ABSTRACT
Introduction: Exon skipping is a therapeutic approach for Duchenne muscular dystrophy (DMD) that has been in development for close to two decades. This approach uses antisense oligonucleotides (AONs) to modulate pre-mRNA splicing of dystrophin transcripts to restore the disrupted DMD reading frame. The approach has moved from in vitro proof of concept studies to the clinical trial phase and marketing authorization applications with regulators. The first AON (eteplirsen) has recently received accelerated approval by the Food and Drug Administration in the US.
Areas covered: In this review the authors explain the antisense-mediated exon skipping approach, outline how it needs be tailored for different DMD mutation types and describe the challenges and opportunities for each mutation type. The authors summarize the clinical development of antisense-mediated exon 51 skipping, and discuss methods to improve efficiency. Finally, the authors provide their opinion on current developments and identify topics for future prioritization.
Expert opinion: Exon skipping development has been a learning experience for all those involved. Aside from an approved therapy, its development has yielded side benefits including the development of tools for clinical trials and has increased collaboration between academics, patients, industry and regulators.
1. Introduction
1.1. Duchenne muscular dystrophy (DMD)
DMD is a genetic disorder that is inherited in an X-linked manner and thus affects primarily boys, although female carriers can be symptomatic as well. The incidence is estimated at 1:5000 newborn boys [Citation1]. Clinical symptoms derive from progressive muscle weakness that generally becomes apparent in the first years of life. Affected boys can have delayed motor milestones, and although they obtain the possibility to walk independently, they cannot keep up with their peers and walking is compromised by frequent falls. They have difficulty climbing stairs, and proper running or hopping is not achieved. Motor performance typically plateaus around the age of 5–7 years, after which a large number of the boys lose ambulation between 8 and 12 years. However, natural history studies have shown that the distance covered in the 6 min walking test (6MWT), used as primary endpoint in all the recent clinical trials, can still increase beyond 7 years, and can even be stable for some time in young teenagers [Citation2,Citation3]. The reasons for this variability are largely unknown. Genetic modifiers involved in inflammatory pathways are being identified and may explain part of this variation [Citation4,Citation5]. It has also become clear that cohorts of patients with certain mutations have a different mean age at which they lose ambulation [Citation6]. In the non-ambulatory phase, further progression of muscle weakness leads to a decline of arm and hand function, the need for respiratory support, and cardiomyopathy. Life expectancy is severely reduced, and sudden death can still occur before the age of 20. Nonetheless, international standards of care probably enable survival beyond the third decade of life for the majority of currently diagnosed children [Citation7]. These standards include the use of corticosteroids, which are usually commenced around the age of 5 years. Corticosteroids have clearly prolonged the ambulatory phase for DMD patients. The median age at loss of ambulation is estimated to be postponed by 3 years (13.0 versus 10.0) by using steroids for more than 1 year in the ambulant phase compared to a combined cohort with steroid treatment less than 1 year and untreated patients [Citation8]. Unfortunately, this study also illustrates the wide variability in treatment regimes. Both prednisone and deflazacort are used at various levels, and dosing can be either daily or intermittent with variable intervals. This non-uniformity in the natural history and treatment of DMD clearly is an important factor to consider when recruiting patients in international clinical trials.
1.2. DMD mutations and the exon skipping approach
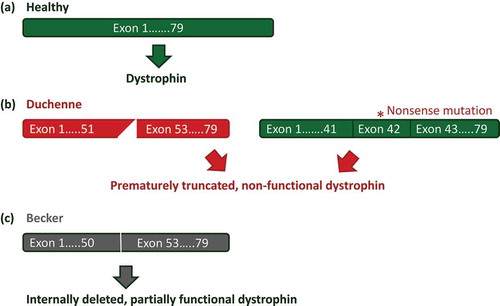
DMD is caused by mutations that abolish the production of functional dystrophin protein, either through frame-shifts or through nonsense mutations [Citation9] (). Dystrophin acts as a molecular shock-absorber and provides muscle fibers with stability during exercise [Citation10,Citation11]. Lacking functional dystrophin, muscle fibers are easily damaged, which eventually leads to replacement of muscle tissue by fibrotic and fat tissue and the progressive loss of muscle function. Notably, in-frame deletions can allow the production of internally deleted, partially functional dystrophin proteins [Citation9] ()). These dystrophins are associated with a less severe and less progressive disease, Becker muscular dystrophy (BMD).
Figure 1. Mutations in the DMD gene. (a) In the healthy situation the dystrophin mRNA transcript encodes a fully functional dystrophin protein. (b) In Duchenne patients, mutations that disrupt the reading frame (e.g. a deletion of exon 52, left panel) or nonsense mutations (e.g. nonsense mutation in exon 42, right panel) lead to a premature truncation of protein translation and non-functional dystrophins. (c) In Becker patients mutations maintain the reading frame (a deletion of exon 51 and 52), allowing production of internally deleted, but partially functional proteins.
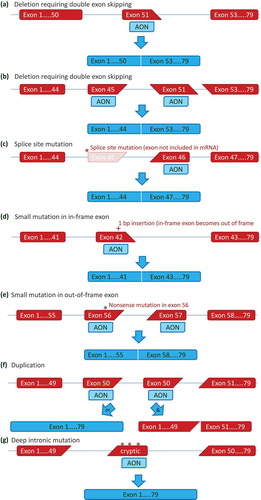
The exon skipping approach aims to restore the disrupted open reading frame for DMD dystrophin transcripts [Citation12] ()). This can be achieved by antisense oligonucleotides (AONs), small modified pieces of DNA or RNA that specifically hybridize to a target exon, hiding said exon from the splicing machinery so that it is spliced out with its flanking introns. While this makes the deletion larger, the restoration of the reading frame allows the production of a partially functional dystrophin like those found in BMD patients [Citation13].
