Abstract
Peficitinib is a Janus kinase (JAK) inhibitor, newly developed and approved in Japan. In contrast to other JAK inhibitors, it is a unique pan-JAK inhibitor, demonstrating inhibition of all JAKs. In patients with rheumatoid arthritis with an inadequate response to previous disease-modifying anti-rheumatic drugs, the efficacy of peficitinib (100 mg and 150 mg) has been confirmed with a comparison to placebo in Phase 2b and 3 trials conducted in Asia. Reportedly, peficitinib was well tolerated for 52 weeks during the trial duration, as well as for the next few years in a subsequent, ongoing long-term extension study. Safety signals, especially, the increased risk of herpes zoster was comparable with other JAK inhibitors.
Introduction
Rheumatoid arthritis (RA) is one of the most common chronic inflammatory autoimmune diseases, mainly targeting synovial tissues, causing progressive joint destruction [Citation1]. The pathogenesis of RA involves several proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 [Citation2,Citation3]. In patients with RA, the advent of biologic agents that directly inhibit these cytokines has dramatically improved prognosis by deterring joint destruction and functional disabilities [Citation4,Citation5]. Janus kinase (JAK) inhibitors are small molecules of disease-modifying anti-rheumatic drugs that have recently been proven as effective biologic agents in RA [Citation6]. Peficitinib is a JAK inhibitor, newly developed and approved in Japan. This review summarizes the efficacy and safety of peficitinib.
JAK inhibitors
JAKs are a family of cytoplasmic protein tyrosine kinases with four members; JAK1, JAK2, JAK3, and TYK2. The binding of cytokines, growth factors, interferons, and peptide hormones to their respective receptors phosphorylates JAKs through the reciprocal interaction of two adjoining JAKs, phosphorylating the signal transducer and activator of transcription (STAT) (). Activated STATs translocate to the nucleus and promote cytokine-responsive gene expression, regulating a wide range of cellular processes, including cell growth, proliferation, differentiation, and apoptosis [Citation7].
Figure 1. JAK family and their related cytokines. The binding of cytokines, growth factors, interferons, and peptide hormones to their respective receptors phosphorylates JAKs through the reciprocal interaction of two adjoining JAKs.
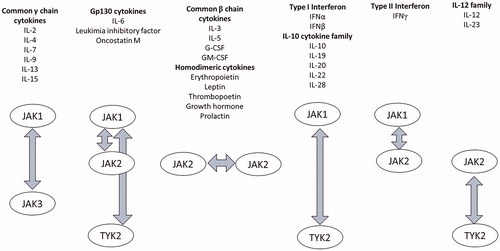
Notably, JAK-STAT pathways are involved in the signaling of various cytokines that play crucial roles in the pathogenesis of RA, and JAK inhibition has been proposed as a promising treatment for RA. Recently, several JAK inhibitors have been developed and approved for RA, including tofacitinib, baricitinib, peficitinib, and upadacitinib. Although all these molecules have been categorized as JAK inhibitors, they differ structurally, as well as demonstrate varying selectivity in JAK suppression () [Citation8–10]. Tofacitinib preferentially inhibits JAK1 and JAK3, baricitinib inhibits JAK1 and JAK2, and upadacitinib is a selective JAK1 inhibitor. In contrast, peficitinib is a pan-JAK inhibitor.
Table 1. JAK Inhibitors approved for RA (not direct comparison).
Pharmacodynamics and pharmacokinetics of peficitinib
Peficitinib was developed in Japan and has been approved for RA in Japan and Korea. It has been classified as a pan-JAK inhibitor as it inhibited JAK activity in a concentration-dependent manner in an in vitro kinase assay, demonstrating half-maximal inhibitory concentrations of 4 nM for JAK1, 5 nM for JAK2, 0.7 nM for JAK3, and 5 nM for TYK2. Reportedly, peficitinib prevents IL-2-induced human T-cell proliferation and STAT5 phosphorylation in a concentration-dependent manner in vitro [Citation9] and suppresses the development of arthritis and bone destruction in a rat adjuvant-induced arthritis model [Citation11].
