Abstract
Objective
Assess patient characteristics, real-world treatment patterns, and health care resource utilization (HCRU) among patients with psoriatic arthritis (PsA) in Japan.
Methods
Patients diagnosed with PsA from April 2009 through July 2017 were identified from the Medical Data Vision database. Patient characteristics, treatment patterns, and HCRU were evaluated for these patients.
Results
A total of 639 patients met inclusion criteria and were included in the analysis for patients with a PsA diagnosis. Over 12 months following diagnosis, patients received oral NSAIDs (61.7%), conventional synthetic disease-modifying antirheumatic drugs (DMARDs) (55.1%), corticosteroids (35.1%), topical NSAIDs (34.0%), adalimumab (14.7%), infliximab (9.7%), secukinumab (5.0%), ustekinumab (4.5%), ixekizumab (1.6%), and golimumab (1.6%). A total of 227 (35.5%) patients initiated biologic DMARDs (bDMARDs) over the median 25.2 months of study follow-up. Compared with the overall group of patients diagnosed with PsA, patients who initiated bDMARDs had higher median total per-patient health care costs ($27,772 vs. $11,316), lower median per-patient hospitalization costs ($31,164 vs. $39,359), and fewer median hospital days per admission (8.0 vs. 12.0 days).
Conclusion
This study presents knowledge of the current state of patient characteristics, treatment patterns, HCRU, and costs among patients with PsA in Japan. Considering the relatively recent guideline recommendations, the preliminary treatment patterns suggest physicians may be following treatment guidelines.
1. Introduction
Spondyloarthritis (SpA) comprises several inflammatory autoimmune conditions that can severely impact affected patients. SpA is uncommon in Japan, with an estimated prevalence of 9.5 per 100,000 person-years [Citation1]. SpA is categorized by predominantly axial SpA or predominantly peripheral SpA. Peripheral SpA includes psoriatic arthritis (PsA), which is associated with stiff, swollen, and painful joints in virtually any location and can progress to structural joint damage [Citation2]. The published prevalence rates of PsA in patients with psoriasis in Japan range from approximately 2% to 15% [Citation3–5]. A recent study examining more than 3,000 patients with psoriasis treated at three tertiary care centers in Japan reported a PsA prevalence of 14.3% [Citation4], whereas another study of patients treated in 73 facilities in Japan found PsA affected 10.5% of newly treated psoriasis patients [Citation5]. A 2015 epidemiology study used a national Japanese database of 429,679 patients with psoriasis to determine a PsA prevalence of 1.9% among patients with psoriasis in Japan [Citation3]. However, the actual prevalence of PsA in Japan may be underrepresented due to variations in diagnosis among different types of specialists [Citation6]. These PsA prevalence rates are consistent with the 5.8% rate found for patients with psoriasis in China [Citation7]. However, the prevalence of PsA is nearly double (30%) for patients with psoriasis in North America and Europe [Citation8].
There is no curative treatment for PsA, so treatment options consist of symptom control, prevention of joint damage, and disease remission. Nonsteroidal anti-inflammatory drugs (NSAIDs) are typically prescribed as first-line treatment for control of pain and stiffness [Citation9]. If NSAIDs are ineffective, biologic disease-modifying antirheumatic drugs (bDMARDs) have been shown to activate a rapid and sustained response in many patients [Citation9,Citation10]. Biologics, including infliximab (approved in 2010), adalimumab (2010), ustekinumab (2011), secukinumab (2014), ixekizumab (2016), brodalumab (2016), guselkumab (2018), certolizumab (2019), and risankizumab (2019), are approved for treatment of PsA in Japan for patients who have not responded to conventional systemic therapies [Citation11]. The Biologics Review Committee of the Japanese Dermatological Association (JDA) has released evidence-based guidance for the treatment of PsA with biologics, which recommends early administration of bDMARDs to counter the progressive effects of joint destruction [Citation11]. The 2015 European League Against Rheumatism (EULAR) guidance recommends administration of a tumor necrosis factor (TNF) inhibitor bDMARD after inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDS) [Citation12]. Additionally, a recent database study explored the persistence rates and costs of bDMARD treatment for psoriasis in Japan [Citation13], but there is a lack of comprehensive data on real-world treatment patterns and costs specifically for PsA and beyond bDMARD usage.
We conducted a retrospective study using a hospital claims database to address the lack of evidence in the literature on the understanding of patient characteristics, treatment patterns, costs, and health care resource utilization (HCRU) among patients with PsA in Japan. The evaluation of treatment patterns included assessment of medication discontinuation, persistence, estimated adherence, augmentation, PsA-related concomitant medication use, and switching among patients who initiated bDMARDs.
