131]. The IL-6R inhibitor, but not the IL-6 inhibitor, may interfere with IL-30 signaling. These characteristics can distinguish between the effects of the IL-6 inhibitor and the IL-6R inhibitor ().
Figure 2 Differential inhibitory function of IL-6 signaling pathways by IL-6 and IL-6R antibody; a possible mechanism of action. Complexes of IL-6/IL-6R, IL-30/IL-6R, and IL-6/EBI3 can activate gp130, a cell surface signal transduction molecule. IL-6R antibody inhibit IL-6/IL-6R and IL-30/IL-6R but not IL-6/EBI3 dependent signal, whereas IL-6 antibody inhibits IL-6/IL-6R and IL-6/EBI3 but not IL-30/IL-6R dependent signal. IL-6: Interlukin-6; IL-6R: interleukin-6 receptor; IL-30: Interleukin-30; EBI3: Epstein-Barr virus-induced 3; WSX-1: interleukin-27 receptor α.
Conclusion
The IL-6 signaling inhibitory strategy for RA treatment is efficacious and tolerable. Features of the IL-6 inhibitors have been elucidated by many studies assessing TCZ treatment. There are currently multiple IL-6 inhibitors from which to choose (TCZ-IV, 4 or 8 mg/kg q4w; TCZ-SC, 162 mg qw/q2w; and SAR, 150 mg/200 mg q2w). The proper use of IL-6 inhibitors requires further determination. Future clinical investigations will help us determine the best use of IL-6 inhibitors.
Conflict of interest
AO received honoraria for speech from Chugai Pharmaceutical Co, Asahi Kasei Pharm Co, Sanofi KK, Janssen Pharmaceutical KK, Pfizer Co, Bristol-Myers Squibb Co, Eisai Co, GlaxoSmithKline KK, Novartis Co, AYUMI Pharmaceutical Co, and consultant fee from Chugai Pharmaceutical Co. YK and SH have no conflicts of interest to disclosure. KY is a patent holder on the applied patent for the clinical use of tocilizumab on Still’s disease. KY also received lecture fee from Chugai Pharmaceutical Co.
Acknowledgement
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- Kishimoto T. Interleukin-6: from basic science to medicine-40 years in immunology. Annu Rev Immunol. 2005;23:1–21.
- Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31(6):784–8.
- Ospelt C. Synovial fibroblasts in 2017. RMD Open. 2017;3(2):e000471.15
- Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum. 1997;40(6):1096–105.
- MacNaul KL, Hutchinson NI, Parsons JN, Bayne EK, Tocci MJ. Analysis of IL-1 and TNF-alpha gene expression in human rheumatoid synoviocytes and normal monocytes by in situ hybridization. J Immunol. 1990;145:4154–66.
- Schinnerling K, Aguillon JC, Catalan D, Soto L. The role of interleukin-6 signalling and its therapeutic blockage in skewing the T cell balance in rheumatoid arthritis. Clin Exp Immunol. 2017;189(1):12–20.
- Hata H, Sakaguchi N, Yoshitomi H, Iwakura Y, Sekikawa K, Azuma Y, et al. Distinct contribution of IL-6, TNF-α, IL-1, and IL-10 to T cell? mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114(4):582–8.
- Sasai M, Saeki Y, Ohshima S, Nishioka K, Mima T, Tanaka T, et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42(8):1635–43.
- Tada Y, Ho A, Koh DR, Mak TW. Collagen-induced arthritis in CD4- or CD8-deficient mice: CD8+ T cells play a role in initiation and regulate recovery phase of collagen-induced arthritis. J Immunol. 1996;156(11):4520–6.
- Kagari T, Doi H, Shimozato T. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J Immunol. 2002;169(3):1459–66.
- Sato K, Tsuchiya M, Saldanha J, Koishihara Y, Ohsugi Y, Kishimoto T, Bendig MM. Reshaping a human antibody to inhibit the interleukin 6-dependent tumor cell growth. Cancer Res. 1993;53(4):851–6.
- Ogata A, Hirano T, Hishitani Y, Tanaka T. Safety and efficacy of tocilizumab for the treatment of rheumatoid arthritis. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:27–42.
- Ogata A, Morita T, Yoshida Y, Tanaka T. Subcutaneous formulation of tocilizumab for treatment of rheumatoid arthritis. Ther Deliv. 2015;6(3):283–95.