Figure 2. Antisense mediated exon skipping for various mutation types. (a) Antisense oligonucleotides (AONs) can hybridize to a target exon during the pre-mRNA splicing process. This will hide the target exon from the splicing machinery, and it will be spliced out with its flanking introns. For a deletion of exon 52, the skipping of exon 51 will restore the reading frame, to allow the production of a Becker-type dystrophin rather than a non-functional dystrophin. (b) Double exon skipping for a deletion mutation. For most deletions the reading frame can be corrected by skipping a single exon (see Figure 2). Some deletions however, such as a deletion of exon 46–50, required the skipping of both flanking exons to restore the reading frame. (c) Exon skipping for a splice site mutation. Most splice site mutations, will cause a single exon deletion on mRNA level (a 3ʹ splice site mutation in intron 44 results in exon 45 no longer being recognized by the splicing machinery in this example). Like with exon deletions, skipping additional exons (exon 46 in this example) can restore the reading frame. (d) Single exon skipping for small mutations. When small mutations are located within an in-frame exon, skipping this exon will bypass the mutation, while maintaining the reading frame. In this example, a 1 basepair insertion in exon 42 disrupts the reading frame. Skipping exon 42 bypasses the frame-disrupting mutation. (e) Double exon skipping for small mutations. When small mutations are located within out-of-frame exons, the mutation containing exon needs to be skipped as well as a flanking exon, to bypass the mutation without affecting the reading frame. In the example a nonsense mutation in exon 56 requires the skipping of both exon 56 and exon 57. (f) Exon skipping for single exon duplications. Single exon skipping for single exon duplications allows restoration of the normal transcript when either the original or duplicated exon is skipped (left panel). However, double exon skipping will lead to an out of frame transcript (right panel). (g) Exon skipping for cryptic splicing mutations. Deep intronic mutations can activate splice sites, leading to the inclusion of part of an intron into the mRNA. These cryptic exons often are out-of-frame and/or contain stop codons. Targeting the cryptic exon with an AON can restore the normal transcript.
2. Applicability
2.1. Exon skipping for deletion mutations
Exon skipping is a mutation-specific approach; which exon to skip depends on the size and location of the mutation [Citation12]. As such, having a genetic diagnosis of the disease is important [Citation14]. Most DMD patients have a deletion of one or more exons (~68%); the reading frame can be restored by single exon skipping for 70% of these deletions ()), while an additional 8% need skipping of two exons to restore the reading frame [Citation12] (example in ()). Because deletions cluster between exon 45 and 55, the skipping of exons in this area applies to larger groups of patients, with exon 51 skipping applying to 14% of patients, exon 45 and exon 53 skipping both applying to an additional 8% of patients and exon 44 applying to an additional 6% [Citation15]. AON-induced single and double exon skipping leading to dystrophin restoration have both been shown feasible in patient-derived cell cultures and animal models (overview given by Aartsma-Rus [Citation13]). Thus far only single exon skipping has been pursued in clinical trials (see later). Double exon skipping requires a combination of two AONs ()). This was quite straightforward in vitro, being almost equally efficient as single exon skipping with regards to exon skipping efficiency and the percentage of dystrophin positive cells [Citation16]. However, it is good to bear in mind that the transfection efficiency in cultured cells is very high (close to 100%). Therefore, most cells will take up both AONs in relatively high amounts, making the chance that two AONs target the same transcript high. It has been observed that double exon skipping is less efficient in vivo, probably requiring higher doses [Citation17,Citation18]. This makes sense, since much less AON will be taken up by individual muscle fiber nuclei after systemic delivery, thus decreasing the chance that two AONs target the same transcript. It therefore follows that when AONs for two different exons are approved, further tests needs to be done before combining these AONs for patients requiring double exon skipping is approved as well [Citation19].
2.2. Exon skipping for patients with small mutations
About 20% of patients have small mutations within exons [Citation15]. Half of these (~10% of all patients) have nonsense mutations (()), while ~35% consist of small deletions or insertions that disrupt the reading frame (7% of all patients). The final 15% (3% of all patients) have splice site mutations. These are anticipated to result in the skipping of the exon involved. As such mutations will generally be similar to deletions on RNA level and skipping of adjacent exons can often restore the reading frame ()). For the insertions, deletions and nonsense mutation, the exon itself will need to be skipped to bypass the mutation (()). When mutations are located in in-frame exons, this can be done without disrupting the reading frame. However, when mutations are located in out-of-frame exons this will require the skipping of two or more exons [Citation12]. As with deletions, single and double exon skipping straightforwardly resulted in dystrophin restoration for small mutations (overview in Aartsma-Rus [Citation13]). However, the same considerations for double exon skipping for deletion mutations apply here. Furthermore, small mutations do not cluster in a hotspot. As such, the applicability of skipping in-frame exons to bypass these mutations by single exon skipping is low (0.02–0.8% of all patients [Citation12]) making the clinical development of such AONs challenging if not impossible [Citation19].
2.3. Exon skipping for duplication mutations
Duplications of one or more exons occur in ~11% of patients and cluster between exon 2 and 11. Single exon duplications are interesting from two perspectives. First, skipping one of the two exons would restore the normal transcript ()). Second, the ‘dose’ of the target is double compared to deletion mutations. However, there is of course also a risk of skipping both exons, leading to an out-of-frame transcript. In patient-derived cell cultures, this in fact can happen, either exclusively or in combination with single exon skipping [Citation20,Citation21]. However, for single exon duplications, it is anticipated that in vivo AON treatment will result in single exon skipping, because the chance that two AONs end up in the same nucleus and target the same transcript is much smaller than in vitro. A recently developed mouse model with a duplication of mouse exon 2 [Citation22] will allow experiments to hopefully shed more light on this.
For duplications involving multiple exons, exon skipping is challenging, because here the effect will be diluted, skipping a duplicated exon will often restore the reading frame, while skipping an original exon will not. Furthermore, splicing for multiexon duplications is often complex and unpredictable [Citation23].
2.4. Exon skipping for deep intronic mutations that affect splicing
Deep intronic mutations giving rise to aberrant inclusion of ‘cryptic exons’ occur in less than 1% of DMD patients. Here, AONs can be designed to skip the cryptic exon (()), which has been successfully done in patient-derived cells [Citation24]. The main challenge is that most of these mutations are unique, which poses challenges on clinical development of these AONs [Citation19].
2.5. Multiexon skipping approach
Skipping a stretch of multiple exons has been proposed as a way to increase applicability of the exon skipping approach [Citation16,Citation25]. Exon 45–55 skipping has been studied most, because this would apply to ~40% of patients in global databases [Citation12,Citation15] and 62% of patients in a French database [Citation25]. This multiexon skipping relies on a combination of 11 AONs targeting each of the individual exons present in the exon 45–55 stretch. In patient-derived cells cultures, skipping this stretch of exons was revealed to be challenging [Citation26]. More success was achieved in a mouse model with a deletion of exon 52, where a cocktail of 10 AONs could induce skipping of exons 45–51 and 53–55 leading to dystrophin restoration [Citation27]. The biggest challenge for multiexon skipping is the fact that in addition to the in-frame intended skipping, multiple out-of-frame transcripts will be induced as well. Thus, there is a dilution of the therapeutic effect to a much larger extent than with the previously described double exon skipping. Another challenge is the dosing, which will have to be higher than for single and double exon skipping. It is as yet unknown whether these high doses will be safe in humans.