2. Materials and methods
2.1. Study design
This retrospective cohort study analyzed patient records from the Medical Data Vision (MDV) database using the methodology described in Tomita et al. [Citation14]. The MDV database is a large database derived from hospital claims data recorded at more than 350 hospitals across Japan [Citation15,Citation16]. The database has diagnosis, procedure, pharmacy, and inpatient and outpatient visits claims data since 2008 for more than 20 million patients. The age distribution of patients’ data in the database population reflects that of the general population in Japan, and data from the MDV has been used in several studies [Citation17–20]. All patients with an observed diagnosis of PsA according to International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM; codes L40.50, L40.51, L40.52, L40.53, L40.54, and L40.59) [Citation21] between 1 April 2009 and 31 July 2017 were screened for study inclusion. Included patients were required to have a second PsA diagnosis at least 30 days after the first PsA diagnosis in order to increase the specificity of the sample. The MDV database designates each diagnosis code as ‘confirmed’ or ‘doubt,’ and only ‘confirmed’ diagnoses were used. The date of the first PsA diagnosis defined the index date. Patients 18 years of age or older at the study index date with at least 3 months of continuous data available before their index date (termed baseline period) and at least 12 months of continuous data available after their index date (to allow for sufficient time to observe the study measures) were included for the study. Patients with PsA diagnoses during the baseline period were excluded from the analysis in order to ensure that only newly diagnosed patients were included. The periods of continuous data availability before and after the index date were established by selecting the earliest and most recent medical encounters between 1 April 2008 and 31 July 2018 (time period for which the database was available when the study was conducted). An additional diagnosis of PsA that was at least 30 days after the index date was required for patients to be eligible for the study. A subgroup of patients with PsA who initiated a bDMARD at any time during the available follow-up post index date was identified by the presence of at least one agent-specific health claim code(s) associated with anatomical therapeutic chemical (ATC) codes for bDMARDs (L04B, L04C, M01C) combined with product generic and brand names (Supplementary Table S1). The first bDMARD identified after the index date was used to define the index bDMARD.
2.2. Assessments
2.2.1. Demographic, clinical, and other characteristics at diagnosis
Age and gender were measured at the index date. The Charlson Comorbidity Index (CCI) score was calculated to obtain a measure of patients’ overall comorbidity burden during the baseline period [Citation22] (Supplementary Table S2). Comorbidities or complications related to PsA that were not captured in the CCI were also examined during the baseline period (Supplementary Table S3). Additionally, the distributions of physician departments and imaging procedures associated with the index diagnosis were reported (Supplementary Table S4). Since the database did not provide the exact day (month and year of the visit was available) when a visit was made to a particular department, a hierarchy-based algorithm was used to assign the index department to each patient. If a patient had multiple visits to different departments during the index month, including a visit to dermatology, the patient was assigned first to dermatology. If no visit to dermatology was observed, the next preference was given to rheumatology, then to orthopedic, then internal medicine, and then other specialties. The total follow-up for a given patient was defined as the time from the index date to the last recorded hospital visit encounter in the MDV database for that patient.
2.2.2. Treatment patterns
Treatment patterns assessment included the number and percentage of patients with PsA-related therapies received during the first 12-month follow-up period along with the average time from the index date to the first observed treatment. To define treatment line, the first prescription fill of PsA-related therapies after PsA diagnosis was defined as the start of a line of treatment. Any additional treatment administered within 30 days of start of treatment defined a combination therapy. End of a specific line of treatment was defined as one of the following: (1) line continued until the end of follow-up period; (2) no drug administered within 90 days of exhaustion of the last administration; or (3) previous line of treatment was interrupted by a new agent/drug class. To assess treatment sequences, this process was repeated for three times during the available follow-up period. Second- and third-line treatments following the top five most frequently administered first-line treatments were described. Treatments were identified based on specific health claims codes that were associated with respective ATC codes and product generic and brand names (Supplementary Table S1). Procedure codes for physical therapy and surgeries are presented in Supplementary Table S4. For this study, corticosteroids were not separately categorized as oral corticosteroids or topical administration.