- Turkstra E, Ng SK, Scuffham PA. A mixed treatment comparison of the short-term efficacy of biologic disease modifying anti-rheumatic drugs in established rheumatoid arthritis. Curr Med Res Opin. 2011;27(10):1885–97.
- Jones G, Darian-Smith E, Kwok M, Winzenberg T. Effect of biologic therapy on radiological progression in rheumatoid arthritis: what does it add to methotrexate? Biologics 2012;6:155–61.
- Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol. 2014;4:15–23.
- ohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012;1261:88–96.
- Maes M, Scharpé S, Meltzer HY, Bosmans E, Suy E, Calabrese J, Cosyns P. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993;49(1):11–27.
- Fragiadaki K, Tektonidou MG, Konsta M, Chrousos GP, Sfikakis PP. Sleep disturbances and interleukin 6 receptor inhibition in rheumatoid arthritis. J Rheumatol. 2012;39(1):60–2.
- Gossec L, Steinberg G, Rouanet S, Combe B. Fatigue in rheumatoid arthritis: quantitative findings on the efficacy of tocilizumab and on factors associated with fatigue. The French multicentre prospective PEPS Study. Clin Exp Rheumatol. 2015;33:664–70.
- Townes SV, Furst DE, Thenkondar A. The impact of tocilizumab on physical function and quality of life in patients with rheumatoid arthritis: a systematic literature review and interpretation. Open Access Rheumatol. 2012;4:87–92.
- Harrold LR, John A, Reed GW, Haselkorn T, Karki C, Li Y, et al. Impact of tocilizumab monotherapy on clinical and patient-reported quality-of-life outcomes in patients with rheumatoid arthritis. Rheumatol Ther. 2017;4(2):405–17.
- Tanaka Y, Kameda H, Saito K, Kaneko Y, Tanaka E, Yasuda S, et al. IL-6 affect the Effect of subcutaneous tocilizumab treatment on work/housework status in biologic-naïve rheumatoid arthritis patients using inverse probability of treatment weighting: FIRST ACT-SC study. Arthritis Res Ther. 2018;20:151.
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–6.
- Nakazawa S, Tomosugi N, Kawabata H, Ishikawa T, Nishikawa T, Yoshizaki K. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood 2010;116(18):3627–34.
- Fève B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5(6):305–11.
- Ogata A, Morishima A, Hirano T, Hishitani Y, Hagihara K, Shima Y, et al. Improvement of HbA1c during treatment with humanised anti-interleukin 6 receptor antibody, tocilizumab. Ann Rheum Dis. 2011;70(6):1164–5.
- Hagihara K, Nishikawa T, Isobe T, Song J, Sugamata Y, Yoshizaki K. IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem Biophys Res Commun. 2004;314:363–9.
- Yoshizaki K. Basic and Clinical Significance of Interleukin 6 (IL-6) AA Amyloidosis. Proceedings of the XIIIth International Symposium on Amyloidosis; 2012 May 6–10, Groningen, Netherlands. p. 394–7.
- Nishida S, Hagihara K, Shima Y, Kawai M, Kuwahara Y, Arimitsu J, et al. Rapid improvement of AA amyloidosis with humanised anti-interleukin 6 receptor antibody treatment. Ann Rheum Dis. 2009;68(7):1235–6.
- Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008;112(10):3959–64.
- Ogata A, Atsumi T, Fukuda T, Hirabayashi Y, Inaba M, Ishiguro NK, et al. Results of switching from intravenous to subcutaneous tocilizumab monotherapy in patients with rheumatoid arthritis: extension of the MUSASHI study. Arthritis Care Res (Hoboken). 2015;67(10):1354–62.
- Saiki O, Uda H. Successful extension of tocilizumab infusion intervals from 4 weeks to 6 or 5 weeks in 90% of RA patients with good response to 4-week intervals. Clin Exp Rheumatol. 2017;35(4):666–70.
- Ogata A, Amano K, Dobashi H, Inoo M, Ishii T, Kasama T, et al. Longterm safety and efficacy of subcutaneous tocilizumab monotherapy: results from the 2-year open-label extension of the MUSASHI study. J Rheumatol. 2015;42(5):799–809.
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37.
- Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363(9410):675–81.
- Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69(1):88–96.
- Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66(9):1162–7.
- Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–9.
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58(10):2968–80.
- Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis.2008;67(11):1516–23.
- Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371(9617):987–97.
- Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63(3):609–21.