2.6. Mutations to which exon skipping is not applicable
While exon skipping would in theory apply to up to 81% of patients, there are notable exceptions. These are directly related to the location of the functional domains of the dystrophin protein (, [Citation28]). Dystrophin connects F-actin to beta-dystroglycan and thus the cytoskeleton to the extracellular matrix [Citation28]. It contains three actin binding domains and a cysteine-rich domain. When in-frame mutations abolish all actin binding domains and/or the dystroglycan binding domains of the dystrophin protein, the resulting protein will not be functional [Citation28]. Furthermore, in-frame deletions in the central rod domain that intersperses the actin- and dystroglycan-binding domains, are generally well tolerated. However, deletions involving more than 36 exons results in non-functional dystrophins. As such it is anticipated that exon skipping will not result in functional dystrophins when mutations abolish all actin-binding domains, involve the dystroglycan binding domain and/or are deletions involving 36 exons or more [Citation28,Citation29].
Figure 3. Dystrophin protein domains relative to the encoding exons (previously published [Citation28], used with permission from IOS press). Dystrophin function relies on the presence of at least one actin binding domain (encoded by exon 2–9 and exon 31–45) and the dystroglycan-binding domain (encoded by exon 64–70). Furthermore, the central rod domain (encoded by exon 10–63) does not tolerate deletions involving 36 or more exons.
![Figure 3. Dystrophin protein domains relative to the encoding exons (previously published [Citation28], used with permission from IOS press). Dystrophin function relies on the presence of at least one actin binding domain (encoded by exon 2–9 and exon 31–45) and the dystroglycan-binding domain (encoded by exon 64–70). Furthermore, the central rod domain (encoded by exon 10–63) does not tolerate deletions involving 36 or more exons.](/cms/asset/156132fe-ae15-48f4-9d15-77b93f3a3291/iebt_a_1271872_f0003_oc.jpg)
3. Clinical development
Clinical trials investigating the effect of exon skipping have until now been performed using two different chemically modified AONs, that is, 2’-O-methyl-phosphorothioates (2OMePS) developed by Prosensa, GlaxoSmithKline (GSK) and finally BioMarin, and phosphorodiamidate morpholino oligomers (PMO), applied by AVI-Biopharma, a company that changed its name to Sarepta Therapeutics in 2012. Modification is done to increase the stability of the AON and endow AONs with good pharmacokinetic properties. Splice modulating AONs further need a modification to prevent cleavage by RNA nucleases of the AON-target hybrid. Different AON chemistries have different pharmacokinetic, pharmacodynamic, and safety profiles. 2OMePS are negatively charged, have a high level of plasma protein binding, and a relatively long plasma half-life ranging from weeks to months upon systemic subcutaneous administration [Citation30]. PMOs are charge-neutral and have a much shorter plasma half-life of several hours after intravenous dosing [Citation31]. Both compounds have limited penetration in cardiomyocytes and do not cross the blood–brain barrier upon systemic administration.
3.1. Results from 2OMePS-mediated exon skipping in DMD
PRO051, developed by Prosensa Therapeutics, was the first 2OMePS AON targeting exon 51 in the DMD gene to be tested in humans. In 2006, intramuscular injection of 0.8 mg into the tibialis anterior muscle of four non-ambulant patients (PRO051-CLIN01) resulted in clear sarcolemmal dystrophin signals in the immunofluorescence analyses performed 28 days later, although formally, no pretreatment biopsies were performed [Citation32]. Next, systemic administration of PRO051 with weekly subcutaneous injections was studied in 12 ambulant patients using a dose escalation design (four groups of three patients per dose) ranging between 0.5 and 6.0 mg/kg for 5 weeks [Citation30]. In this study, starting in 2008 (PRO051-CLIN02, ClinicalTrials.gov Identifier NCT01910649), muscle biopsies from the tibialis anterior muscle were available at baseline and 2 weeks after the last injection from the three patients included in 0.5 mg/kg dosing cohort, and at 2 and 7 weeks after the last injection for the patients treated with 2.0, 4.0, and 6.0 mg/kg. Although the study duration was short considering the plasma and tissue half-life of PRO051, exon skipping was observed on RNA level in all six patients receiving 4.0 and 6.0 mg/kg. Immunofluorescence analyses in the 6.0 mg/kg cohort 2 weeks after treatment revealed an increase in dystrophin signal intensity ranging from 4.7% to 15.5% of that of a control sample. Importantly, from this and other studies it became increasingly appreciated that if advanced immunohistochemistry techniques are applied, nearly all DMD patients show traces of dystrophin in their biopsies [Citation33,Citation34]. Variations between different techniques, within single DMD muscle biopsies, and between controls complicate the use of detailed dystrophin quantification as biomarker [Citation28].
After the dose escalation phase and a pause of 6–15 months, all 12 patients resumed dosing at 6 mg/kg per week subcutaneously for 72 weeks followed by an interruption of several weeks and intermittent dosing. By then, PRO051 was licensed in by GSK changing the compound code to GSK2402968. The generic name drisapersen was introduced and the trial code changed to DMD114673 (ClinicalTrials.gov identifier NCT01910649). Although two boys lost ambulation before the open label extension (OLE) study was initiated, and another two lost ambulation within 1.5 years after the onset of the OLE study, the remaining eight showed a stable course of the ambulatory capacity. At 177 weeks, the median change from baseline of the OLE phase was +8 m on the 6MWT at an age range of 9.3–15.4 years [Citation35].
Upon acquisition of the PRO051 license, GSK initiated a number of studies planned and conducted more or less in parallel. An exploratory study conducted only in the US (DMD114876, ClinicalTrials.gov Identifier NCT01462292) compared weekly subcutaneous dosing of 3 and 6 mg/kg to placebo for 24 weeks, but failed to demonstrate a statistically significant difference in the 6MWT. The randomized controlled trial DEMAND 2 (DMD 114117, ClinicalTrials.gov Identifier NCT01153932) was defined as a phase 2 study, and compared two subcutaneous dosing regimens (an initial loading dose of six doses in 3 weeks followed by 6 mg/kg weekly or 6 mg/kg intermittently, adding up to nine doses per 10 weeks) to placebo in 53 ambulant boys who had a baseline distance in the 6MWT of more than 75 m, and who could rise from the floor within 7 s. Until now, it is the only published trial from the drisapersen program showing a promising mean difference from placebo in the 6MWT of 35.09 m at week 25 (p = 0.014), and 35.84 m at week 49 (p = 0.051) in the 18 boys on continuous dosing [Citation36]. By contrast, no significant changes were found in the intermittent dosing group despite similar pharmacokinetic exposure. The 17 boys in this group were slightly older and heavier at baseline. Results of the muscle biopsies, performed at week 25, are reported qualitatively, and do not provide insight in absolute RNA or protein levels of the skipped product. Nevertheless, an effect on RNA and/or protein was observed for the majority of drisapersen-treated patients.