In the single-dose study, peficitinib absorption was rapid under fasted conditions; the median time to maximum plasma concentration (tmax) was 1.0–1.8 h, increasing with the number of doses [Citation12]. Furthermore, the maximum plasma concentration (Cmax) and area under the plasma concentration-time curve were dose-dependent. Administration with food delayed tmax by 1.5–6.0 h [Citation12], increasing the Cmax and area under the plasma concentration-time curve by 57% and 37%, respectively [Citation13]. In a multiple-dose study under fed conditions, steady-state peficitinib was achieved by day 3, and mean t1/2 was established between 10 and 18 h on day 14 [Citation12]. Peficitinib primarily undergoes metabolism, but approximately 10% is excreted in the urine in the unchanged form. Additionally, the plasma concentration-time profile of peficitinib was similar between individuals with normal and impaired renal function [Citation14].
Efficacy of peficitinib
To date, results from five randomized, placebo-controlled trials and two long-term extension studies have been reported [Citation15–21]. Among the five randomized, placebo-controlled trials, two were Phase 2b trials conducted as global studies, one was a Phase 2b trial and one a Phase 3 trial both conducted in Japan, and one was a Phase 3 trial conducted in Japan, Korea, and Taiwan.
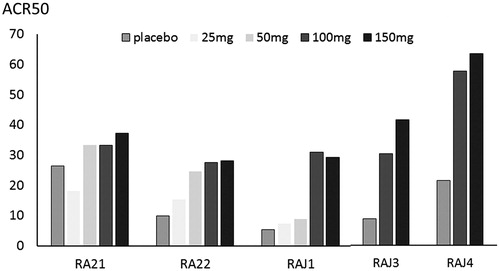
RA21 was a global, randomized, placebo-controlled Phase 2b trial [Citation14]. In the RA21 study, 387 patients demonstrating inadequate response to methotrexate and prior exposure to TNF inhibitors permitted in North America, including Mexico, Latin America, and Europe received a placebo or peficitinib (25 mg, 50 mg, 100 mg, or 150 mg) in combination with methotrexate for 12 weeks. The primary endpoint was defined as the American College of Rheumatology (ACR) 20 response rate at week 12 and was reported as 50.0% in the placebo group, which failed to statistically differ from 43.9%, 61.5%, 46.4%, and 57.7% documented in the peficitinib 25 mg, 50 mg, 100 mg, and 150 mg groups, respectively. The non-significance was partially attributed to high ACR20 response rates observed in placebo groups enrolled in North America (50.0%) and Latin America (75.0%). RA22 was a global, randomized, placebo-controlled Phase 2b study [Citation16], conducted in North America, including Mexico, and Europe, and enrolled 289 patients presenting an inadequate response to disease-modifying anti-rheumatic drugs (DMARDs), with prior use of biologic agents allowed. The participants received either a placebo or peficitinib 25 mg, 50 mg, 100 mg, or 150 mg, alone or in combination with conventional synthetic (cs) DMARDs. The primary endpoint of ACR20 response rates at week 12 was 29.7% in the placebo group and 22.0%, 36.8%, 48.3%, and 56.3% in the 25 mg, 50 mg, 100 mg, and 150 mg peficitinib groups, respectively. The efficacy of 100 mg and 150 mg peficitinib was significantly superior to the placebo. RAJ1 was a Phase 2b investigation conducted in Japan [Citation17]. Overall, 281 patients with active RA were enrolled regardless of previous treatment and were randomized to receive a placebo or peficitinib (25 mg, 50 mg, 100 mg, or 150 mg) monotherapy for 12 weeks. The primary endpoint of ACR20 response rates at week 12 were 10.7% in the placebo group and 23.6%, 31.6%, 54.5%, and 65.5% in the 25 mg, 50 mg, 100 mg, and 150 mg peficitinib groups, respectively. The 100 mg and 150 mg peficitinib groups demonstrated significantly higher ACR20 response rates than the placebo group.
