Abstract
Umbilical cord blood unit (CBU) “quantity” continues to grow internationally, while cord blood transplantation (CBT) “quality” remains poorly defined and subject to uncertainty. CBT “quality” is affected by both the product (i.e. CBUs) and CBT processes, with “best practice” varying across countries. To improve overall CBT “quality”, we need to better understand the uncertainty associated with CBUs and CBT processes and how staff manage it. In this qualitative study, we conducted in-depth semi-structured interviews with individuals working in CBT in UK and Japan. We found that understanding of CBT quality by the cord blood community is underpinned by the quality of the CBU, the expertise and collaboration of scientific and clinical stakeholders, trust in collection and testing processes and international accreditation. Importantly, we found that local and individual experience is used to manage uncertainty within CBT, and we propose that selection guidelines should acknowledge the extent of uncertainty in decision-making.
Introduction
Since Gluckman’s first successful trials in Fanconi’s anemia in 1988, umbilical cord blood units (CBUs) have become an important source of hematopoietic stem cells (HSCs) for various hematological conditions (Gluckman et al. Citation1989). CBUs can act as a source of HSCs for patients who would otherwise struggle to find a bone marrow match, such as those from ethnic minority communities and those without genetic siblings (Dessels, Alessandrini, and Pepper Citation2018; Hough et al. Citation2016). CBUs require less accurate Human Leukocyte Antigen (HLA)-matching to be useful for a wider population compared to bone marrow (Dessels, Alessandrini, and Pepper Citation2018). Furthermore, CBUs are already physically stored when the decision is taken to use them for transplant, whereas there is a “necessary and time-consuming” gap for the bone marrow to be harvested from matched donors (Brown, Machin, and McLeod Citation2011). Hence, the rapidly available and “off-the-shelf” nature of CBUs for a broad patient population is particularly attractive in clinical emergencies and when procurement becomes difficult, such as during the SARS-Cov2 pandemic (Kindwall-Keller and Ballen Citation2020).
Yet, cord blood transplantation (CBT) can still be considered a novel technology. It accounted for only 6% of allografts in the UK, and the number of CBUs shipped globally fell by nearly one-third between 2012 and 2019 (World Marrow Donor Association Citation2020). Conversely, the number of internationally banked CBUs has continued to rise, resulting in a growing surplus. The standards for CBUs to be acceptable for transplantation have developed over time, which is captured in the work of Eurocord; a cord blood registry and study group that works with banks and registries to analyze outcomes of CBT (http://eurocord.org/index.php). The exact number of CBUs currently stored that would be acceptable for transplantation today is unknown. These evolving standards reflect the uncertainties and complexities surrounding determining “quality” with CBUs. Consequently, there have been calls from within the cord blood community to focus upon and better understand defining and improving “quality” in CBUs (Querol et al. Citation2010). To date, there has been limited exploration of the notion of “quality” in CBT that goes beyond the scientific community or that adopts a qualitative approach to the topic.
“Quality” cord blood
The notion of “quality” in cord blood is rarely complicated in the existing literature, and instead an objective depiction of the “scientific” characteristics is presented. CBT “quality” can be split in to intrinsic CBU product “quality,” relating to the biological factors of the CBU, and process “quality,” which includes collection, processing, procurement and treatment. For clarity, an overview of CBT processes is shown in . Collection “quality” and the associated ethical conflicts of CBU collection have been discussed elsewhere (see Machin Citation2016) and will therefore not be discussed further in this paper. Instead, we will summarize the current understanding of CBT “quality” in the literature and in clinical practice.
Figure 1. Cord Blood Treatment Process. The Cord Blood Treatment Process that was developed following interviews. A number of factors that contribute to the “systems” uncertainty have been noted for each phase of the process.
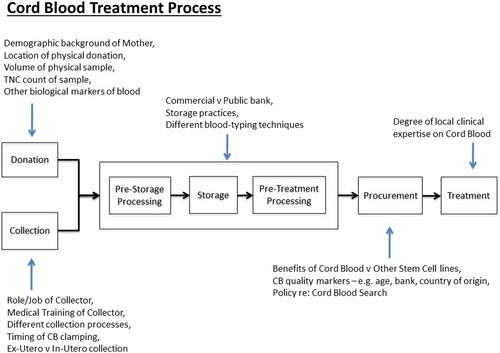
The concept of CBT “quality” is defined by international standards and national guidelines. International standards were first created in 2000, when the International NetCord Foundation (NetCord) and the Foundation for the Accreditation of Cellular Therapy (FACT) first collaborated as NetCord-FACT. These standards cover all areas of CBT; NetCord-FACT provide accreditation for banks who meet these “quality” standards, providing some level of international standardization of CBU banks. The most recent standards enact CBU testing requirements for banks, which can be split into safety requirements (e.g. viral screening, ABO/Rh) and markers of CBU “quality” (NetCord-FACT Citation2019). These “quality” markers include CD34 + count, Total Nucleated Cell (TNC) count, TNC viability, CD34 + viability, Colony Forming Units and HLA typing. National guidelines differ across countries and often prioritize different measures of CBU “quality.” While findings from Eurocord have led to the modification of cell dose thresholds to improve CBT outcomes, uncertainty remains and varies across different professional domains and stakeholder groups regarding the most accurate “quality” measure, minimum acceptable cell counts for CBT success and how best to perform CBU selection (see for key “quality” markers). However, these are “known unknowns” and conscious debate on these issues is ongoing, both in the literature and between different nation’s guidelines (Barker, Byam, and Scaradavou Citation2011; Dehn et al. Citation2019; Hough et al. Citation2016; Politikos et al. Citation2020; Rich Citation2015; Shaw et al. Citation2009).
Table 1. Examples of uncertainties surrounding key “quality” markers for CBU based on published literature.
Quality in laboratory medicine has been defined as “the guarantee that each activity throughout the total testing process is correctly performed, providing valuable medical decision-making and effective patient care” (Lippi et al. Citation2013). Although the quality of analytic processing has improved significantly throughout laboratory medicine, pre- (i.e. CBU collection) and post-analytical (i.e. CBU storage) stages remain targets for improvement. This is primarily by identifying quality indicators (Lippi et al. Citation2013). Yet, intrinsic CBU product, CBU process “quality,” and its’ impact on clinical outcomes remains poorly understood in the literature. For example, the impact of the collection bag material and the maximal appropriate duration of CBU storage is “still not defined” (Querol et al. Citation2010), highlighting inadequate understanding surrounding quality indicators in post-analytical CBU processing. Studies report no differences in clinical outcomes relating to the processing methods used by different banks (Ballen et al. Citation2015; Nikiforow et al. Citation2017; Saccardi et al. Citation2016; Santos et al. Citation2016). Detailed descriptions of processing methods are provided in NetCord-FACT standards, although the impact of variation within such methods remains unknown, and further “unknown unknown” factors may yet exist undiscovered.