The pivotal phase 3 trial DEMAND 3 (DMD114044, ClinicalTrials.gov Identifier NCT01254019), started in parallel in 2010, included 186 boys worldwide and compared 48 weeks of subcutaneous dosing at 6 mg/kg weekly in 125 patients to 61 controls. In this study, no differences in the 6MWT as primary endpoint nor in any of the secondary clinical endpoints were met, leading to a discontinuation of the whole drisapersen program in September 2013, and to GSK handing over the DMD research data back to Prosensa Therapeutics. In this pivotal trial, the inclusion criteria were not as strict as those in the DEMAND 2 study, that is, the boys were required to walk more than 75 m on the 6MWT, but the requirement of rising from the floor within 7 s was left out. This resulted in a difference at baseline between the subjects in the DEMAND 2 and 3 studies (mean age 7.2 versus 8.0 years, and mean 6MWT distance 337 versus 428 m), favoring the smaller DEMAND 2 study [Citation35]. By then it had become clear from natural history studies and placebo cohorts from these and other clinical trials that the 6 MWT did not show any consistent linear correlation with age. A baseline distance below 330–350 m, and an age of >7 are predictors of an increased decline in walking or even the loss of ambulation within 1–2 years [Citation37,Citation38]. This example illustrates why ideally one has natural history data of the primary endpoint available before planning a trial. This will allow powering of the study and selection of cohorts in which a therapeutic can be best assessed [Citation39].
Side effects from the subcutaneous dosing of drisapersen included mainly injection-site reactions, proteinuria and thrombocytopenia. The latter two were most often subclinical, and resolved upon temporarily withholding the dosing. The injection-site reactions included pain, erythema, swelling and hematomas in the acute phase. However, long-term use led to discoloration, induration of the skin, sclerotic changes, and subcutaneous lipodystrophy that did not seem to resolve after cessation of dosing. Although structural reports and studies are lacking, this seems to be a class effect of AONs with a phosphorothioate backbone [Citation40]. It directly led to the exploration of intravenous administration of drisapersen, but also PRO044, PRO045, and PRO053. These latter compounds, selected to skip exons 44, 45, or 53, respectively, had been tested in smaller phase 1/2 studies. Aim of these studies was to study the safety, pharmacodynamics and pharmacokinetics in a small group of patients while waiting for the results of the drisapersen program, and with the possibility to expand the inclusion to demonstrate clinically significant benefit later on.
The pooled data from all drisapersen results was acquired by the end of 2014 by BioMarin ltd. This included long-term follow-up from the initial 12 patients and patients from the DEMAND 2 and 3 trials who had continued dosing in an open-label extension study (ClinicalTrials.gov Identifier NCT01480245) up to September 2013. Data were presented to the US and European regulators. The US Food and Drug Administration (FDA) indicated that the data were as yet not convincing of a positive risk–benefit balance for this compound. BioMarin ltd. withdrew the marketing authorization application from the European Medicines Agency in May 2016. The company announced that they would stop the 2OMePS AON programs for all DMD-related products in the summer of 2016 and would focus on the next generation of AONs.
3.2. Results from PMO mediated exon skipping in DMD
The first study in humans testing a PMO AON targeting exon 51 used AVI-4658, which was developed by AVI-BioPharma. The study was done in seven DMD patients between 10 and 17 years old through intramuscular injections of 0.09 (n = 2) and 0.9 mg (n = 5) into the extensor digitorum brevis (EDB) muscle (ClinicalTrials.gov Identifier NCT00159250). Saline injections in the contralateral muscle were used as a control [Citation41]. Muscle biopsies taken 3–4 weeks later showed dystrophin expression in the five patients treated with the higher dose. Signal intensity was related to that of healthy control muscle and increased from 4% to 14% in the saline-treated EDB to 22–32%. Next, systemic treatment was studied in 19 boys between 6 and 13 years old using 12 weekly intravenous doses of AVI-4658 in a dose escalation study ranging from 0.5 to 20 mg/kg per dose (ClinicalTrials.gov Identifier NCT00844597). Muscle biopsies were taken 2 weeks after the last dose from the biceps brachii muscle and compared to a pretreatment biopsy available from the diagnostic procedure (n = 3), or from the contralateral biceps (n = 16) [Citation42]. Efficacy of the treatment was assessed using three parameters: percentage of dystrophin-positive fibers, mean fluorescence intensity per fiber, and dystrophin expression calculated by western-blotting. Although at least one of these parameters favored treatment in all eight patients dosed at 10 or 20 mg/kg, this study again confirmed that untreated patients express some dystrophin, with up to 11% mean fluorescence intensity per fiber. When looking at this parameter in more detail, the three patients with the highest response (one patient in each cohort of 2, 10, and 20 mg/kg, respectively) had an increase from 5–9% pretreatment to 19–27% post-treatment. By contrast, even in the two cohorts dosed with 10 and 20 mg/kg, some patients showed more limited or no change in the percentage of dystrophin positive fibers, the mean fluorescence intensity or western blotting, and no formal dose response relation could be established from this study.