- Ogata A, Tanimura K, Sugimoto T, Inoue H, Urata Y, Matsubara T, et al. Phase III study of the efficacy and safety of subcutaneous versus intravenous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(3):344–54.
- Burmester GR, Rubbert-Roth A, Cantagrel A, Hall S, Leszczynski P, Feldman D, et al. Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA). Ann Rheum Dis. 2016;75(1):68–74.
- Kivitz A, Olech E, Borofsky M, Zazueta BM, Navarro-Sarabia F, Radominski SC, et al. Subcutaneous tocilizumab versus placebo in combination with disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(11):1653–61.
- Ogata A, Tanaka Y, Ishii T, Kaneko M, Miwa H, Ohsawa S, et al. A randomized, double-blind, parallel-group, phase III study of shortening the dosing interval of subcutaneous tocilizumab monotherapy in patients with rheumatoid arthritis and an inadequate response to subcutaneous tocilizumab every other week: results of the 12-week double-blind period. Mod Rheumatol. 2018;28:76–84.
- Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, Mola EM, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis. 2013;72(1):43–50.
- Kaneko Y, Atsumi T, Tanaka Y, Inoo M, Kobayashi-Haraoka H, Amano K, et al. Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study). Ann Rheum Dis. 2016;75(11):1917–23.
- Burmester GR, Rigby WF, van Vollenhoven RF, Kay J, Rubbert-Roth A, Kelman A, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75(6):1081–91.
- Bijlsma JWJ, Welsing PMJ, Woodworth TG, Middelink LM, Pethö-Schramm A, Bernasconi C, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016;388(10042):343–55.
- Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–50.
- Dougados M, Kissel K, Conaghan PG, Mola EM, Schett G, Gerli R, et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study. Ann Rheum Dis. 2014;73(5):803–9.
- Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–77.
- Burmester GR, Choy E, Kivitz A, Ogata A, Bao M, Nomura A, et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(6):1078–85.
- Leffers HC, Ostergaard M, Glintborg B, Krogh NS, Foged H, Tarp U, et al. Efficacy of abatacept and tocilizumab in patients with rheumatoid arthritis treated in clinical practice: results from the nationwide Danish DANBIO registry. Ann Rheum Dis. 2011;70(7):1216–22.
- Hishitani Y, Ogata A, Shima Y, Hirano T, Ebina K, Kunugiza Y, et al. Retention of tocilizumab and anti-tumour necrosis factor drugs in the treatment of rheumatoid arthritis. Scand J Rheumatol. 2013;42(4):253–9.
- Choy EH, Bernasconi C, Aassi M, Molina JF, Epis OM. Treatment of rheumatoid arthritis with anti-tumor necrosis factor or tocilizumab therapy as first biologic agent in a global comparative observational study. Arthritis Care Res (Hoboken). 2017;69(10):1484–94.
- Nishimoto N, Amano K, Hirabayashi Y, Horiuchi T, Ishii T, Iwahashi M, et al. Drug free REmission/low disease activity after cessation of tocilizumab (Actemra) Monotherapy (DREAM) study. Mod Rheumatol. 2014;24(1):17–25.
- Huizinga TW, Conaghan PG, Martin-Mola E, Schett G, Amital H, Xavier RM, et al. Clinical and radiographic outcomes at 2-years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT-RAY study. Ann Rheum Dis. 2015;74(1):35–43.
- Kaneko Y, Kato M, Tanaka Y, Inoo M, Kobayashi-Haraoka H, Amano K, et al. Tocilizumab discontinuation after attaining remission in patients with rheumatoid arthritis who were treated with tocilizumab alone or in combination with methotrexate: results from a prospective randomised controlled study (the second year of the SURPRISE study). Ann Rheum Dis. 2018;77(9):1268–75.
- Nishimoto N, Amano K, Hirabayashi Y, Horiuchi T, Ishii T, Iwahashi M, et al. Retreatment efficacy and safety of tocilizumab in patients with rheumatoid arthritis in recurrence (RESTORE) study. Mod Rheumatol. 2014;24(1):26–32.
- Fortunet C, Pers YM, Lambert J, Godfrin-Valnet M, Constant E, Devilliers H, et al. Tocilizumab induces corticosteroid sparing in rheumatoid arthritis patients in clinical practice. Rheumatology (Oxford). 2015;54(4):672–7.