2.2.3. Treatment patterns with biologic disease-modifying antirheumatic drugs
Treatment discontinuation, persistence, estimated adherence, augmentation, PsA-related concomitant medication use, and switching were evaluated for the first bDMARD therapy. A patient was considered to have discontinued treatment with a specific bDMARD if there was no evidence of a refill within 90 days of completion of the dispensed drug supply. Prescribing guidelines were used to assign days’ supply to each of the injection-based therapies. The date of exhaustion of the days’ supply from the last prescription before the initiation of an observed treatment gap (if applicable) was considered the date of treatment discontinuation. Treatment persistence was defined as continuation of bDMARD treatment at the end of 12 months and 24 months of study follow-up. Estimated treatment adherence was defined using the medication possession ratio (MPR), which was defined as the proportion of patients’ time on drug with medication supply on hand (i.e. sum of days’ supply of all fills for the drug divided by total patient time on drug; time on drug was defined as the days between the initiation of a bDMARD and the end of the days’ supply of the last administration). The MPR was computed for the overall study follow-up as well as at the end of 12 months and 24 months of study follow-up. Switching was defined as receipt of a new prescription for a different bDMARD and/or csDMARD following discontinuation of the index bDMARD. Therapies under the csDMARD category include methotrexate, sulfasalazine, leflunomide, hydroxychloroquine, and gold salts. If applicable, combination regimens (e.g. infliximab + methotrexate, methotrexate + sulfasalazine) were regimens that were initiated within 30 days of the first agent that the patient switched to after discontinuation of the index bDMARD. Augmentation was defined as the uptake of additional PsA therapies after 90 days of treatment with an index bDMARD along with continued use of the index bDMARD [Citation21,Citation23]. All other PsA-related treatments that the patient received during first 90 days after initiating the index bDMARD were considered as concomitant medications to the index bDMARD. There was no limit to the time window for switching.
2.2.4. Health care resource utilization and costs
Health care resource utilization and costs were reported overall and for the subset of patients who initiated a bDMARD anytime during the study follow-up. All-cause resource utilization and costs were stratified by the service sector (i.e. inpatient visits, hospital outpatient visits, hospital outpatient pharmacy) in which they occurred based on the inpatient/outpatient designation and claim categorization codes associated with each claim. The subset of HCRU and costs specific to PsA-related hospitalizations and PsA-related outpatient pharmacy dispenses, identified as claims associated with a PsA diagnosis or relevant treatment, were reported separately. The Consumer Price Index for Japan [Citation24] was used to update cost data to 2018 Japanese yen before being converted to United States (US) dollars using the 2018 mid exchange rate of 1 US dollar = 110.8 Japanese yen [Citation25].
2.2.5. Statistical analysis
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). Descriptive analyses displayed mean values, medians, ranges, and standard deviations (SDs) of continuous variables and frequency distributions for categorical variables. Treatment discontinuation was assessed using the Kaplan-Meier method. The censored group included those who had treatment ongoing at the end of study follow-up, and the event group included those with a discontinuation. The association between patient demographics, baseline comorbidities of interest, baseline medications received, and the subsequent initiation of a bDMARD in the 12 months after diagnosis of PsA was assessed using a multivariable logistic regression model. Covariates were determined based on clinical importance and a literature review [Citation26].
3. Results
3.1. Patient population
A total of 639 patients met inclusion criteria and were included in the overall analysis for patients with a PsA diagnosis (). Of these patients, 227 (35.5%) had initiated bDMARDs during the entire study follow-up period and were included in a subanalysis population. Demographics and patient characteristics can be found in . Patients had a mean (SD) age of 59.3 (14.6) years, and a slight majority were male (55.4%). All patients had a minimum of 12 months’ follow-up, with follow-up duration ranging from 12.1 to 108.7 months and a median of 25.2 months. Most patients (75.6%) received at least one imaging procedure during the baseline period. Patients had a mean (SD) CCI score of 1.3 (1.8). Psoriasis (38.2%), gastroesophageal reflux disease (GERD) (25.0%), and hypertensive conditions (24.6%) were the most prevalent comorbidities identified at baseline among all patients diagnosed with PsA (). Approximately half of patients diagnosed with PsA had an index diagnosis attributable to a physician department of dermatology (56.3%), followed by orthopedics (17.1%), internal medicine (13.3%), rheumatology (9.7%), other specialties (3.3%), and unknown specialty (0.3%). The subset of 227 patients who initiated bDMARDs had similar age and sex ratios, with a mean (SD) age of 56.9 (14.0) years and a sex composition of 56.0% male. The categorization of physician departments associated with the diagnosis had the highest percentage of diagnoses attributable to dermatology (68.7%), followed by internal medicine (12.3%), orthopedics (7.9%), rheumatology (7.9%), and other specialties (3.1%).