Data on more long-term systemic dosing of this compound came from 12 boys randomized to three cohorts of weekly intravenous dosing at 30 and 50 mg/kg per week and placebo for 24 weeks in the 4658-us-201 study (ClinicalTrials.gov Identifier NCT01396239) [Citation31]. AVI-4658 had by then received the generic name of eteplirsen. After 24 weeks, the placebo cohort was randomized to 30 (n = 2) and 50 (n = 2) mg/kg per week in the 4658-us-202 extension study (ClinicalTrials.gov Identifier NCT01540409). Biopsies from the biceps muscle were taken at baseline and at 12 weeks for the 50 mg/kg cohort and two controls, and at 24 weeks for the 30 mg/kg cohort and two controls. Muscle biopsies from the deltoid muscle were taken in all patients at 48 weeks. The main biopsy parameter that was reported was the percentage of dystrophin fibers assigned as positive by a pathologist in blinded fashion. This percentage had increased significantly at 24 weeks of 30 mg/kg eteplirsen treatment by 15.9–22.9%, but not at 12 weeks of 50 mg/kg eteplirsen treatment. At week 48, the deltoid muscle showed an increase of 29.8–60.3% positive fibers in the cohorts treated with 30 and 50 mg/kg. All patients continued treatment using the same dose as given at 48 weeks. Clinical data from these 12 boys have been published recently [Citation43]. Two boys in the original 30 mg/kg cohort had lost ambulation soon after enrolment. After 3 years of treatment, however, none of the remaining 10 boys had lost ambulation at an age of 10–13 years. Although this is not uncommon for steroid-treated DMD patients, it differed from a selected population of 13 historical Italian and Belgian controls, matched by age and carrying mutations amendable to exon 51 skipping. In this cohort, six patients had lost ambulation within 3 years of follow-up. Eteplirsen had a good safety profile, no systemic reactions or treatment-related serious adverse events were reported, and none of the adverse events led to treatment interruptions of dose adjustments [Citation43]. Sarepta filed for accelerated approval with the FDA based on data obtained in these 12 patients (referred to as the 201/202 study results). Upon request of FDA, dystrophin was quantified using western blot in biopsies obtained after 180 weeks of treatment. Since baseline tissue was not available for all patients, tissues from six untreated patients amenable to exon 51 skipping were added to this baseline analysis. This revealed an increase of dystrophin protein levels from 0.08% to 0.93% [Citation44]. In June 2016, FDA indicated that they were unable to decide on eteplirsen until Sarepta provided them with western blotting data on additional muscle biopsies from patients involved in a currently ongoing phase 3 clinical trial for eteplirsen (4658–301 or PROMOVI study, Clinicaltrials.gov Identifier: NCT02255552) [Citation45]. At that time, 13 patients had been treated for at least 48 weeks. Analysis of biopsies taken before and after 48 weeks of treatment revealed an increase of 0.28% of dystrophin for about half of the patients, while no increase was observed for the rest [Citation46]. On 19 September 2016, FDA decided to give eteplirsen accelerated approval based on the dystrophin data, which were considered ‘reasonably likely to predict a clinical benefit’ [Citation47]. At the same time, FDA stated that ‘a clinical benefit of eteplirsen, including improved motor function, has not been established’, and that Sarepta will have to confirm a functional effect in future trials. The decision was made after extensive internal debate of which details have been made public [Citation46] (for a commentary see Aartsma-Rus and Krieg [Citation48]).
4. Alternative approaches
What is clear from the above is that the exon skipping approach would benefit from ways to increase the efficiency of AONs and/or the longevity of the effect.
4.1. Alternative chemistries
Ongoing efforts aim to optimize AON chemistry [Citation49]. Tricyclo DNA (tcDNA) was recently reported as an alternative chemistry to induce dystrophin exon skipping [Citation50]. The tcDNA chemistry is a constrained oligonucleotide analogue that is more hydrophobic than regular DNA and RNA and has a very high affinity for target RNA. Therefore, shorter AONs can be used (14–16mer, compared to 18–22mer 2OMePS, and 25–30mer PMOs). Treatment with tcDNA in mdx mice resulted in exon skipping and dystrophin restoration in skeletal muscle and heart at levels that were significantly higher than in mdx mice treated with equimolar levels of 2OMePS and PMO AONS [Citation50]. Furthermore, detectable levels of exon skipping and dystrophin restoration were also observed in brain, partly correcting behavioral defects observed in mdx mice. However, high doses of tcDNA were needed to achieve exon skipping in brain, and this was accompanied by mild toxicity [Citation50]. As this chemistry has not been tested in humans yet, tolerability in humans is as yet unknown. As such, it is not yet known whether the therapeutic window will allow treatment effects in the brain in humans.
The 2ʹdeoxy 2ʹfluoro phosphorothioate (2FPS) was recently reported to recruit interleukin enhancer binding factors 2 and 3, and in this manner to increase exon skipping levels, probably through increased steric hindrance by the bulky pre-mRNA–AON–protein complex, thus preventing splicing factors to bind the target exon [Citation51]. We evaluated 2FPS AONs for DMD exon skipping and found that indeed in vitro these AONs are extremely efficient, leading to enhanced exon skipping levels for most 2OMePS counterparts tested [Citation52]. However, upon systemic treatment in the mdx mouse model, the 2FPS AONs did not induce any exon skipping. Furthermore, a decrease in weight and splenomegaly was observed for 2FPS-treated animals.
4.2. Conjugates
Because the PMO chemistry is uncharged, it is relatively straightforward to conjugate peptides to these AONs. The addition of arginine rich peptides leads to a significant increase in uptake by muscle, and to a larger extent heart and for some peptides even by the central nervous system, accompanied by high levels of exon skipping and dystrophin restoration in muscle and heart [53–59]. A plethora of arginine rich peptides have been generated and tested in DMD mouse models [Citation55,Citation56,Citation59]. Only one peptide has so far been tested in non-human primates. Unfortunately, this peptide-PMO caused severe kidney toxicity in injected monkeys already at levels below those needed to induce exon skipping in muscle [Citation60]. Safety has yet to be evaluated for additional peptides, but as the toxicity may have been driven by the amount of arginines, it is likely that most of these compounds are not tolerated well by higher species. Hopefully, peptides with less arginines will have a better safety profile, while still leading to a significant increase in uptake by muscle and heart.
Attempts have also been made to improve muscle and heart-specific uptake by conjugating homing peptides to the AONs [Citation57,Citation61]. For PMOs, addition to muscle-specific peptides by themselves did not result in increased uptake [Citation57]. However, combining them with arginine rich peptides did increase uptake and exon skipping beyond what was achieved with the arginine rich peptide. However, this only was observed when the muscle-specific peptide was placed between the arginine rich peptide and the PMO, suggesting that it may be possible the peptide is acting more as a hydrophic spacer (which has been shown to increase PMO efficiency) than directing PMO homing to muscle. For 2OMePS AONs, arginine rich peptides cannot be conjugated due to the negative charge of the backbone. However, the addition of a 7-mer peptide heart-homing identified by phage display biopanning did result in increased uptake and exon skipping after injection of a 2OMePS AON in the mdx mouse model [Citation61].
4.3. Formulations
In addition to conjugates and chemical modifications, recent reports have shown that formulating AONs in high hexose solutions (5% glucose/fructose or 5% fructose) can increase uptake, exon skipping levels and dystrophin restoration as well [Citation62,Citation63]. For the glucose/fructose solution this effect occurred for 2OMePS and PMO AONs and siRNA in mdx mice [Citation63]. The effect was shown to be the result of an impaired muscle energy metabolism in mdx mice [Citation63]. It is known that mdx muscles have relatively good quality muscle due to very efficient regeneration and that these mice are extremely hypertrophic (~50% more muscle than wild-type mice) [Citation64]. It is possible that the hypertrophy and/or regeneration processes drive the increased uptake of AONs in a sugar formulation. As these processes are occurring at much lower levels in humans, it is possible that uptake effect will not translate to humans. Experiments in the DBA/mdx mouse (which is not hypertrophic [Citation64]) will have to elucidate this further.