- Kremer JM, Rigby W, Singer NG, Birchwood C, Gill D, Reiss W, et al. Sustained response following discontinuation of methotrexate in patients with rheumatoid arthritis treated with subcutaneous tocilizumab: results from a randomized, controlled trial. Arthritis Rheumatol. 2018;70(8):1200.
- Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011;13(5):R141.
- Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20(3):222–32.
- Mohan S, Michalska M, Yourish J, Pei J, Gale S, Birchwood C, et al. Long-term safety of tocilizumab from large clinical trial and postmarketing populations. Ann Rheum Dis. 2017;76 (suppl 2):95.
- Bykerk VP, Ostör AJ, Alvaro-Gracia J, Pavelka K, Ivorra JA, Graninger W, et al. Tocilizumab in patients with active rheumatoid arthritis and inadequate responses to DMARDs and/or TNF inhibitors: a large, open-label study close to clinical practice. Ann Rheum Dis. 2012;71(12):1950–4.
- Kivitz A, Olech E, Borofsky MA, Zazueta B, Navarro-Sarabia F, Radominski SC, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45(4):456–64.
- Choy E, Caporali R, Xavier R, Fautrel B, Sanmarti R, Bao M, et al. Subcutaneous tocilizumab in rheumatoid arthritis: findings from the common-framework phase 4 study programme TOZURA conducted in 22 countries. Rheumatology (Oxford). 2018;57(3):499–507.
- Atsumi T, Fujio K, Yamaoka K, Tomobe M, Kuroyanagi K, Kameda H. Safety and effectiveness of subcutaneous tocilizumab in patients with rheumatoid arthritis in a real-world clinical setting. Mod Rheumatol. 2018;28(5):780–88.
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;(2):CD008794.
- Campbell L, Chen C, Bhagat SS, Parker RA, Östör AJ. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford). 2011;50(3):552–62.
- Sakai R, Cho SK, Nanki T, Watanabe K, Yamazaki H, Tanaka M, et al. Head-to-head comparison of the safety of tocilizumab and tumor necrosis factor inhibitors in rheumatoid arthritis patients (RA) in clinical practice: results from the registry of Japanese RA patients on biologics for long-term safety (REAL) registry. Arthritis Res Ther. 2015;17:74.
- Fujiwara H, Nishimoto N, Hamano Y, Asanuma N, Miki S, Kasayama S, et al. Masked early symptoms of pneumonia in patients with rheumatoid arthritis during tocilizumab treatment: a report of two cases. Mod Rheumatol. 2009;19(1):64–8.
- Atsumi T, Ando Y, Matsuda S, Tomizawa S, Tanaka R, Takagi N, et al. Prodromal signs and symptoms of serious infections with tocilizumab treatment for rheumatoid arthritis: text mining of the Japanese postmarketing adverse event-reporting database. Mod Rheumatol. 2018;28(3):435–43.
- Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis. 2014;58(12):1649–57.
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford). 2018;57(6):997–1001.
- Yun H, Xie F, Delzell E, Chen L, Levitan EB, Lewis JD, et al. Risks of herpes zoster in patients with rheumatoid arthritis according to biologic disease modifying therapy. Arthritis Care Res (Hoboken). 2015;67(5):731–6.
- Sout A, Maneiro JR, Salgado E, Carmona L, Gomez-Reino JJ. Risk of tuberculosis in patients with chronic immune-mediated inflammatory diseases treated with biologics and tofacitinib a systematic review and meta-analysis of randomized controlled trials and long-term extension studies. Rheumatology 2014;53:1872–85.
- Ogata A, Mori M, Hashimoto S, Yano Y, Fujikawa T, Kawai M, et al. Minimal influence of tocilizumab on IFN-gamma synthesis by tuberculosis antigens. Mod Rheumatol. 2010;20(2):130–3.
- Mori S, Sugimoto M. Pneumocystis jirovecii infection: an emerging threat to patients with rheumatoid arthritis. Rheumatology (Oxford). 2012;51(12):2120–30.
- Chen LF, Mo YQ, Jing J, Ma JD, Zheng DH, Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis. 2017;20(7):859–69.
- Ahn SS, Jung SM, Song JJ, Park YB, Park JY, Lee SW. Safety of tocilizumab in rheumatoid arthritis patients with resolved hepatitis B virus infection: data from real-world experience. Yonsei Med J. 2018;59(3):452–6.