These “unknowns” when contemplating CBT quality highlight “the limitation of our knowledge” and the epistemic uncertainty that results (Indrayan Citation2020). Epistemic uncertainty, due to an incomplete knowledge of the particular system in question, is ubiquitous within medicine, particularly when considering technological advancement and novel treatments such as CBT. Process “quality” has arguably been discursively and pragmatically constructed by prominent actors, including NetCord-FACT and those in transplant centers drawing on their clinical experiences and as such varies across professional domains. Within the UK CBT community, governmental (Department of Health’s Stem Cell Strategy Oversight Committee) and medical groups (British Society of Blood and Marrow Transplantation Cord Blood Working Group) have led stakeholders to form a clear and unified narrative of the current “state of play.” In turn, stakeholders steer clinical practice and research forming a “collective production of (un)certainty”; presenting what is known about CBT with confidence and highlighting what needs to be known for practice to develop and advance (Cambrosio et al. Citation2006; Moreira, May, and Bond Citation2009).
Yet, CBT operates within a complex bionetwork, which is defined as consisting of a “plurality of actors … working across geographical spaces, regulatory regimes and social institutions” (Sleeboom-Faulkner and Patra Citation2011). CBT product and process “quality,” and their associated uncertainties, form only the content of the CBT bionetwork; the diverse range of stakeholders add further complexity to narratives surrounding CBT. In current CBT practice, different “truths” seem to simultaneously co-exist, as shown by variation between national guidelines and local selection policies (Barker, Byam, and Scaradavou Citation2011; Hough et al. Citation2016). Consequently, what determines quality in one domain such as registries and biobanks, may not satisfy the criteria for those in transplant centers. The interplay therefore between national and international interest groups, including their differing recommendations, motives, and relationships, creates a “shifting landscape” (Williams Citation2018). The aims of these stakeholders may not overlap and networking means that each player’s role becomes blurred (Chang Citation2016). This can frustrate transplant centers as they must put these differing recommendations into practice and evolve in response to the “shifting landscape” (Williams Citation2018). In addition, transplant centers “have very little control over what happens in other jurisdiction(s)”; NetCord-FACT standards allow significant variation within acceptable practice, giving transplant centers little control over the “quality” of processing methods (Brown, Machin, and McLeod Citation2011). Thus, a successful CBT bionetwork relies on collaboration between stakeholders, including transplant centers; to build trust, increase confidence in policy and ultimately navigate the complexity of the CBT bionetwork.
Collaborations and networks
For collaborations to be successful, professional boundaries need to be transcended, which can lead to a loss of autonomy for all those involved (D’Amour et al. Citation2005; Freeth Citation2001). Furthermore, complex relationships can form between the different occupational groups, sometimes reflecting the implicit power imbalances that can emerge when working collaboratively (D’Amour et al. Citation2005; Gachoud et al. Citation2012). In response to the perceived loss of power and autonomy, informal processes can form surrounding the interactions between professional groups, which facilitate the continuation of the collaboration as well as upholding professional boundaries (Heldal Citation2010). Through these informal processes, we can observe professional “territories of care,” each with their own priorities and patterns of working (Hardey et al. Citation2001) and distinct areas of knowledge and practice (Langan-Fox and Cooper Citation2014). Knowledge is deemed central to professional control (Abbott Citation1988), and therefore “discursive” battles can result as occupational groups attempt to claim or maintain their jurisdiction and expertise (Covaleski, Dirsmith, and Rittenberg Citation2003). One occupational group can attribute characteristics to another group in order to distinguish themselves more favorably, for example, work activities can be portrayed as “scientific” or “non-scientific” (Gieryn Citation1983). Such discursive boundary work (Gieryn Citation1983) observed in the context of CBU banking (see Machin, Brown, and McLeod Citation2012) has similarly been explored within the CBT community. Beltrame (Citation2020a; Citation2020b) addresses the jurisdictional disputes in relation to the practices of valuing the clinical quality of cord blood.
Collaborative networks have been key to CBT since its inception, with national and international banking and matching networks including Eurocord and National Marrow Donors Association (Brown, Machin, and McLeod Citation2011). Nations and their donors have often been viewed as altruistic participants, donating “gifts” to an inclusive and collaborative community (Brown, Machin, and McLeod Citation2011). Whilst the majority of CBT practice exists within a public international community, notable exceptions are private CBU banks (where individuals store CBUs for their own familial use only) and nations with a self-sufficient CBU supply, such as Japan (who generally do not engage with international CBU trade). Different nations, such as Japan and the UK can therefore hold different positions within the CBT bionetwork, often influenced by culture and strategy.
CBT in Japan and the UK
Japan were early adopters and innovators in CBT, creating a CBT committee in the mid-1990s and national bank network in 1999 (Takanashi et al. Citation2011). This coincided with the Japanese government’s Millennium Project, part of an expansive plan to become “the scientific and technological nation” (Fukushima Citation2016). Contradicting the international trend, Japan has continued to perform more CBTs year-on-year (Japanese Red Cross Society Citation2021). Underpinning the Japanese successful CBT program are a strong national collaboration via the Japanese Cord Blood Bank Network, and a prioritization of scientific rather than political goals. The potential damaging influence of politics in science is reflected in the failed Japanese Protein 3000 Project, which had significant political involvement and therefore struggled with high societal expectations of the scientific achievements resulting from political over-promises (Sleeboom-Faulkner Citation2011). For Japanese scientists then, widespread collaboration, or high political involvement could equate to unrealistic public and professional expectations of science and a decreased ability to exert control over a project. That said, the importance of CBT for the country was reflected in 2014, when the Act for Appropriate Provision of HSC to be Used in Transplantations was enacted in Japan, which aimed to increase efforts to “ensure the quality of CB(Us) used in transplants.”