4.4. CRISPR/Cas9
Since exon skipping AONs target the pre-mRNA, the effect is transient and repeated injections are required. CRISPR/Cas9 technology allows editing of the DMD gene to bring about permanent exon skipping (). This technology uses guide RNAs that target Cas9 enzymes to generate double stranded breaks in the DNA. These will be repaired by non-homologous enjoining or, when a template is provided, by homologous recombination.
Figure 4. Genome editing approaches for DMD. (a) Deletion of a mutated or frame-disrupting exon. Using a pair of guide RNAs targeting the introns flanking an exon containing a mutation (the nonsense mutation in exon 23 from the mdx mouse is shown in this example), the exon can be removed on DNA level when the generated ends in intron 22 and intron 23 are linked by non homologous end joining. (b) Splice site mutations. A single guide RNA can be used to generate a double strand DNA break at a splice site. Since the non homologous end joining process is error prone, the repaired DNA will often involve a mutated splice site. Thus the exon containing the mutation is no longer recognized and skipped on RNA level, thus bypassing the mutation or restoring the reading frame. (c) Gene correction. In stem cells, double strand DNA breaks can activate homologous recombination (in addition to non homologous end joining). Homologous recombination requires a template with the correct information, that is provided with the guideRNA and CRISPR/Cas9. Homologous recombination between the homologous DNA at the borders of the DNA break and the template will result in a corrected DNA (exon 52 is restored in this example).
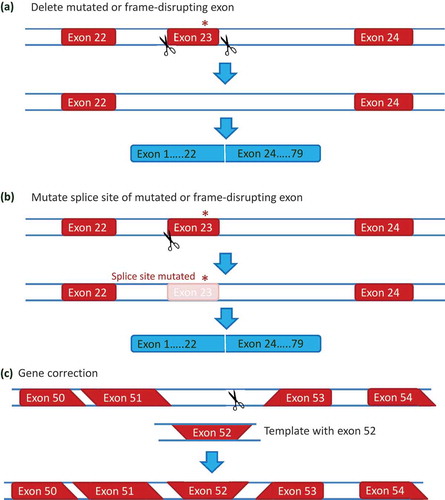
It has been shown that genome editing for the DMD gene is feasible in cell culture and mouse models [Citation65–Citation74]. Most studies use a pair of guide RNAs to target intron 22 and intron 23 in the mdx DMD gene, and generate an exon 23 deletion on DNA level, thus removing the mutation while maintaining the reading frame [Citation68–Citation71] (()). Alternatively, a single guide RNA targeting the 3ʹ or 5ʹ splice site has been used successfully as well. Here, the splice site will be mutated in a subset of targeted cells due to the error-prone non-homologous end joining process, resulting in a permanent skip on mRNA level ()). It has been shown feasible to correct the mutation in patient-derived myoblasts and iPS cells using the same strategies [Citation65–Citation67,Citation72,Citation73]. To increase the applicability, CRISPR/Cas9 has also been used to delete larger genomic regions, for example, an exon 45–55 deletion [Citation65,Citation72], which would apply to larger groups of patients and is associated with a mild phenotype in Becker patients [Citation15,Citation75] or to generate an exon 50–54 fusion exon [Citation66]. Gene correction, that is, replacing the mutated exon for a correct one via homologous recombination (()), has been shown in iPS cells [Citation73]. However, homologous recombination is a very inefficient process, and most likely not occurring in postmitotic cells such as muscle tissues, so this finding probably has limited translational value.
5. Conclusions
Exon skipping for DMD is a mutation-specific approach that would in theory apply to over 80% of patients. Exon 51 skipping AONs, which would apply to the largest group of DMD patients have been developed further and two AON chemistries have been tested in phase 2 and 3 clinical trials and one has now received accelerated approval in the United States after extensive internal dispute within the FDA. Key findings from the clinical development of AON mediated exon skipping in DMD are that these compounds are able to reach the target tissue through intramuscular, subcutaneous or intravenous admission. Both 2OMePS and PMO AONs induce skipped transcripts and the expression of internally deleted dystrophins. At the same time, it has become clear that all muscle fibers of untreated DMD patients show variable traces of dystrophin, and that methodology to quantify the levels of localized and functional protein is still in development.
No randomized placebo control trial has been able to demonstrate a clinically relevant benefit of AON treatment up to 48 weeks in ambulant DMD patients. Small uncontrolled studies suggest that long-term systemic dosing of both eteplirsen and drispersen might prolong the ambulatory phase in DMD. Long-term subcutaneous treatment with drisapersen led to local injection-site reactions of the skin that may prove to be permanent. Eteplirsen treatment was safe and well tolerated in patients treated so far. As yet, the number of patients exposed is a lot smaller than the drisapersen-treated cohort and further studies will need to be done to confirm a functional effect.
It is clear that there is room for improvement for this approach. Currently, work focuses on the development of more effective AON chemistries, increasing distribution of AONs to muscle or having a permanent reading frame restoration using CRISPR technology. However, these approaches have their own challenges, such as lack of safety data in humans.
6. Expert opinion
At the onset of these clinical trials, which were among the first randomized controlled trials in DMD, no detailed data were available on the natural history of the disease, especially regarding the clinical endpoints such as the 6MWT and timed function tests. The 6MWT was not developed for DMD, but for adult patients with chronic lung disease [Citation76]. The 6MWT was recommended by the FDA for one of the first DMD trials studying a read-through compound [Citation77]. By studying the placebo cohorts from these trials, the variability of this scale, the nonlinear decline towards loss of ambulation, and the fact that younger children can still show an increase in the distance within 1 year has posed questions for the clinical relevance of this outcome parameter. It is also questionable if the clinical effect from compounds that aim to restore dystrophin, and thus to prevent muscle damage rather than increase muscle power, can be captured by such a scale over a relatively short period of one to two years. This period seems the maximum that is feasible for placebo controlled studies in children. Over the last years, intensive international collaboration between scientists, commercial partners, patients organizations, and regulators has led to joined efforts to further standardize clinical care, to develop clinically relevant outcome parameters (including those that can be used in non-ambulant patients), but also to look at alternative outcome parameters such as serum biomarkers or muscle MRI that are able to demonstrate treatment effects over shorter periods [Citation39]. Since the primary endpoint in clinical trials aims to measure clinical benefit of a therapy, it is important that outcome measures assess functions that are deemed important by patients. Unlike the 6MWT that was not developed specifically for DMD, the performance upper limb scale was developed in collaboration with patients and the items of this scale reflect functions that DMD patients indicated they find important [Citation78,Citation79].