- Moots RJ, Sebba A, Rigby W, Ostor A, Porter-Brown B, Donaldson F, et al. Effect of tocilizumab on neutrophils in adult patients with rheumatoid arthritis: pooled analysis of data from phase 3 and 4 clinical trials. Rheumatology (Oxford). 2017;56:541–9.
- Bernad A, Kopf M, Kulbacki R, Weich N, Koehler G, Gutierrez-Ramos JC. Interleukin-6 is required in vivo for the regulation of stem cells and committed progenitors of the hematopoietic system. Immunity. 1994;1(9):725–31.
- Wright HL, Cross AL, Edwards SW, Moots RJ. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology (Oxford). 2014;53(7):1321–31.
- Suwa T, Hogg JC, English D, Van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol. 2000;279(6):H2954–60.
- Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9):2720–5.
- Gainsford T, Nandurkar H, Metcalf D, Robb L, Begley CG, Alexander WS. The residual megakaryocyte and platelet production in c-mpl-deficient mice is not dependent on the actions of interleukin-6, interleukin-11, or leukemia inhibitory factor. Blood. 2000;95:528–34.
- Kim SC, Solomon DH, Rogers JR, Gale S, Klearman M, Sarsour K, et al. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi-database cohort study. Arthritis Rheumatol. 2017;69:1154–64.
- Xie F, Yun H, Levitan EB, Muntner P, Curtis JR. Tocilizumab and the risk for cardiovascular disease: a direct comparison among biologic disease-modifying antirheumatic drugs for rheumatoid arthritis patients. Arthritis Care Res (Hoboken). 2018 [Sep 3]. [Epub ahead of print]. doi: 10.1002/acr.23737
- Ljung L, Rantapää-Dahlqvist S, Jacobsson LT, Askling J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann Rheum Dis. 2016;75(12):2087–94.
- Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford). 2014;53(12):2143–54.
- Hashizume M, Mihara M. TNF-α, IL-6 and serum from RA patients each atherogenic effects of TNF-α and IL-6 via up-regulation of scavenger receptors. Cytokine. 2012;58(3):424–30.
- Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110(22):3493–500.
- Genovese MC, Kremer JM, van Vollenhoven RF, Alten R, Scali JJ, Kelman A, et al. Transaminase levels and hepatic events during tocilizumab treatment: pooled analysis of long-term clinical trial safety data in rheumatoid arthritis. Arthritis Rheumatol. 2017;69(9):1751–61.
- Takeuchi T, Thorne C, Karpouzas G, Sheng S, Xu W, Rao R, F, et al. Sirukumab for rheumatoid arthritis: the phase III SIRROUND-D study. Ann Rheum Dis. 2017;76(12):2001–8.
- Nishikawa T, Hagihara K, Serada S, Isobe T, Matsumura A, Song J, et al. Transcriptional complex formation of c-Fos, STAT3, and hepatocyte NF-1 alpha is essential for cytokine-driven C-reactive protein gene expression. J Immunol. 2008;180(5):3492–501.
- Streetz KL, Luedde T, Manns MP, Trautwein C. Interleukin 6 and liver regeneration. Gut. 2000;47(2):309–12.
- Xie F, Yun H, Bernatsky S, Curtis JR. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 2016;68(11):2612–7.
- Strangfeld A, Richter A, Siegmund B, Herzer P, Rockwitz K, Demary W, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis. 2017;76(3):504–10.
- Jin X, Zimmers TA, Zhang Z, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2010;59(2):186–96.
- Kuhn KA, Manieri NA, Liu TC, Stappenbeck TS. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One. 2014;9(12):e114195.
- Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212.
- Rubbert-Roth A, Sebba A, Brockwell L, Kelman A, Porter-Brown B, Pulley J, et al. Malignancy rates in patients with rheumatoid arthritis treated with tocilizumab. RMD Open. 2016;2(1):e000213.
- Kim SC, Pawar A, Desai RJ, Gale S, Klearman M, Sarsour K, et al. No difference in the risk of malignancy in tocilizumab versus TNF inhibitor initiators in patients with rheumatoid arthritis: a multi-database cohort study. Ann Rheum Dis. 2018;77(2):50.
- Chang Q, Daly L, Bromberg J. The IL-6 feed-forward loop: a driver of tumorigenesis. Semin Immunol. 2014;26(1):48–53.
- Tsukamoto H, Fujieda K, Senju S, Ikeda T, Oshiumi H, Nishimura Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci. 2018;109(3):523–30.