The UK government were slower than the Japanese government in their uptake and focus on cord blood, with a national strategy on CBU banking not developed until 2010. Data showed that 90% of CBUs given to UK patients were imported, with each unit being “prohibitively expensive” (Brown, Machin, and McLeod Citation2011). The UK government promoted collaboration between the main UK CBU banks Anthony Nolan and the National Health Service Blood and Transplant agency (Williams Citation2015). The interests of these two CBU banks were aligned as a result of financial incentives, which prompted a switch from international “competition” to national “collaboration” (Williams Citation2015). As a result, the UK planned to increase their domestic CBU supply with the hope of achieving self-sufficiency (All Party Parliamentary Group on Stem Cell Transplantation Citation2012; UK Stem Cell Strategic Forum Citation2010). In 2012, the matter of quality CBUs arose, when the UK All Party Parliamentary Group on Stem Cell Transplantation proposed a best practice tariff to reimburse hospitals for securing “high quality collections.” Given the high level of imports and HLA diversity of the UK population, as with most Western countries, complete self-sufficiency has been deemed to be an unrealistic target (Williams Citation2015). Japan, however, is considered unique when described as the “only country [that can be CBU self-sufficient] … because they’re such a homogeneous group”; in 2008, Japan did not import a single CBU (Brown, Machin, and McLeod Citation2011). As such, Japan has sat outside the international CBT community and appears immune to international fluctuations in cord blood policy or supply (Brown and Williams Citation2015). Japan and the UK hold vastly different positions within the CBT bionetwork, particularly their national policies on CBT. Their positions are affected by (geo)politics, history (of scientific advancement), culture and ethnic diversity within each country. Consequently, their definitions of CBT “quality” may differ.
To date, there has been limited primary qualitative research on CBT “quality.” A focus on CBU “quality” (rather than “quantity”) and qualitative understandings of CBT generally may support progress within CBT (Querol et al. Citation2010). In this paper, we therefore aim to broaden understandings surrounding “quality” within CBT and promote wider discussion within the literature. We propose CBT “quality” (and its’ individual elements) cannot be accurately captured within singular definitions. Instead, descriptions of “quality” may be discursively constructed from differing perspectives within the “shifting landscape” of the CBT bionetwork (Williams Citation2015) and are therefore vulnerable to the ubiquitous epistemic uncertainty within CBT practice. It is noteworthy that the matter of “quality” CBU from the perspectives of those working in transplant centers have rarely been explored in-depth. We suggest that transplant centers act as “regime(s) of truth” describing the situation as it is, rather than how it should be in ideal circumstances, making their perspective of particular interest (Martin, Brown, and Turner Citation2008; Moreira and Palladino Citation2005; Williams Citation2015). In this paper, we provide insight into how some stakeholders working in transplant centers understand CBT “quality” in practice. We explore how trust, collaboration, uncertainty, expertise and experience can inform and shape the varying perceptions of “quality” of these stakeholders. We conclude that the cord blood community uses their own experience and that of trusted colleagues, to manage uncertainty within CBT to varying extents. In turn, the role of experience, expertise and collaboration in decision-making is affirmed and arguably valued.
Materials and methods
The study took place at hospitals in Japan and the UK during July 2014 and July 2015. Participants were recruited because of their close and prominent involvement in CBT. This included two transplant consultants from different sites in the UK, three transplant consultants from three different sites in Japan and four other transplant team members, all of whom are from the same site as UK Consultant 2 (a tissue typer, a transplant co-ordinator, a lab manager and a clinical nurse specialist). Recruitment was via an email invitation disseminated by departmental secretaries and participation was on a voluntary basis. Nine in-depth semi-structured interviews were conducted; each lasting between 30 and 90 min. Consent was gained prior to interview. Institutional ethical approval was granted for the research to be conducted in Japan (National Institute of Public Health, Tokyo) and in the UK (Lancaster University Research Ethics Committee). Governance approvals were also gained from each hospital’s Research and Development Department.
An active interview approach was adopted (Holstein and Gubrium Citation1995). The interviews focused on health professionals’ decision-making relating to CBT, and their perceptions of the roles and responsibilities of others in the wider cord blood community. The transcribed data from the interviews were read multiple times and coded using Nvivo software. The codes were then grouped into over-arching themes and a thematic analysis was undertaken; the analysis was, therefore, an iterative process (Braun and Clarke Citation2006).
Results
Our data highlighted that understandings surrounding “quality” within CBT could be divided into the following higher themes: product, expertise and collaboration, trust, and local experience and international accreditation. Epistemic uncertainty was present throughout the data.
Product
Participants declared the importance of cell dose and HLA match in CBU selection. However, participants challenged the certainty with which the importance of cell dose and HLA match has been presented in the cord blood community, and the priority given to one factor over the other in CBT.
There are different views on the importance of two factors [cell dose and HLA match], which one is how important. (Japan Consultant 2)
Regarding the acceptable minimum cell dose for successful CBT, participants highlighted the lack of certainty and scientific rigor in determining this number. Participants commented on differences between minimum cell doses, and the supporting research, used in the UK and Japan in particular.
So there becomes a point, and there’s a magic number that has been somewhat plucked out of mid-air of four and everybody uses this. Why do we use four? Well, because we’ve always used it. Where’s your proof of it? (UK Transplant Nurse)
Nobody knows what is the true lower limit [of viable cell dose]. (Japan Consultant 1)
Whilst cell dose and HLA match were considered of importance by all participants, regardless of the associated epistemic uncertainty, they did not consistently agree whether other factors were associated with CBU “quality” or not. CBU age and collection method were two such examples.
But we do try to look for ones that are newer because they tend to be better quality. (UK Consultant 2)
We don’t generally [consider the age of the cord as significant], no. (UK Consultant 1)
Beyond these recognized factors, participants suggested there were many other potentially important factors that were not yet known to the cord blood community.
There must be more factors other than HLA matching … However, these are still in a black box and yet to be understood. We believe these factors are valid, but not for sure. (Japan Consultant 2)
Participants highlighted CBT research with contradictory outcomes conducted in Japan and the UK. Population differences between the two countries, such as ethnicity were presented as justification for these differences, alongside the unknown yet important factors affecting CBT.
There is inconsistency in evidence, we cannot explain it medically or scientifically … Because transplantation treatment is extremely complicated, a slightly different conduct, which may not look different, can often cause a completely different result. (Japan Consultant 2)
Expertise and collaboration
Both UK and Japanese participants identified “expert knowledge” as an important factor in determining CBU “quality.” UK participants portrayed transplant consultants and tissue typers as “the experts” within the CBT multidisciplinary team. Both expert groups acknowledged the importance of the other, which indirectly affirmed the significance of their own role within the team and allowed little space for others to gain authority. Transplant consultants from both countries displayed little knowledge of how CBUs were processed, for example, they were unable to explain how bank laboratory staff processed a CBU following collection. This created a boundary between “processing” and “clinical” expertise in CBT, as well as the importance of collaborative knowledge.