Figure 1. Sample selection flow chart. DMARD: disease-modifying antirheumatic drug; PsA: psoriatic arthritis.
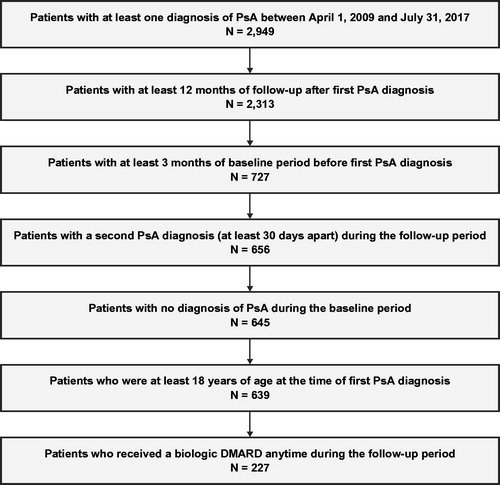
Table 1. Patient demographics and clinical characteristics at baseline.
Table 2. Comorbidity burden at baseline using the charlson comorbidity index and other comorbidities of interest.
3.2. Treatment patterns
The majority of patients (89.2%) received treatment for PsA during the 12-month follow-up period, with the initiation of the first treatment occurring at a median of 15.0 days from the index date (). Over the course of the 12-month follow-up period, patients received oral NSAIDs (61.7%), csDMARDs (55.1%), corticosteroids (35.1%), topical NSAIDs (34.0%), bDMARDs (32.2%), physical therapy (8.8%), and surgery (4.1%) (). Adalimumab, infliximab, secukinumab, ustekinumab, golimumab, and ixekizumab were the six most administrated bDMARDs and were used by 14.7%, 9.7%, 5.0%, 4.5%, 1.6%, and 1.6% of all PsA patients, respectively, over the 12-month follow-up period. Methotrexate, sulfasalazine, and cyclosporine comprised the majority of csDMARD treatments, which were used by 34.0%, 13.8%, and 13.5% of patients, respectively. First treatments administered to patients during the 12-month follow-up period included oral NSAIDs (44.9%), corticosteroids (21.1%), methotrexate (18.6%), topical NSAIDs (16.0%), cyclosporine/ciclosporin (9.1%), sulfasalazine (7.7%), adalimumab (6.0%), infliximab (3.6%), secukinumab (2.7%), ustekinumab (1.6%), and apremilast (0.9%) (). Multiple combinations of first treatments administered were observed (first-line combinations), including NSAIDs only (23.2%), NSAIDs + csDMARD (14.1%), csDMARD only (10.0%), corticosteroid + NSAIDs + csDMARD (7.0%), and bDMARD monotherapy (6.4%) for the top five first-line combinations (). Of the 41 patients who received first-line bDMARD monotherapy, 37 (90.2%) had an index diagnosis attributable to a physician department of dermatology. The logistic regression analysis () demonstrated that index diagnosis from orthopedics (odds ratio [OR]: 0.22; 95% confidence interval [CI]: 0.11–0.42), rheumatology (OR: 0.42; 95% CI: 0.21–0.84), or internal/general medicine specialties (OR: 0.54; 95% CI: 0.29–0.99) was associated with a statistically significant lower odds of a patient receiving bDMARDs within 12 months of being diagnosed with PsA as compared with a index diagnosis by a dermatologist.
Figure 2. Treatment sequencing following the top five first-line combinations for all patients with PsA diagnosis. cs: conventional synthetic; DMARD: disease-modifying antirheumatic drug; NSAID: nonsteroidal anti-inflammatory drug; PsA: psoriatic arthritis. Notes. Sixty-nine patients (10.8%) had no observed treatment during available follow-up, and 24.9% had other treatment sequences not shown in the top 5 treatments. ‘Other treatments’ comprises second-line treatments received by fewer than five patients. Patients in the NSAID second-line treatment category following first-line treatment of NSAID only are patients who discontinued and then restarted NSAID treatment.