Two Phase 3 trials were conducted in Asia. The randomized, placebo-controlled RAJ3 study was performed in Japan, Korea, and Taiwan, enrolling 507 patients with inadequate response to DMARDs, with a permissible history of biologic agents [Citation18]. Patients received a placebo, peficitinib (100 mg or 150 mg), or open-label etanercept, alone or in combination with csDMARDs for 52 weeks. After 12 weeks, patients in the placebo group were switched to peficitinib 100 mg or 150 mg in a blinded manner. Etanercept was designated as the open-label reference to evaluate the safety of peficitinib. The primary endpoint of ACR20 response rates at week 12 was significantly higher in the peficitinib 100 mg (57.7%) and 150 mg (74.5%) groups when compared with the placebo group (30.7%). The ACR20 response rate was maintained or even increased through week 52; the ACR20 response rates at week 52 with last observation carried forward analysis were 65.4% in the 100 mg group, 84.3% in the 150 mg group, 67.4% in the placebo vs. 100 mg group, and 74.5% in the placebo vs. 150 mg group (as a reference, 86.5% in the etanercept group). RAJ4 was a randomized, placebo-controlled study conducted in Japan, enrolling 519 patients with inadequate response to methotrexate [Citation19]. In combination with methotrexate, the primary endpoint of ACR20 response rates at week 12 was significantly higher in the peficitinib 100 mg group (58.6%) and 150 mg group (64.4%) when compared to the placebo group (21.8%). The clinical efficacy was increased toward week 24 and maintained until week 52. Additionally, RAJ4 evaluated the efficacy of peficitinib on inhibition of joint destruction. The change in the modified total Sharp score from baseline to week 28, a co-primary endpoint, was significantly reduced in the peficitinib 100 mg group (1.6) and 150 mg group (1.0) when compared with the placebo group (3.4). At week 52, the proportion of patients demonstrating a change of ≤0.5 in the modified total Sharp score was 64.0% in the peficitinib 100 mg group and 68.9% in the peficitinib 150 mg group; it was 42.5% in the placebo group.
The ACR50 response rates in the three Phase 2b and two Phase 3 studies are presented in [Citation17–21].
Figure 2. ACR50 achievement of placebo and each peficitinib dose in the five randomized, placebo-controlled trials. RA21 and RA22 were global phase 2b trials. RAJ1 and RAJ were conducted in Japan, and RAJ3 was conducted in Japan, Korea, and Taiwan. ACR, American College of Rheumatology. The approved peficitinib dose for rheumatoid arthritis in Japan was 100 mg and 150 mg daily.
Safety of peficitinib
The incidence of total adverse events and adverse events of interest in the five randomized, placebo-controlled trials are summarized in [Citation17–21].
Table 2. Summary of adverse events in the five randomized, placebo-controlled trials.
During the 12-week observation periods of the two Phase 2b global trials (RA21 and RA22) [Citation17,Citation18], the adverse event incidence was comparable between the placebo and combined peficitinib groups. However, the incidence of serious infection was higher in the peficitinib groups than in the placebo group, with a total of four patients presenting herpes zoster in the peficitinib groups in RA21 and RA22. The other Phase 2b trial, RAJ1 conducted in Japan [Citation19], demonstrated similar safety signals; the total incidence of treatment-emergent adverse events was similar between the placebo and peficitinib groups; however, herpes zoster was observed in four patients in the peficitinib group, with no patient in the placebo group reporting a similar infection.
In the Phase 3 RAJ3 study [Citation20], the incidence of adverse events during the 12 weeks of double-blinded phase was 53.5% in the placebo group, 55.3% in the peficitinib 100 mg and 150 mg groups, and 59.5% in the etanercept groups. During the 52 weeks, the incidence of adverse events was 87.9% in the peficitinib group and 89.0% in the etanercept group. Herpes zoster was reported in nine patients in the peficitinib group, and five patients in the etanercept group during the 52-week study period. Notably, 5 of the 90 patients switched from the placebo to peficitinib developed herpes zoster after the treatment change. In the Phase 3 RAJ4 study [Citation21], the incidence of adverse events during the 12 weeks of double-blinded phase was 49.4% in the placebo group and 55.5% in the peficitinib 100 mg and 150 mg groups. During the 52-week study period, herpes zoster was documented in 12 patients in the peficitinib group, and in 2 of the 148 patients switched from placebo to peficitinib post switching. No patient in the placebo group developed herpes zoster during the 12 weeks of placebo administration. As for blood cells, neutrophil counts were decreased in the peficitinib groups by around 500/μL at week 52 with a tendency of peficitinib 150mg showing larger change than peficitinib 100mg (data not shown). No marked decrease in hemoglobin and platelet counts were observed. Serum creatinine and transaminase levels did not change. Serum creatine phosphokinase levels were slightly increased. Although there exist concerns regarding the increased risk of thromboembolic events and malignancy with JAK inhibitors [Citation22], no thromboembolic events and few malignant tumor were observed in patients treated with peficitinib during these trials.