Yeah, that’s kind of their job. That’s what they do … ultimately I’m not going to be there going through and typing. I’m going to be a complete Luddite with it. I’ve got a patient, they need a transplant, provide me with something to transplant. (UK Consultant 2)
Then, if I was asked … how to collect samples or which method of storage is considered good, it would be difficult for me to give an answer. (Japan Consultant 2)
Tissue typers were depicted by participants as providing “scientific” expertise regarding the “quality” of available CBU products in a supportive yet “really integral” (UK Consultant 1) role, which assisted the transplant consultants in their decision-making. That tissue typers “provide advice for other centres” (UK Consultant 1) was perceived by participants as a sign of their excellence, and (international) collaboration demonstrated an individual’s expertise. Yet, it also distanced the tissue typer from the “core” transplant team. Transplant consultants were “guided by what our tissue typing labs … tell us that is the best cord unit” (UK Transplant Coordinator). To some extent, the transplant consultant presented their own expertise as limited in comparison to tissue typers. Yet, it also enabled the transplant consultants to present themselves as providing leadership, and having an ability to manage the complexity that arises within CBT; the complex decisions were therefore left “mostly to the consultants and what they want” (UK Transplant Coordinator). These portrayals set professional boundaries within the team; with knowledge forming around “expert” and “non-expert,” “scientific” and “clinical,” and “core” and “adjunct” team membership. By constructing differences between their roles in this way, transplant consultants and tissue typers cemented their own niche contributions within the team and prevented their role and expertise being encroached upon by the other. In this way, tissue typers were deemed as “scientific” experts, whereas transplant consultants were “non-scientific,” patient-centred clinical leaders, with little overlap between the two. Equally, these divisions in knowledge mapped onto participants perceived “territories of care” with the laboratory as a territory whereby care was provided during the CBU during processing (Hardey et al. Citation2001). In contrast, the transplant center was the site of care for the patient receiving the CBT.
British and Japanese participants presented decision-making as requiring the human oversight and experience of the transplant consultant, as they can “make a comprehensive judgement, rather than focusing on one point” (Japan Consultant 2). The desire to make one’s own independent decisions, rather than rigidly following guidelines, was presented as a “natural response” by a UK Transplant Nurse, “Nobody likes being told this is the only way to do it.” Japanese participants portrayed themselves as having greater trust in individuality in decision-making, and therefore were considered to benefit from greater clinical freedom and flexibility as a result. In turn, their British counterparts were depicted as restricted by the guidelines and policies of international organizations.
If these people say it is 2 or 2.5 [cell count limit], even though they are in the countries of individualism … all the others say the same. Interestingly in Japan … it seems to me that they make a little allowance. (Japan Consultant 1)
Japanese transplant consultants collaborated with individuals, although there was no mention of group or team discussion, as happened in UK selection meetings. In contrast, UK participants valued collaboration and regular communication with individuals within “very small network(s)” (UK Transplant Nurse), both locally and internationally. The perceived benefits were that collaboration promoted trust, improved relationships, and reduced uncertainty in decision-making. Additionally, the formation and protection of professional boundaries did not impede but enabled collaboration between tissue typers and transplant consultants. They shared close relationships; trust and mutual respect built up over time as they adopted “an open attitude” (UK Consultant 1) towards each other.
Trust and uncertainty in the process
UK transplant consultants discussed their attempts to manage CBU uncertainty. They described the strategies they put in place to prepare for potential issues in order to ensure overall CBT “quality,” e.g. assessing the viability of a thawed vial, double-checking HLA typing of the CBU itself once it arrived, and ordering back-up cords. These strategies were usually discussed with implicit or explicit reference to the consultants’ inability to measure all the variables that could influence CBU processing. When patients had poor outcomes from their CBT, UK participants claimed they found it difficult to know where or when errors had occurred.
Well, we’ve never quite sussed it out. It could have been from there or it could have been waiting around too long. It could have been the freezing process. (UK Lab Manager)
These uncertainties mainly related to post-analytical issues, rather than analytical errors made by laboratory staff, and therefore highlighted the lack of quality indicators that transplant centers used to measure “quality.” Conversely, UK teams felt vindicated in their strategies by positive CBT results. Put simply, the ends justified the means. The “success” of a CBT was reinforced by the UK teams’ portrayals of processing methods as difficult to change, and requiring validation, and team members required to adjust or learn new skills. The UK participants displayed less interest in methodological detail than their Japanese colleagues, who presented themselves as interested in the “technical aspect” (Japan Consultant 3) of CBU processing.
So they need to find a better method and standardise if possible but at the end of the day the stuff that we’re getting is actually okay so we’re not hugely worried about it. (UK Lab Manager)
Transplant consultants trusted CBU banks for different, but often related reasons. Trust was built through direct experience with a specific bank, the volume of and ease of access to information they received about a CBU, as well as a bank’s reputation. Japanese participants’ trust in banks increased if they perceived “quality” methods were used. This sense of trust was confirmed for Japanese participants when they checked the banks’ methods, “I do not believe anything until I check it by myself” (Japan Consultant 3). In turn, Japanese participants developed trust for specific experts within a particular CBU bank, “I think it is difficult unless you know people inside the institution” (Japan Consultant 3). Essentially, people were deemed the most important quality indicator; hence, trust (in people) and quality were presented as synonymous. Positive experiences would encourage participants to use a bank again. In contrast, UK transplant centers had placed trust in international banks that they used based on the accreditation system, as it was impractical for them to visit every bank and assess its methods. This trust assumed a shared professionalism from all involved.
The quality of what ends up being thawed after having been frozen, you have no idea. All you’ve got is a document that tells you what was put in the freezer … The handling of that unit when it’s thawed out and washed by the stem cell laboratories, you’re going to trust that they do it well and are competent to do it. There is … a lot of trust along the way with this. (UK Tissue Typer)
Yet, UK participants undermined CBU selection when they claimed an inability to eradicate all uncertainties surrounding CBT. Once transplant centers had selected their “most suitable” cord(s), UK participants expressed frustration that up to half of all patients would be affected by an unviable CBU having been selected and the delays that followed as a result.