In parallel to efforts to optimize the tools needed for clinical trials, research focuses on optimizing the tools to induce exon skipping. New chemistries are reported on a regular basis and many of these could in theory be used for DMD exon skipping. However, in our opinion, two things should be considered ideally in an early stage. First, whether the manufacturing allows upscaling. Since skeletal muscle makes up 30–40% of our body mass and repeated injections are required, high doses of AONs will be needed. So, when upscaling is impossible, ineffective or very expensive, this may not be compatible with developing this chemistry for DMD exon skipping. The second consideration is whether the chemistry is safe. Mice are not very predictive for AON safety, so ideally very promising chemistries are screening at an early stage for safety in a pilot experiment in a more relevant animal such as rat.
The CRISPR technology has been used to restore the reading frame on DNA level rather than RNA level, thus effecting a permanent change. Expectations about the therapeutic potential for this technology are high. However, we should not forget that in order for this approach to work in DMD patients, the guide RNAs and CRISPR/Cas9 will need to be delivered to muscle efficiently. In animal models, this has now been achieved with the adeno-associated virus or using cell transplantation of ex vivo corrected cells. However, these approaches (gene and cell therapy) have been in development for DMD for decades, so far without success [Citation80]. This is mainly due to the abundance and inaccessibility of skeletal muscles, that precludes sufficient delivery of genes or cells to muscle.
Another challenge (one that faces all therapies) is that the time of intervention will influence the therapeutic effect. When disease progresses muscle tissue is replaced by fat and adipose tissues. These tissues do not express dystrophin, so AONs will not have a target transcript to hybridize to and when genome editing would occur in these tissues this will not lead to a therapeutic effect. Thus, early intervention is anticipated to result in a larger therapeutic effect. The majority of DMD patients are non-ambulant. While exon skipping will not restore the ability to walk for these patients, slowing down the loss of arm, hand and respiratory function would have therapeutic value as it would increase autonomy and independence and quality of life.
In conclusion, the exon skipping development has been a learning experience for everyone involved. While exon skipping is not yet an approved treatment yet, the clinical development of this approach has already yielded side benefits in the form of the development of tools for clinical trials and increased collaboration between academics, patients, industry and regulators [Citation39], which will hopefully expedite the development of current and future therapeutic approaches for DMD.
Article highlights
Duchenne muscular dystrophy is a severe, progressive muscle wasting disease with unmet medical need
Antisense-mediated exon skipping is a therapeutic approach that aims to increase production of partially functional dystrophin proteins
Antisense-mediated exon skipping is a mutation specific approach
Clinical development of exon skipping compounds has been challenging due to the lack of natural history data and clinical trial tools
Stakeholder collaboration in the DMD field has generated new tools for clinical trials that hopefully will facilitate future therapy development for DMD
This box summarizes key points contained in the article.
Declaration of interest
A Aartsma-Rus reports grant from the Duchenne Parent Project, ZonMw, an EU FP7 grant, AFM (The French Muscular Dystrophy Association), the Parent Project Muscular Dystrophy (PPM) and the Prinses Beatrix Spierfonds. She is also employed by the Leiden University Medical Center (LUMC), which has patents on exon skipping technology. As co-inventor of some patents, she is entitled to royalties. She also serves as an ad hoc consultant for several companies (BioClinica, Grunenthal, BioMarin, Summit, PTC Therapeutics, Global Guidepoint, GLC Consulting and Deerfield Consulting. Renumeration for these activities and speaker honoraria go to Leiden University Medical Center. EH Niks reports grants from the Duchenne Parent Project, ZonMW and the French Muscular Dystrophy Association and has received trial support from BioMarin, GlaxoSmithKline, Eli Lilly & Company and Santhera. He also reports acting as a consultant for BioMarin and Summit. Again, all reimbursements were received by the LUMC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy.. Ann Neurol. 2012;71:304–313.
- Pane M, Mazzone ES, Sivo S, et al. Long term natural history data in ambulant boys with Duchenne muscular dystrophy: 36-month changes. PLoS One. 2014;9:e108205.
- Goemans N, Vanden Hauwe M, Signorovitch J, et al. Individualized prediction of changes in 6-minute walk distance for patients with Duchenne muscular dystrophy. PLoS One. 2016;11:e0164684.
- Van Den Bergen JC, Hiller M, Bohringer S, et al. Validation of genetic modifiers for Duchenne muscular dystrophy: a multicentre study assessing SPP1 and LTBP4 variants. J Neurol Neurosurg Psychiatry. 2014.
- Bello L, Flanigan KM, Weiss RB, et al. Association study of exon variants in the NF-kappaB and TGFbeta pathways identifies CD40 as a modifier of Duchenne muscular dystrophy. Am J Hum Genet. 2016. DOI:10.1016/j.ajhg.2016.08.023
- Ricotti V, Ridout DA, Pane M, et al. The NorthStar ambulatory assessment in Duchenne muscular dystrophy: considerations for the design of clinical trials. J Neurol Neurosurg Psychiatry. 2016;87:149–155.
- Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93.
- Bello L, Morgenroth LP, Gordish-Dressman H, et al. DMD genotypes and loss of ambulation in the CINRG Duchenne Natural History Study. Neurology. 2016;87:401–409.
- Monaco AP, Bertelson CJ, Liechti-Gallati S, et al. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95.
- Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228.
- Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchene muscular dystrophy locus.1987. Biotechnology. 1992;24:457–466.
- Aartsma-Rus A, Fokkema I, Verschuuren J, et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum Mutat. 2009;30:293–299.
- Aartsma-Rus A. Antisense-mediated modulation of splicing: therapeutic implications for Duchenne muscular dystrophy. RNA Biol. 2010;7:453–461.
- Aartsma-Rus A, Ginjaar IB, Bushby K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J Med Genet. 2016;53:145–151.
- Bladen CL, Salgado D, Monges S, et al. The TREAT-NMD DMD global database: analysis of more than 7,000 Duchenne muscular dystrophy mutations. Hum Mutat. 2015; DOI:10.1002/humu.22758
- Aartsma-Rus A, Janson AA, Kaman WE, et al. Antisense-induced multiexon skipping for Duchenne muscular dystrophy makes more sense. Am J Hum Genet. 2004;74:83–92.
- Mitrpant C, Fletcher S, Iversen PL, et al. By-passing the nonsense mutation in the 4 CV mouse model of muscular dystrophy by induced exon skipping. J Gene Med. 2009;11:46–56.
- Yokota T, Lu QL, Partridge T, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676.
- Aartsma-Rus A, Ferlini A, Goemans N, et al. Translational and regulatory challenges for exon skipping therapies. Hum Gene Ther. 2014;25:885–892.
- Greer KL, Lochmüller H, Flanigan K, et al. Targeted exon skipping to correct exon duplications in the dystrophin gene. Mol Ther Nucleic Acids. 2014;3:e155.
- Aartsma-Rus A, Janson AA, Van Ommen GJ, et al. Antisense-induced exon skipping for duplications in Duchenne muscular dystrophy. BMC Med Genet. 2007;8:43.