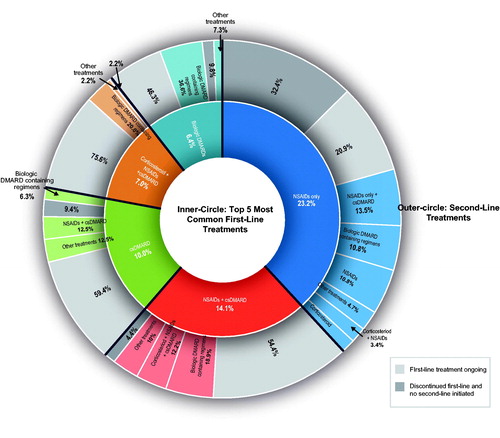
Figure 3. Logistic regression analysis to assess factors associated with receipt of biologic DMARDs in the 12 months after diagnosis of PsA. CCI: Charlson Comorbidity Index; CI: confidence interval; cs: conventional synthetic; DMARD: disease-modifying antirheumatic drug; NSAID: nonsteroidal anti-inflammatory drug; PsA: psoriatic arthritis; ref.: reference.
Table 3. Overall treatment categories during the 12-month follow-up perioda.
Table 4. First treatments administered to patients during the 12-month follow-up perioda.
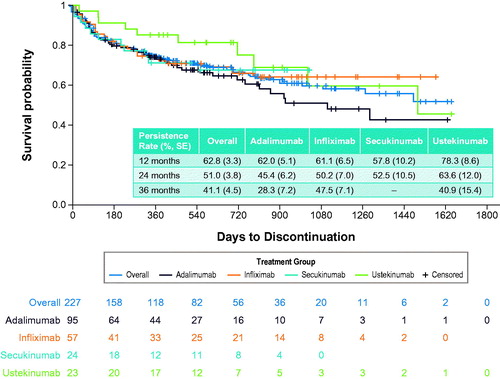
The majority of patients receiving adalimumab, infliximab, secukinumab, and ustekinumab bDMARDs remained on treatment, with persistence rates of 62.8% and 51.0% observed at 12 and 24 months’ follow-up, respectively (). Persistence rates were observed to be similar for adalimumab, infliximab, secukinumab, and overall bDMARDs at multiple time points through 24 months, while the persistence rate for ustekinumab was substantially greater (). The proportion of patients adherent (MPR ≥ 0.8) to the index bDMARD at 12 months (n = 172) was 83.1% and at 24 months (n = 89) was 87.6%. Patients were less adherent to secukinumab (70.0%) compared with the other bDMARDs (85.4%–95.0%) at 12 months, although the difference in adherence rates among these four bDMARDs was similar at 24 months (84.9%–93.8%) (). Among the 110 patients (48.5%) who discontinued bDMARD treatment, 89 (80.9%) switched to a different treatment or treatment combination. Of the patients treated with bDMARDs, 163 (71.8%) exhibited use of PsA-related concomitant medication, which included oral NSAID use in 97 patients (59.5%), methotrexate use in 81 patients (49.7%), corticosteroid use in 73 patients (44.8%), topical NSAIDs use in 40 patients (24.5%), and sulfasalazine use in 26 patients (16.0%) (Supplementary Table S5).
Table 5. Treatment patterns during all available follow-up among patients receiving biologic DMARDsa.
3.3. Health care resource utilization
All-cause HCRU and costs were investigated for all patients with a PsA diagnosis and a smaller subset of patients with PsA who initiated bDMARDs (). The overall PsA patient group had lower median annual per-patient costs compared with patients who initiated bDMARDs ($11,316 vs. $27,772). Both groups had similar percentages of patients who experienced at least one hospital admission and who had at least one hospital outpatient visit. Most of the patients (98.1%) in the overall PsA group had at least one prescription filled at an outpatient pharmacy. The average admitted patient in the overall PsA group compared with the bDMARD initiation group spent more days in the hospital per visit (median, 12.0 vs. 8.0 days), and median hospitalization costs per patient (among patients who had a hospitalization) were higher by approximately $8,000 ($39,359 vs. $31,164). Comparable trends were seen for specific HCRU and costs related to PsA (). Patients who were hospitalized and were in the overall group of patients diagnosed with PsA spent more days receiving PsA-related care as admitted hospital patients than did patients who initiated bDMARDs (median, 11.5 days vs. 6.0 days, respectively) and had higher annual PsA-related hospitalization costs of $32,885 per patient compared with $26,847.
Table 6. All-Cause and Other specific health care resource utilization and costs during anytime in the follow-up period.