When you’re presented with 100 units and a combination of any of those 100 units would fit the bill, the number of variations you’re going to get is enormous. So there isn’t necessarily an absolute right answer every time there can be two or three right answers. (UK Tissue Typer)
Local experience and international accreditation
UK transplant consultants portrayed NetCord-FACT accreditation as the initial, yet essential, criteria that CBU banks must meet in order to gain a level of trust. Participants presented the accreditation as a pragmatic solution to assess the banks’ methods of CBU collection, processing and storage. UK participants deemed CBUs from banks without accreditation to be inferior in “quality” to those from banks with accreditation.
It gives you that Kite mark, if you like. It’s like if you go into the shop and you see something that’s properly packaged and has Marks & Spencer written on it then there’s a feeling that it’s okay. (UK Consultant 1)
However, a Japanese transplant consultant claimed NetCord-FACT standards “cannot measure the technical aspect” (Japan Consultant 3) of processing. For this participant, these standards did not measure “quality” per se. Even within accredited banks, it was considered that the “quality” varied because of the range of different methods used. Not all accredited banks provided every piece of information mandated by the standards. Consequently, some UK participants claimed “some [banks] are better than others” (UK Consultant 2). All participants positioned the “flexibility” within the standards arising from banks using different methods to claim accreditation as assuring “safe processing” rather than “quality.”
The JACIE [NetCord-FACT] criteria are written so that they can be moved a little bit. It’s not you must absolutely do this, this and this it’s a guideline to say these are the things we expect you to do. Can you prove that you do these things in the right way to give you the right result at the end? (UK Transplant Nurse)
Both UK and Japanese participants prioritized banks with which they had previously used and the experience had been positive. Yet, it was “the quality of [a] person’s experience [and] the degree of experience” (Japan Consultant 1), including both failures and successes, that influenced and informed participants’ trust in a bank. One Japanese participant suggested that experience could compensate for cell doses lower than the acknowledged lower limit to ensure outcomes perceived as “quality” CBT.
Both UK and Japanese participants displayed preferences in the banks from which they received CBUs, although their preferences were not based on evidence available in published literature or international guidance. Japanese centers were depicted by participants as having a preference for CBUs from domestic banks because international transportation was perceived as increasing the risk of accidents, “Japanese banks are more familiar and their regulations are known” (Japan Consultant 2). Equally, with CBUs from abroad, “there are unknown elements there” (Japan Consultant 2). By comparison, UK transplant centers chose domestic CBUs for their lower associated costs.
Japanese transplant consultants’ preferences were described as “spontaneously formed … not a policy” (Japan Consultant 2) and based on their own local experiences.
In reality, we have [preferences amongst CBU banks]. Officially, we, like others, do not have any preference. (Japan Consultant 2)
Discussion
The international CBT community is known to exist as a complex bionetwork, with overlapping roles played by different stakeholders, and individual countries having varied positions within the network. However, understanding of the CBT bionetwork has tended to focus on research settings rather than clinical settings (Sleeboom-Faulkner and Patra Citation2011). Our findings therefore extend understanding of the CBT bionetwork by focusing on the perspectives of and relationships between different stakeholders, including transplant centers and tissue typers ().
Table 2. A comparison of UK and Japanese consultants’ decision-making when contemplating “quality” cord blood units.
In our study, Japanese transplant consultants did not discuss local collaboration in the way UK participants did. Instead, Japanese participants highlighted the importance of generally having trust in individuals performing key tasks (including CBU processing). UK consultants displayed greater faith in the knowledge of others than their Japanese colleagues, who required a personal experience of an individual to develop trust. The trust of Japanese transplant consultants was built on a one-to-one and direct basis, with more pre-requisites and not extended to organizations or groups of individuals (particularly on an international level). This distrust of international organizations may be a consequence of previously failed projects in Japan (e.g. Protein 3000 Project), where political interference significantly contributed to project failure (Fukushima Citation2016; Sleeboom-Faulkner Citation2011). Furthermore, our Japanese participants’ more individualistic approach is likely to be informed by the country’s CBU self-reliance, whereas UK consultants actively engaged with international colleagues and valued international policies (e.g. NetCord-FACT standards, national guidelines), and their resulting positions in the bionetwork as identified by others (All Party Parliamentary Group on Stem Cell Transplantation Citation2012; Brown and Williams Citation2015; Titmuss Citation1970; Williams Citation2015).
On CBT “quality,” transplant consultants from both countries highlighted the importance of trust in colleagues, in processing, and in information, as well as in collaboration. Our participants from UK centers set clear professional boundaries between “experts” (tissue typers and transplant consultants), which were portrayed as central to successful local collaboration. Both sets of experts were secure with these boundaries as it permitted meaningful collaboration to occur, resulting in these boundaries being transcended (D’Amour et al. Citation2005; Freeth Citation2001). The strength of the transplant consultant-tissue typer relationship was presented by UK participants as key to the “quality” of local decision-making. Tissue typers were trusted by transplant consultants with the “science” of CBU selection as their area of expertise. However, leadership of the selection meeting and wider MDT relied on the knowledge of clinical factors (e.g. transplant urgency), so could only be provided by transplant consultants; this leadership was uncontested. So, while “scientific” roles have been portrayed as superior and holding authority (Gieryn Citation1983), transplant consultants downplayed any scientific aspects of their role. They comfortably presented tissue typers as “scientific” experts, which maintained professional boundaries and cemented their own role as a clinical leader. In turn, the clinical setting was subtly presented as of equal standing to the “scientific” laboratory domain. This is in keeping with the interplay witnessed between counselors, scientists, and embryologists and the constructed boundaries of expertise surrounding the “clinical” and “scientific” in the context of embryo donation for stem cell research (Machin and Williams Citation2017).
Whilst NetCord-FACT accreditation is valued highly within CBU banks and provides some standardization between banks, our study participants suggested that the standards were imperfect, with variable compliance by CBU banks to every requirement set out in the standards. The difference in the value attributed to NetCord-FACT accreditation by those in transplant centers and in CBU banks reinforces the importance of perspective and position within the bionetwork and highlights the discursive nature of CBT “quality.” CBU banks have independent control of their processing methods, within the broad boundaries of NetCord-FACT standards (Brown, Machin, and McLeod Citation2011), while transplant centers hold minimal power over processing CBUs. Our findings, therefore, highlight one of the many felt vulnerabilities by transplant centers in the context of cord blood quality. Accreditation was used by participants in the transplant centers as a proxy for trust in CBU banks’ methods, as it assumed a degree of professionalism from CBU banks, until transplant centers have developed sufficient experience and trust with a bank. This further highlights the importance of trust and experience within CBT “quality,” as well as how “quality” is interpreted differently depending on one’s position within the bionetwork. Thus, trust was used as by participants from both countries as a measure of “quality” within CBT decision-making.