Additionally, two reports have documented the long-term safety of peficitinib from open-label extension studies [Citation20,Citation22]. In one extension study, patients who participated in either one of the two global Phase 2b studies (RA21 and RA22) were included and received 100 mg of peficitinib daily. Of the 611 patients included, herpes zoster developed in 14 patients (1.5/100 patient-years [PY]), and malignancies were recorded in six patients (0.6/100PY) [Citation22]. The second extension study (RAJ2) enrolled patients who participated in the RAJ1, RAJ3, and RAJ4 trials and is currently ongoing; however, the interim analysis of 843 patients documented that the incidence of adverse events of special interest was 2.3/100PY for serious infections, 6.8/100PY for herpes zoster, and 1.1/100PY for malignancies. Overall, no new safety signals have been identified.
Discussion
Peficitinib, a novel JAK-inhibitor, has been approved for RA in Japan, Korea, and Taiwan. The efficacy and safety of peficitinib have been confirmed in clinical trials, and peficitinib is considered a good option in the management of RA.
In the past two decades, the management of RA has undergone dramatic improvements. Methotrexate and biologic agents have played a great role in that improvements, and JAK inhibitors, anti-rheumatic drugs with a novel mode of action, have built an additional position. At present, four JAK inhibitors have been approved for RA in Japan; tofacitinib, baricitinib, peficitinib, and upadacitinib. While inhibition of individual kinases by each JAK inhibitor is characteristic experimentally, trials on those JAK inhibitors have shown similar efficacy and safety profile. For example, the ACR50 achievement of JAK inhibitors in combination with methotrexate in patients with inadequate response to methotrexate was 51.5% of tofacitinib 5mg twice daily versus 25.3% of methotrexate at 6 months from the ORAL Scan study [Citation23], 70% of baricitinib 4 mg daily vs 40% of methotrexate at 12 weeks from the RA-BEAM study [Citation24], 74.5% of peficitinib 150 mg daily versus 30.7% of methotrexate at 12 weeks from the RAJ3 study) [Citation18], and 71% of upadacitinib 150 mg daily versus 36% of methotrexate at 12 weeks from the SELECT-COMPARE study [Citation25]. Serious adverse events occurred in 3.7% of tofacitinib during 12 weeks, 4.7% of baricitinib during 24 weeks, 2.0% of peficitinib during 12 weeks, and 3.7% of upadacitinib during 26 weeks [Citation18,Citation23–25]. The effect on blood cells also appears similar.
Switching from a JAK inhibitor to another one is a different matter. Unmet needs still exist as some patients fail to achieve or maintain clinical remission with biologic agents or JAK inhibitors [Citation26]. If patients fail to respond to a biologic agent, several reports from registry data and observational studies as well as a randomized controlled trial, have suggested that switching to a drug with a different mode of action may improve efficacy, rather than switching to a second drug with a similar mode of action [Citation27–30]. However, another JAK inhibitor may be effective when treatment failure is observed with a previous JAK inhibitor because, as described above, each JAK inhibitor presents a different suppression profile for individual JAKs, resulting in possible different effect on cytokines and immune cell function. Peficitinib is a unique pan-JAK inhibitor, demonstrating substantial efficacy and tolerable safety and can provide a valuable treatment option in the management of RA.
Furthermore, it should be noted that peficitinib demonstrated superior performance in clinical trials conducted in Asia compared to those conducted globally. In the global trials, the low effect size of peficitinib is partly attributed to the high ACR20 response rates of the placebo group. Another possible explanation is differing body weights between patients in global trials and those in Asian trials. The daily dose of peficitinib 150 mg may be sufficient in Asian patients but may have been inadequate in North American, Latin American, and European patients. Additionally, genetic factors may have contributed to the differential responses observed with peficitinib. This remains to be seen in further studies.
In conclusion, peficitinib is a good therapeutic option for patients with RA demonstrating inadequate responses to previous DMARDs. The long-term safety and optimal usage of peficitinib after achieving remission need to be further investigated in future studies.
Acknowledgments
None.
Conflicts of interest
YK has received grants or speaking fees from AbbVie, Astellas, Ayumi, Bristol–Myers Squibb, Chugai, Eisai, Eli Lilly, Hisamitsu, Jansen, Kissei, Kirin, Novartis, Pfizer, Sanofi, Takeda, Taisho, Tanabe-Mitsubishi, and UCB.
Additional information
Funding
References
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38.
- Winthrop KL, Weinblatt ME, Bathon J, Burmester GR, Mease PJ, Crofford L, et al. Unmet need in rheumatology: reports from the Targeted Therapies meeting 2019. Ann Rheum Dis. 2020;79(1):88–93.
- O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591–602.
- Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33(6):679–707.
- Takeuchi T, Kameda H. The Japanese experience with biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. 2010;6(11):644–52.