4. Discussion
This database study describes the diagnosis, treatment patterns, and HCRU for PsA in Japan using real-world data. The patient characteristics observed in this study reflected the literature. A recent Japanese database study using the Japanese national database of health insurance claims observed a mean age of 55.5 years and a male composition percentage of 53.0% among 8,360 patients with PsA [Citation3]. These findings were comparable to our own findings of 59.3 years and 55.4% male, respectively. Psoriasis (other than PsA), GERD, and hypertensive conditions were the three most prevalent comorbidities among all patients diagnosed with PsA. The approach for confirming a PsA diagnosis in clinical practice and the subsequent documentation in the MDV database could explain the documentation of rheumatologic disease as a differential diagnosis for patients with PsA. While patients in the bDMARD group had higher annual all-cause HRCU costs ($27,772) and higher outpatient pharmacy costs ($3,560) than all patients with PsA diagnosis ($11,316 and $1,872, respectively), it should be noted that patients in the bDMARD group had nearly one-half the median days of hospital care per PsA-related hospitalization event as patients in the overall PsA group (6.0 days versus 11.5 days). The annual mean per-patient cost of $27,772 (2018 USD) for all-cause HCRU of bDMARD users is comparable to the annual all-cause per-patient cost of $29,621 (2018 USD) that Merola et al. [Citation27] found for patients with PsA in the US, although this study did not report the composition of bDMARD users.
Some patients received treatments that were off-label for PsA during the study window, including methotrexate (34.0%), sulfasalazine (13.8%), and golimumab (1.6%), but these medications could have been prescribed to treat comorbidities or PsA-associated conditions and not necessarily PsA. Most patients had an index PsA diagnosis attributable to a physician department of dermatology (56.3%). Oral NSAIDs were the most frequently prescribed first-lined treatment, followed by corticosteroids and methotrexate. Approximately one-third of patients (32.2%) used bDMARDs within 12 months following diagnosis. The majority of patients who received bDMARDs were administered adalimumab and/or infliximab (75.7%, 156/206), which reflects the bDMARD treatment patterns observed by Kishimoto et al. [Citation28] for patients with psoriasis and concomitant PsA. Notably, the persistence rate for bDMARDs at 12 months (62.8%) was higher than the 12-month persistence rate (44.5%) reported for PsA patients on bDMARDs in the US [Citation21]. Patients who received bDMARDs had greater annual per-patient costs, spent fewer days in the hospital per visit, and had lower hospitalization costs compared with the overall cohort of patients with PsA.
The most frequently prescribed first-line treatments, NSAID only (23.2%), NSAID + csDMARD (14.1%), and csDMARD (10.0%), are in accordance with the 2016 EULAR recommendation to initiate treatment with NSAID or csDMARDs, depending on the extent of joint damage [Citation12]. The administration of methotrexate and sulfasalazine, the two most frequently prescribed csDMARDs, is especially notable since these prescriptions indicate that physicians were following EULAR recommendations despite methotrexate and sulfasalazine being off-label in Japan during most of the study window. Methotrexate was not approved for PsA indication in Japan until 2018, and sulfasalazine had not received approval as of early 2020. First-line bDMARD monotherapy administration, which is not recommended by the EULAR guidance, was observed in 6.4% of patients. Most of these administrations (90.2%) were associated with an index PsA diagnosis from the dermatology specialty, which could indicate dermatologists are prescribing first-line bDMARDs earlier than other physician specialties. This potential association between physician specialty and first-line bDMARD administration is supported by our logistic regression analysis, which found the probability of a patient receiving bDMARDs within 12 months of being diagnosed with PsA was significantly decreased with an index diagnosis from rheumatology, orthopedics, or internal/general medicine specialties as compared with dermatology specialty (p < .05). We have included additional details on the differences in treatment patterns between dermatologists and other physician specialties in Supplementary Tables S6 and S7 of the Supplementary Material. However, the database used, study design, and methodology are not suitable for extracting or analyzing comparison data between physician specialties. The primary decision maker for a patient’s treatment, as well as the prescriber of biologics, cannot be confirmed using the dataset. First-line bDMARD administrations could also potentially be assigned because of an immunologic comorbidity, such as concurrent psoriasis, or, possibly, because of the nature of a database study whereby the database does not accurately capture the first PsA diagnosis (i.e. patient may be referred to a hospital included in MDV database from other hospitals). The rates of first-line bDMARD administration are considerably lower than that reported by a US database study, where 42.8% of patients with PsA initiated treatment with a bDMARD [Citation29]; however, this should be interpreted with caution, as it is not clear if the first-line treatment category excluded patients who initiated with NSAIDs. The 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of PsA [Citation30] recommends first-line administration of TNF inhibitors instead of oral small-molecule treatments, including methotrexate, sulfasalazine, and cyclosporine, in treatment-naive patients. The Biologics Review Committee of the JDA [Citation11] recommend early administration of bDMARDs that have demonstrated evidence of preventing joint damage in order to minimize a decline in activities of daily living. However, it is unclear if early administration encompasses first-line administration. The delayed usage of bDMARDs could have implications for patient healing, as the efficacy of TNF-α inhibitors may decrease when administered in later treatment lines [Citation31]. These observations suggest a potential need for monitoring the utilization of bDMARDs in addition to increasing communication of the guidelines among the different physician specialties who treat PsA in Japan.