Epistemic uncertainty was accepted by transplant consultants from Japan and the UK as an unavoidable part of CBT, and even generated by them during interviews. The presentation of “definitive” limits within selection guidelines, such as the minimum acceptable cell doses, contradicts this uncertainty and ignores the existence of the “black box” factors described by our participants in transplant centers. This is exacerbated by the variation between different country’s guidelines and frequent changes to guidelines (Barker, Byam, and Scaradavou Citation2011; Dehn et al. Citation2019; Hough et al. Citation2016; Politikos et al. Citation2020; Rich Citation2015; Shaw et al. Citation2009). When defining “quality” in CBT, selection guidelines assume a level of detailed information from the banks that our participants claimed was not always provided. Guidelines cannot account for nuance in individual patient factors or clinicians’ personal preferences. By generating and affirming epistemic uncertainty, transplant consultants created space for themselves to exercise clinical autonomy and justified using their own experiences rather than available data and literature alone. Transplant consultants attempted to “own” the epistemic uncertainty of CBT by accepting responsibility for decision-making from their colleagues and the risks that are associated with decision-making. In doing so, they acquired the trust of their colleagues, reduced uncertainty within the team, and retained clinical autonomy.
Guidelines cannot support consultants’ decision-making when pragmatism is required, as it so often is in clinical practice with uncertainty. For example, what should transplant centers do when multiple (or no) CBUs meet the requirements of the selection criteria? Instead, transplant consultants make collaborative and pragmatic decisions informed by prior experience. This creates a constantly changing and adapting model for decision-making. Our study affirms the existence of a CBT bionetwork, made up of “rapidly changing landscapes”; hence, this adaptive, experience-based decision-making model is more appropriate than fixed selection criteria (Sleeboom-Faulkner and Patra Citation2011; Williams Citation2018). The unwritten “house rule(s)” of local tacit knowledge augment official policies and are exerted within the boundaries of selection guidelines. Application of experience and local tacit knowledge ensures that decision-making works effectively in the real world; it helps transplant consultants to manage epistemic uncertainty when making decisions, where guidelines cannot. The sum value of local tacit knowledge arguably exceeds the sum of the individuals forming each team and, therefore, should be valued when planning national services in the future. Transplant centers should continue sharing best practice based on their experience and to safely push the boundaries provided by current guidance.
This paper broadens our understandings of CBT “quality,” looking beyond CBU “quality” alone. We have explored perspectives from UK and Japanese transplant centers, as “regime(s) of truth” on current clinical practice (Martin, Brown, and Turner Citation2008; Moreira and Palladino Citation2005; Williams Citation2015), and what “quality” means to their staff in practice. While guidelines take a quantitative approach and scientific focus to “quality” (Hough et al. Citation2016; Politikos et al. Citation2020), little attention has been paid to the role of qualitative factors influencing and informing CBT “quality” for those working in clinical practice. We support Querol et al.’s (Citation2010) call for a shift in focus from “quantity” to “quality” in CBUs and argue for further qualitative research to advance understandings surrounding CBT “quality.” Trust, collaboration, (un)certainty, expertise and experience are some of the key components that have been identified in our study as influential on understanding “quality.” Given the main limitation of this study was the small sample size, we anticipate that different transplant centers and stakeholders may hold varying perspectives of CBT “quality” in addition to those we have identified in our study. Further large-scale qualitative research is needed to explore these identified concepts involving transplant center teams and to delve deeper into the qualitative aspects of CBT “quality.”
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbott, A. 1988. The System of Professions: An Essay on the Division of Expert Labour. London, UK: The University of Chicago Press.
- All-Party Parliamentary Group on Stem Cell Transplantation. 2012. “Cord Blood Transplantation: Meeting the Unmet Demand.” http://www.appg-stemcell.org.uk/uploads/4/4/4/7/44471153/cord_blood_transplantation_-_meeting_the_unmet_demand.pdf.
- Ballen, K., B. Logan, M. Laughlin, W. He, D. Ambruso, S. Armitage, R. Beddard, et al. 2015. “Effect of Cord Blood Processing on Transplantation Outcomes After Single Myeloablative Umbilical Cord Blood Transplantation.” Biology of Blood and Marrow Transplantation 21 (4): 688–695. doi:10.1016/j.bbmt.2014.12.017.
- Barker, J., C. Byam, and A. Scaradavou. 2011. “How I Treat: The Selection and Acquisition of Unrelated Cord Blood Grafts.” Blood 117 (8): 2332–2339. doi:10.1182/blood-2010-04-280966.
- Beltrame, L. 2020a. “Anchoring Hopes in a Regime of Truth. The Legitimizing Use of Cord Blood-Derived Products in Italy.” New Genetics and Society 39 (2): 173–190. doi:10.1080/14636778.2019.1686969.
- Beltrame, L. 2020b. “Values in Tension. Clinical Quality and Civic Participation in Umbilical Cord Blood Banking in Italy.” Sociology of Health and Illness 42 (4): 689–704. doi:10.1111/1467-9566.13049.
- Braun, V., and V. Clarke. 2006. “Using Thematic Analysis in Psychology.” Qualitative Research in Psychology 3 (2): 77–101. doi:10.1191/1478088706qp063oa.
- Brown, N., L. L. Machin, and D. McLeod. 2011. “Immunitary Bioeconomy: The Economisation of Life in the International Cord Blood Market.” Social Science and Medicine 72 (7): 1115–1122. doi:10.1016/j.socscimed.2011.01.024.
- Brown, N., and R. Williams. 2015. ““Cord Blood Banking-Bio-Objects on the Borderlands Between Community and Immunity.” Life Sciences, Society and Policy 11 (11): 1–18. doi:10.1186/s40504-015-0029-8.
- Cambrosio, A., P. Keating, T. Schlich, and G. Weisz. 2006. “Regulatory Objectivity and the Generation and Management of Evidence in Medicine.” Social Science and Medicine 63 (1): 189–199. doi:10.1016/j.socscimed.2005.12.007.
- Chang, H. C. 2016. “The Multiple Roles of Cord Blood Banks in Taiwan: Competition and Collaboration.” New Genetics and Society 25 (3): 1–21. doi:10.1080/14636778.2016.1209106.