- Singh N, Hofmann TJ, Gershenson Z, Levine BL, Grupp SA, Teachey DT, et al. Monocyte lineage-derived IL-6 does not affect chimeric antigen receptor T-cell function. Cytotherapy. 2017;19(7):867–80.
- Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–30.
- Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14(15):2525–31.
- Yoshizaki K, Nishimoto N, Matsumoto K, Tagoh H, Taga T, Deguchi Y, et al. Interleukin 6 and expression of its receptor on epidermal keratinocytes. Cytokine. 1990;2(5):381–7.
- Goodman SM, Springer B, Guyatt G, Abdel MP, Dasa V, George M, et al. 2017 American College of Rheumatology/American Association of hip and knee surgeons guideline for the perioperative management of antirheumatic medication in patients with rheumatic diseases undergoing elective total hip or total knee arthroplasty. Arthritis Care Res (Hoboken). 2017;69(8):1111–24.
- Hirao M, Hashimoto J, Tsuboi H, Nampei A, Nakahara H, Yoshio N, et al. Laboratory and febrile features after joint surgery in patients with rheumatoid arthritis treated with tocilizumab. Ann Rheum Dis. 2009;68(5):654–7.
- Momohara S, Hashimoto J, Tsuboi H, Miyahara H, Nakagawa N, Kaneko A, et al. Analysis of perioperative clinical features and complications after orthopaedic surgery in rheumatoid arthritis patients treated with tocilizumab in a real-world setting: results from the multicentre TOcilizumab in Perioperative Period (TOPP) study. Mod Rheumatol. 2013;23(3):440–9.
- Hoeltzenbein M, Beck E, Rajwanshi R, Gøtestam Skorpen C, Berber E, Schaefer C, et al. Tocilizumab use in pregnancy: analysis of a global safety database including data from clinical trials and post-marketing data. Semin Arthritis Rheum. 2016;46(2):238–45.
- Nakajima K, Watanabe O, Mochizuki M, Nakasone A, Ishizuka N, Murashima A. Pregnancy outcomes after exposure to tocilizumab: a retrospective analysis of 61 patients in Japan. Mod Rheumatol. 2016;26(5):667–71.
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368(6469):339–42.
- Sakurai T, Takai R, Bürgin H, Ishihara K, Sakamoto Y, Amano J, et al. The effects of interleukin-6 signal blockade on fertility, embryo-fetal development, and immunization in vivo. Birth Defects Res B Dev Reprod Toxicol. 2012;95(4):304–17.
- Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. 2012;95(1–2):1–14.
- June RR, Olsen NJ. Room for more IL-6 blockade? Sarilumab for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2016;16(10):1303–9.
- Raimondo MG, Biggioggero M, Crotti C, Becciolini A, Favalli EG. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des Devel Ther. 2017;11:1593–603.
- Fontes JA, Rose NR, Čiháková D. The varying faces of IL-6: from cardiac protection to cardiac failure. Cytokine. 2015;74(1):62–8.
- McGinnis GR, Ballmann C, Peters B, Nanayakkara G, Roberts M, Amin R, et al. Interleukin-6 mediates exercise preconditioning against myocardial ischemia reperfusion injury. Am J Physiol Heart Circ Physiol. 2015;308(11):H1423–33.
- Fahmi A, Smart N, Punn A, Jabr R, Marber M, Heads R. p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cell Signal. 2013;25(4):898–909.
- McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184(12):7219–28.
- Sommer J, Engelowski E, Baran P, Garbers C, Floss DM, Scheller J. Interleukin-6, but not the interleukin-6 receptor plays a role in recovery from dextran sodium sulfate-induced colitis. Int J Mol Med. 2014;34(3):651–60.
- Chehboun S, Labrecque-Carbonneau J, Pasquin S, Meliani Y, Meddah B, Ferlin W, et al. Epstein-Barr virus-induced gene 3 (EBI3) can mediate IL-6 trans-signaling. J Biol Chem. 2017;292(16):6644–56.
- Garbers C, Spudy B, Aparicio-Siegmund S, Waetzig GH, Sommer J, Hölscher C, et al. An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 protein receptor homodimer. J Biol Chem. 2013;288(6):4346–54.
- Petes C, Mariani MK, Yang Y, Grandvaux N, Gee K. Interleukin (IL)-6 inhibits IL-27- and IL-30-Mediated inflammatory responses in human monocytes. Front Immunol. 2018;9:256.