There are some limitations with this study. Results from our study may not be generalizable to all patients with PsA in Japan, since only participating facilities provide data to the MDV database. Although this study required that patients have a minimum of 3 months without documentation of PsA before the first observed PsA diagnosis, patients may still have received a diagnosis of or been treated for PsA at a nonparticipating facility. These unavailable data could have resulted in these patients being misclassified as having newly received a PsA diagnosis. Similarly, some comorbidities (e.g. depression) may have been underreported if these conditions were not treated at a hospital, since hospital databases were used for this study. The primary analysis was conducted using a 3-month baseline period and a 12-month follow-up period. Increasing the durations of the baseline and follow-up periods would have restricted the available sample sizes for this study. Patients who were treated at more than one hospital would appear as multiple unique individuals since hospitals in the MDV database did not link individual patient data. Additionally, five predominant bDMARDs were approved for PsA indication in Japan after the start of the study selection window in April 2009. Infliximab and adalimumab were approved for PsA indication in 2010, ustekinumab in 2011, secukinumab in 2014, and ixekizumab in 2016. Because the study window ended in 2018, not all currently approved bDMARDs may be reflected in the study since guselkumab was approved in 2018 and both certolizumab and risankizumab in 2019. Another limitation is the difficulty of analyzing actual adherence using claims data; however, we were able to estimate adherence using MPR data [Citation21]. Finally, data on laboratory test results, radiographical information, and severity of PsA were not available for this study. Accordingly, assessments of background characteristics relied only on ICD-10 coding, which could have resulted in an underestimation of the prevalence of these conditions. The definition of PsA in the claims data in Japan has not been validated, so another limitation was the reliance on ICD-10 coding to define PsA.
5. Conclusion
This database study presents knowledge of the current state of patient characteristics, treatment patterns, HCRU, and costs among patients with PsA in Japan. Considering the relatively recent guideline recommendations, the preliminary treatment patterns suggest physicians may be following treatment guidelines.
Supplemental Material
Download MS Word (43.6 KB)Acknowledgements
Eli Lilly Japan K. K. provided financial support for the study. The authors thank Brian Samsell of RTI Health Solutions for medical writing assistance.
Conflicts of interest
MS, SK, and HTI are Eli Lilly Japan K. K. employees and hold stock in Eli Lilly Japan K. K. KI is a paid consultant for Eli Lilly Japan K. K. but did not receive compensation for participation in this study. EE and RP are employees of RTI Health Solutions, an independent nonprofit research organization that does work for government agencies and pharmaceutical companies.
References
- Hukuda S, Minami M, Saito T, Mitsui H, Matsui N, Komatsubara Y, et al. Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J Rheumatol. 2001;28(3):554–9.
- Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–ii7.
- Kubota K, Kamijima Y, Sato T, Ooba N, Koide D, Iizuka H, et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5(1):e006450.
- Ohara Y, Kishimoto M, Takizawa N, Yoshida K, Okada M, Eto H, et al. Prevalence and clinical characteristics of psoriatic arthritis in Japan. J Rheumatol. 2015;42(8):1439–42.
- Yamamoto T, Ohtsuki M, Sano S, Igarashi A, Morita A, Okuyama R, et al. Epidemiological analysis of psoriatic arthritis patients in Japan. J Dermatol. 2016;43(10):1193–6.
- Tanaka YP. arthritis in Japan: difference in clinical features and approach to precision medicine. Clin Exp Rheumatol. 2016;34(4 Suppl 98):49–52.
- Yang Q, Qu L, Tian H, Hu Y, Peng J, Yu X, et al. Prevalence and characteristics of psoriatic arthritis in Chinese patients with psoriasis. J Eur Acad Dermatol Venereol. 2011;25(12):1409–14.
- Mease PJ, Gladman DD, Papp KA, Khraishi MM, Thaci D, Behrens F, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–35.
- Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374(26):2563–74.