- Covaleski, M. A., M. W. Dirsmith, and L. Rittenberg. 2003. “Jurisdictional Disputes Over Professional Work: The Institutionalization of the Global Knowledge Expert.” Accounting Organizations and Society 28 (4): 323–355. doi:10.1016/S0361-3682(02)00029-6.
- D’Amour, D., M. Ferrada-Videla, L. S. M. Rodriguez, and M. D. Beaulieu. 2005. “The Conceptual Basis for Interprofessional Collaboration: Core Concepts and Theoretical Frameworks.” Journal of Interprofessional Care 19 (Suppl. 1): 116–131. doi:10.1080/13561820500082529.
- Dehn, J., S. Spellman, C. Hurley, B. Shaw, J. Barker, L. Burns, D. Confer, et al. 2019. “Selection of Unrelated Donors and Cord Blood Units for Hematopoietic Cell Transplantation: Guidelines from the NMDP/CIBMTR.” Blood 134 (12): 924–934. doi:10.1182/blood.2019001212.
- Dessels, C., M. Alessandrini, and M. Pepper. 2018. “Factors Influencing the Umbilical Cord Blood Stem Cell Industry: An Evolving Treatment Landscape.” Stem Cells Translational Medicine 7 (9): 643–650. doi:10.1002/sctm.17-0244.
- Eapen, M., J. P. Klein, G. F. Sanz, S. Spellman, A. Ruggeri, C. Anasetti, M. Brown, et al. 2011. “Effect of Donor-Recipient HLA Matching at HLA A, B, C, and DRB1 on Outcomes After Umbilical-Cord Blood Transplantation for Leukaemia and Myelodysplastic Syndrome: A Retrospective Analysis.” The Lancet Oncology 12 (13): 1214–1221. doi:10.1016/S1470-2045(11)70260-1.
- Freeth, D. 2001. “Sustaining Interprofessional Collaboration.” Journal of Interprofessional Care 15: 37–46. doi:10.1080/13561820020022864.
- Fukushima, M. 2016. “Constructing Failure in Big Biology: The Socio-Technical Anatomy of Japan’s Protein 3000 Project.” Social Studies of Science 46 (1): 7–33. doi:10.1177/0306312715612146.
- Gachoud, D., M. Albert, A. Kuper, L. Stroud, and S. Reeves. 2012. “Meanings and Perceptions of Patient-Centeredness in Social Work, Nursing and Medicine: A Comparative Study.” Journal of Interprofessional Care 26: 484–490. doi:10.3109/13561820.2012.717553.
- Gieryn, T. F. 1983. “Boundary-Work and the Demarcation of Science from Non-Science: Strains and Interests in Professional Ideologies of Scientists.” American Sociological Review 48 (6): 781–795. doi:10.2307/2095325.
- Gluckman, E., H. Broxmeyer, A. Auerbach, H. Friedman, G. Douglas, A. Devergie, H. Esperou, D. Thierry, G. Socie, and P. Lehn. 1989. “Hematopoietic Reconstitution in a Patient with Fanconi’s Anemia by Means of Umbilical-Cord Blood from an HLA-Identical Sibling.” New England Journal of Medicine 321 (17): 1174–1178. doi:10.1056/NEJM198910263211707.
- Hardey, M., S. Payne, J. Powell, S. Hawker, and C. Kerr. 2001. “Professional Territories and the Fragmented Landscape of Elderly Care.” Journal of the Royal Society for the Promotion of Health 121 (3): 159–164. doi:10.1177/146642400112100312.
- Heldal, F. 2010. “Multidisciplinary Collaboration as a Loosely Coupled System: Integrating and Blocking Professional Boundaries with Objects.” Journal of Interprofessional Care 24: 19–30. doi:10.3109/13561820903078322.
- Holstein, J., and J. Gubrium. 1995. The Active Interview. London: SAGE Publications. doi:10.4135/9781412986120
- Hough, R., R. Danby, N. Russell, D. Marks, P. Veys, B. Shaw, R. Wynn, et al. 2016. “Recommendations for a Standard UK Approach to Incorporating Umbilical Cord Blood Into Clinical Transplantation Practice: An Update on Cord Blood Unit Selection, Donor Selection Algorithms and Conditioning Protocols.” British Journal of Haematology 172 (3): 360–370. doi:10.1111/bjh.13802.
- Indrayan, A. 2020. “Aleatory and Epistemic Uncertainties Can Completely Derail Medical Research Results.” Journal of Postgraduate Medicine 66 (2): 94–98. doi:10.4103/jpgm.JPGM_585_19.
- Jaime-Pérez, J., R. Monreal-Robles, L. N. Rodríguez-Romo, C. Mancías-Guerra, J. L. Herrera-Garza, and D. Gómez-Almaguer. 2011. “Evaluation of Volume and Total Nucleated Cell Count as Cord Blood Selection Parameters: A Receiver Operating Characteristic Curve Modeling Approach.” American Journal of Clinical Pathology 136 (5): 721–726. doi:10.1309/AJCPFB6EXO7BJVLR.
- Japanese Red Cross Society. 2021. “Cord Blood Bank Total.” https://www.bs.jrc.or.jp/bmdc/cordblooddonor/m3_04_statistics.html.
- Kindwall-Keller, T., and K. Ballen. 2020. “Umbilical Cord Blood: The Promise and the Uncertainty.” Stem Cells Translational Medicine 9 (10): 1153–1162. doi:10.1002/sctm.19-0288.
- Langan-Fox, J., and C. L. Cooper. 2014. Boundary-Spanning in Organizations: Network, Influence, and Conflict. New York, NY: Routledge.
- Lippi, G., K. Becan-McBride, D. Behúlová, R. A. Bowen, S. Church, J. Delanghe, K. Grankvist, et al. 2013. “Preanalytical Quality Improvement: In Quality We Trust.” Clinical Chemistry of Laboratory Medicine 51 (1): 229–241. doi:10.1515/cclm-2012-0597.
- Machin, L. L. 2016. “The Collection of ‘Quality’ Umbilical Cord Blood for Stem Cell Treatments: Conflicts, Compromises, and Clinical Pragmatism.” New Genetics and Society 35 (3): 307–326. doi:10.1080/14636778.2016.1209109.
- Machin, L. L., N. Brown, and D. McLeod. 2012. “‘Two’s Company – Three’s a Crowd’: The Collection of Umbilical Cord Blood for Commercial Stem Cell Banks in England and the Midwifery Profession.” Midwifery 28 (3): 358–365. doi:10.1016/j.midw.2011.05.002.