- Tanaka Y. Recent progress and perspective in JAK inhibitors for rheumatoid arthritis: from bench to bedside. J Biochem. 2015;158(3):173–9.
- Choy EH. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology (Oxford). 2019;58(6):953–62.
- Clark J, Flanagan ME, Telliez J-B. Discovery and development of Janus Kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57(12):5023–38.
- Hamaguchi H, Amano Y, Moritomo A, Shirakami S, Nakajima Y, Nakai K, et al. Discovery and structural characterization of peficitinib (ASP015K) as a novel and potent JAK inhibitor. Bioorg Med Chem. 2018;26(18):4971–83.
- Parmentier JM, Voss J, Graff C, Schwartz A, Argiriadi M, Friedman M, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2:23
- Ito M, Yamazaki S, Yamagami K, Kuno M, Morita Y, Okuma K, et al. A novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J Pharmacol Sci. 2017;133(1):25–33.
- Cao YJ, Sawamoto T, Valluri U, Cho K, Lewand M, Swan S, et al. Pharmacokinetics, pharmacodynamics, and safety of ASP015K (peficitinib), a new Janus kinase inhibitor, in healthy subjects. Clin Pharmacol Drug Dev. 2016;5(6):435–49.
- Pharmaceuticals and Medical Devices Agency (PMDA) Japan. Smyraf tablets® 50 mg and 100 mg: interview form v5 2020. Available from: https://www.info.pmda.go.jp/go/interview/1/800126_3999046F1023_1_005_1F.pdf
- Miyatake D, Shibata T, Shibata M, Kaneko Y, Oda K, Nishimura T, et al. Pharmacokinetics and safety of a single oral dose of peficitinib (ASP015K) in Japanese subjects with normal and impaired renal function. Clin Drug Investig. 2020;40(2):149–59.
- Genovese MC, Greenwald M, Codding C, Zubrzycka‐Sienkiewicz A, Kivitz AJ, Wang A, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69(5):932–42.
- Kivitz AJ, Gutierrez‐Ureña SR, Poiley J, Genovese MC, Kristy R, Shay K, et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol. 2017;69(4):709–19.
- Takeuchi T, Tanaka Y, Iwasaki M, Ishikura H, Saeki S, Kaneko Y. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75(6):1057–64.
- Tanaka Y, Takeuchi T, Tanaka S, Kawakami A, Iwasaki M, Song YW, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3). Ann Rheum Dis. 2019;78(10):1320–32.
- Takeuchi T, Tanaka Y, Tanaka S, Kawakami A, Iwasaki M, Katayama K, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann Rheum Dis. 2019;78(10):1305–19.
- Genovese MC, Greenwald MW, Gutierrez-Ureña SR, Cardiel MH, Poiley JE, Zubrzycka-Sienkiewicz A, et al. Two-year safety and effectiveness of peficitinib in moderate-to-severe rheumatoid arthritis: a phase IIb, open-label extension study. Rheumatol Ther. 2019;6(4):503–20.
- Takeuchi T, Tanaka Y, Tanaka S, Kawakami A, Song Y-W, Chen Y-H, et al. Safety and effectiveness of peficitinib (ASP015K) in patients with rheumatoid arthritis: interim data (22.7 months mean peficitinib treatment) from a long-term, open-label extension study in Japan, Korea, and Taiwan. Arthritis Res Ther. 2020;22(1):47.
- Scott IC, Hider SL, Scott DL. Thromboembolism with Janus kinase (JAK) inhibitors for rheumatoid arthritis: how real is the risk? Drug Saf. 2018;41(7):645–53.
- van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65(3):559–70.
- Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–62.
- Fleischmann R, Pangan AL, Song I‐H, Mysler E, Bessette L, Peterfy C. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–800.
- Conigliaro P, Triggianese P, De Martino E, Fonti GL, Chimenti MS, Sunzini F, et al. Challenges in the treatment of rheumatoid arthritis. Autoimmun Rev. 2019;18(7):706–13.
- Emery P, Gottenberg JE, Rubbert-Roth A, Sarzi-Puttini P, Choquette D, Martínez Taboada VM, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74(6):979–84.
- Gottenberg J-E, Brocq O, Perdriger A, Lassoued S, Berthelot J-M, Wendling D, et al. Non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. JAMA. 2016;316(11):1172–80.
- Gottenberg J-E, Morel J, Perrodeau E, Bardin T, Combe B, Dougados M, et al. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study. BMJ. 2019;364:l67
- Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