- Ohtsuki M, Terui T, Ozawa A, Morita A, Sano S, Takahashi H, et al. Japanese guidance for use of biologics for psoriasis (the 2013 version). J Dermatol. 2013;40(9):683–95.
- Saeki H, Terui T, Morita A, Sano S, Imafuku S, Asahina A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 version). J Dermatol. 2020;47(3):201–22.
- Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510.
- Sruamsiri R, Iwasaki K, Tang W, Mahlich J. Persistence rates and medical costs of biological therapies for psoriasis treatment in Japan: a real-world data study using a claims database. BMC Dermatol. 2018;18(1):5.
- Tomita T, Sato M, Esterberg E, Parikh RC, Hagimori K, Nakajo K. Treatment patterns and health care resource utilization among Japanese patients with ankylosing spondylitis: a hospital claims database analysis. Mod Rheumatol 2020;2020:1–11.
- Akazawa M, Database, research and regulation in Japan. Presented at the ISPOR Asia Pacific 2018, Tokyo, Japan. 2018.
- Medical.Data.Vision. Introducing MDV Database. 2020. Available from: https://www.mdv.co.jp/mdv_database/english/ [last accessed 28 Feb 2020].
- Sato M, Ye W, Sugihara T, Isaka Y. Fracture risk and healthcare resource utilization and costs among osteoporosis patients with type 2 diabetes mellitus and without diabetes mellitus in Japan: retrospective analysis of a hospital claims database. BMC Musculoskelet Disord. 2016;17(1):489.
- Fuji T, Akagi M, Abe Y, Oda E, Matsubayashi D, Ota K, et al. Incidence of venous thromboembolism and bleeding events in patients with lower extremity orthopedic surgery: a retrospective analysis of a Japanese healthcare database. J Orthop Surg Res. 2017;12(1):55.
- Tanaka K, Hamada K, Nakayama T, Matsuda S, Atsumi A, Shimura T, et al. Risk for cardiovascular disease in Japanese patients with rheumatoid arthritis: a large-scale epidemiological study using a healthcare database. Springerplus. 2016;5(1):1111.
- Wang F, Mishina S, Takai S, Le TK, Ochi K, Funato K, et al. Systemic treatment patterns with advanced or recurrent non-small cell lung cancer in Japan: a retrospective hospital administrative database study. Clin Ther. 2017;39(6):1146–60.
- Walsh JA, Adejoro O, Chastek B, Palmer JB, Hur P. Treatment patterns among patients with psoriatic arthritis treated with a biologic in the United States: descriptive analyses from an administrative claims database. J Manag Care Spec Pharm. 2018;24(7):623–31.
- Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61(12):1234–40.
- Walsh JA, Adejoro O, Chastek B, Park Y. Treatment patterns of biologics in US patients with ankylosing spondylitis: descriptive analyses from a claims database. J Comp Eff Res. 2018;7(4):369–80.
- Japan’s consumer price index annual report. 2018. Available from: https://www.stat.go.jp/english/data/cpi/index.html [last accessed 4 Dec 2019].
- Pound Sterling Live. The U.S. Dollar to Japanese Yen Historical Exchange Rates Conversion Page for 2018. 2019. Available from: https://www.poundsterlinglive.com/best-exchange-rates/best-us-dollar-to-japanese-yen-history-2018 [last accessed 30 Mar 2020].
- Shah K, Paris M, Mellars L, Changolkar A, Mease PJ. Real-world burden of comorbidities in US patients with psoriatic arthritis. RMD Open. 2017;3(2):e000588.
- Merola JF, Peterson S, Dennis N, Chakravarty SD, Mesana L, Lin I, et al. Retrospective study examining health care utilization and costs for patients with psoriasis and psoriatic arthritis in the US. ISPOR 2020. Orlando, FL, USA.
- Kishimoto M, Komine M, Kamiya K, Sugai J, Mieno M, Ohtsuki M. Drug survival of biologic agents for psoriatic patients in a real-world setting in Japan. J Dermatol. 2020;47(1):33–40.
- Lee MP, Lii J, Jin Y, Desai RJ, Solomon DH, Merola JF, et al. Patterns of systemic treatment for psoriatic arthritis in the US: 2004–2015. Arthritis Care Res. 2018;70(5):791–6.
- Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. Special article: 2018 American College of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019;71(1):5–32.
- Reddy SM, Crean S, Martin AL, Burns MD, Palmer JB. Real-world effectiveness of anti-TNF switching in psoriatic arthritis: a systematic review of the literature. Clin Rheumatol. 2016;35(12):2955–66.