- Machin, L. L., and R. A. Williams. 2017. Interprofessional Spanning and Building Boundaries When Supporting Potential Embryo Donors to Stem Cell Research.” Journal of Interprofessional Care 31 (3): 342–350. doi:10.1080/13561820.2016.1253546.
- Martin, P., N. Brown, and A. Turner. 2008. “Capitalising Hope: The Commercial Development of Umbilical Cord Blood Stem Cell Banking.” New Genetics and Society 27 (2): 127–143. doi:10.1080/14636770802077074.
- Moreira, T., C. May, and J. Bond. 2009. “Regulatory Objectivity in Action: Mild Cognitive Impairment and the Collective Production of Uncertainty.” Social Studies of Science 39 (5): 665–690. doi:10.1177/0306312709103481.
- Moreira, T., and P. Palladino. 2005. “Between Truth and Hope: On Parkinson’s Disease, Neurotransplantation and the Production of the ‘Self’.” History of the Human Sciences 18: 55–82. doi:10.1177/0952695105059306.
- NetCord-FACT. 2019. NetCord-FACT International Standards for CORD BLOOD Collection, Banking, and Release for Administration. 7th ed. doi:10.1038/sj.bmt.1701677.
- Nikiforow, S., S. Li, K. Snow, D. Liney, G. Kao, R. Haspel, E. Shpall, et al. 2017. “Lack of Impact of Umbilical Cord Blood Unit Processing Techniques on Clinical Outcomes in Adult Double Cord Blood Transplant Recipients.” Hematopoietic Stem Cell Transplants 19 (2): 272–284. doi:10.1016/j.jcyt.2016.10.016.
- Politikos, I., E. Davis, M. Nhaissi, J. Wagner, C. Brunstein, S. Cohen, E. Shpall, et al. 2020. “Guidelines for Cord Blood Unit Selection.” Biology of Blood and Marrow Transplantation 26 (12): 2190–2196. doi:10.1016/j.bbmt.2020.07.030.
- Querol, S., S. Gomez, A. Pagliuca, M. Torrabadella, and J. Madrigal. 2010. “Quality Rather Than Quantity: The Cord Blood Bank Dilemma.” Bone Marrow Transplantation 45 (6): 970–978. doi:10.1038/bmt.2010.7.
- Rich, I. 2015. “Improving Quality and Potency Testing for Umbilical Cord Blood: A New Perspective.” Stem Cells Translational Medicine 4 (9): 967–973. doi:10.5966/sctm.2015-0036.
- Saccardi, R., L. Tucunduva, A. Ruggeri, I. Ionescu, G. Koegler, S. Querol, G. Grazzini, et al. 2016. “Impact of Cord Blood Banking Technologies on Clinical Outcome: A Eurocord/Cord Blood Committee (CTIWP), European Society for Blood and Marrow Transplantation and NetCord Retrospective Analysis.” Transfusion 56 (8): 2021–2029. doi:10.1111/trf.13661.
- Santos, S., S. Barros, M. Santos, L. Marti, A. Ribeiro, A. Kondo, and J. Kutner. 2016. “Predictors of High-Quality Cord Blood Units.” Transfusion 56 (8): 2030–2036. doi:10.1111/trf.13653.
- Scaradavou, A., K. M. Smith, R. Hawke, A. Schaible, M. Abboud, N. A. Kernan, J. W. Young, and J. N. Barker. 2010. “Cord Blood Units with Low CD34+ Cell Viability Have a Low Probability of Engraftment After Double Unit Transplantation.” Biology of Bone Marrow Transplantation 16 (4): 500–508. doi:10.1016/j.bbmt.2009.11.013.
- Shaw, B. E., P. Veys, A. Pagliuca, J. Addada, G. Cook, C. F. Craddock, A. R. Gennery, et al. 2009. “Recommendations for a Standard UK Approach to Incorporating Umbilical Cord Blood Into Clinical Transplantation Practice: Conditioning Protocols and Donor Selection Algorithms.” Bone Marrow Transplantation 44: 7–12. doi:10.1038/bmt.2008.420.
- Sleeboom-Faulkner, M. 2011. “Regulating Cell Lives in Japan: Avoiding Scandal and Sticking to Nature.” New Genetics and Society 30 (3): 227–240. doi:10.1080/14636778.2011.598052.
- Sleeboom-Faulkner, M., and P. K. Patra. 2011. “Experimental Stem Cell Therapy: Biohierarchies and Bionetworking in Japan and India.” Social Studies of Science 41 (5): 645–666. doi:10.1177/0306312711409792.
- Takanashi, M., H. Tanaka, M. Kohsaki, K. Nakajima, K. Tadokoro, and M. Nakabayashi. 2011. “A Suggested Total Size for the Cord Blood Banks of Japan.” Bone Marrow Transplantation 46 (7): 1014–1015. doi:10.1038/bmt.2010.216.
- Titmuss, R. 1970. The Gift Relationship: From Human Blood to Social Policy. London: Allen and Unwin.
- UK Stem Cell Strategic Forum. 2010. “The Future of Unrelated Donor Stem Cell Transplantation in the UK. Part 1. Findings and Recommendations.” https://silo.tips/download/part-1-findings-and-recommendations.
- Wagner, A., J. N. Barker, T. E. DeFor, K. S. Baker, B. R. Blazar, C. Eide, A. Goldman, et al. 2002. “Transplantation of Unrelated Donor Umbilical Cord Blood in 102 Patients with Malignant and Non-Malignant Diseases: Influence of CD34 Cell Dose and HLA Disparity on Treatment-Related Mortality and Survival.” Blood 100 (5): 1611–1618. doi:10.1182/blood-2002-01-0294.
- Williams, R. 2015. “Cords of Collaboration: Interests and Ethnicity in the UK’s Public Stem Cell Inventory.” New Genetics and Society 34 (3): 319–337. doi:10.1080/14636778.2015.1060116.
- Williams, R. 2018. “Bloody Infrastructures!: Exploring Challenges in Cord Blood Collection Maintenance.” Technology Analysis and Strategic Management 30 (4): 473–483. doi:10.1080/09537325.2017.1337888.
- World Marrow Donor Association. 2020. “WMDA GLOBAL TRENDS REPORT 2020.” https://wmda.info/wp-content/uploads/2021/05/GTR-2020-Summary-slides.pdf.