- Vulin A, Wein N, Simmons TR, et al. The first exon duplication mouse model of Duchenne muscular dystrophy: a tool for therapeutic development. Neuromuscul Disord. 2015;25:827–834.
- White SJ, Aartsma-Rus A, Flanigan KM, et al. Duplications in the DMD gene. Hum Mutat. 2006;27:938–945.
- Gurvich OL, Tuohy TM, Howard MT, et al. DMD pseudoexon mutations: splicing efficiency, phenotype, and potential therapy. Ann Neurol. 2008;63:81–89.
- Beroud C, Tuffery-Giraud S, Matsuo M, et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum Mutat. 2007;28:196–202.
- Van V, De Winter CL, Van Deutekom JC, et al. Assessment of the feasibility of exon 45-55 multiexon skipping for duchenne muscular dystrophy. BMC Med Genet. 2008;9:105.
- Echigoya Y, Aoki Y, Miskew B, et al. Long-term efficacy of systemic multiexon skipping targeting dystrophin exons 45-55 with a cocktail of vivo-morpholinos in mdx52 mice. Mol Ther Nucleic Acids. 2015;4:e225.
- Aartsma-Rus A. Dystrophin analysis in clinical trials. J Neuromuscular Dis. 2014;1:41–53.
- Aartsma-Rus A, Van Deutekom JCT, Fokkema IF, et al. Entries in the leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34:135–144.
- Goemans NM, Tulinius M, Van Den Akker JT, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011;364:1513–1522.
- Mendell JR, Rodino-Klapac LR, Sahenk Z, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013;74:637–647.
- Van Deutekom JC, Janson AA, Ginjaar IB, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686.
- Beekman C, Sipkens JA, Testerink J, et al. A sensitive, reproducible and objective immunofluorescence analysis method of dystrophin in individual fibers in samples from patients with Duchenne muscular dystrophy. PLoS One. 2014;9:e107494. DOI:10.1371/journal.pone.0107494
- Anthony K, Arechavala-Gomeza V, Taylor LE, et al. Dystrophin quantification: Biological and translational research implications. Neurology. 2014;83:2062–2069.
- Goemans NM, Tulinius M, Van Den Hauwe M, et al. Long-term efficacy, safety, and pharmacokinetics of drisapersen in Duchenne muscular dystrophy: results from an Open-Label extension study. PLoS One. 2016;11:e0161955. DOI:10.1371/journal.pone.0161955
- Voit T, Topaloglu H, Straub V, et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): an exploratory, randomised, placebo-controlled phase 2 study. Lancet Neurol. 2014;13:987–996.
- Mazzone ES, Pane M, Sormani MP, et al. 24 month longitudinal data in ambulant boys with Duchenne muscular dystrophy. PLoS One. 2013;8:e52512.
- McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve. 2013;48:343–356.
- Straub V, Balabanov P, Bushby K, et al. Stakeholder cooperation to overcome challenges in orphan medicine development: the example of Duchenne muscular dystrophy. Lancet Neurol. 2016;15:882–890.
- Van ML, Moerland M, Gallagher J, et al. Injection site reactions after subcutaneous oligonucleotide therapy. Br J Clin Pharmacol. 2016;82(2):340–351.
- Kinali M, Arechavala-Gomeza V, Feng L, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928.
- Cirak S, Arechavala-Gomeza V, Guglieri M, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605.
- Mendell JR, Goemans N, Lowes LP, et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 2016;79:257–271.
- http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/peripheralandcentralnervoussystemdrugsadvisorycommittee/ucm497064.pdf. 2016.
- http://investorrelations.sarepta.com/phoenix.zhtml?c=64231&p=irol-newsArticle&ID=2175522. 2016.
- http://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/206488_summary%20review_Redacted.pdf. 2016.
- http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm521263.htm. 2016.
- Aartsma-Rus A, Krieg AM. FDA approves eteplirsen for Duchenne muscular dystrophy - the next chapter in the eteplirsen saga. Nucleic Acid Ther. 2016. DOI:10.1089/nat.2016.0657
- Järver P, O’Donovan L, Gait MJ. A chemical view of oligonucleotides for exon skipping and related drug applications. Nucleic Acid Ther. 2014;24:37–47.
- Goyenvalle A, Griffith G, Babbs A, et al. Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat Med. 2015;21:270–275.
- Rigo F, Hua Y, Chun SJ, et al. Synthetic oligonucleotides recruit ILF2/3 to RNA transcripts to modulate splicing. Nat Chem Biol. 2012;8:555–561.
- Jirka SM, Tanganyika-De Winter CL, Boertje-Van Der Meulen JW, et al. Evaluation of 2ʹ-deoxy-2ʹ-fluoro antisense oligonucleotides for exon skipping in Duchenne muscular dystrophy. Mol Ther Nucleic Acids. 2015;4:e265.
- Betts CA, Saleh AF, Carr CA, et al. Prevention of exercised induced cardiomyopathy following Pip-PMO treatment in dystrophic mdx mice. Sci Rep. 2015;5:8986.
- Shabanpoor F, McClorey G, Saleh AF, et al. Bi-specific splice-switching PMO oligonucleotides conjugated via a single peptide active in a mouse model of Duchenne muscular dystrophy. Nucleic Acids Res. 2015;43:29–39.
- Betts C, Saleh AF, Arzumanov AA, et al. Pip6-PMO, a new generation of Peptide-oligonucleotide conjugates with improved cardiac exon skipping activity for DMD treatment. Mol Ther Nucleic Acids. 2012;1:e38.
- Yin H, Saleh AF, Betts C, et al. Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice.. Mol Ther. 2011;19:1295–1303.
- Yin H, Moulton HM, Betts C, et al. Functional rescue of dystrophin-deficient mdx mice by a chimeric peptide-PMO. Mol Ther. 2010;18:1822–1829.
- Yin H, Moulton HM, Seow Y, et al. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet. 2008;17:3909–3918.
- Wu B, Moulton HM, Iversen PL, et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci U S A. 2008;105:14814–14819.
- Moulton HM, Moulton JD. Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim Biophys Acta. 2010;1798:2296–2303.
- Jirka SM, Heemskerk H, Tanganyika-De Winter CL, et al. Peptide conjugation of 2ʹ-O-methyl phosphorothioate antisense oligonucleotides enhances cardiac uptake and exon skipping in mdx mice. Nucleic Acid Ther. 2014;24:25–36.
- Cao L, Han G, Lin C, et al. Fructose promotes uptake and activity of oligonucleotides with different chemistries in a context-dependent manner in mdx mice. Mol Ther Nucleic Acids. 2016;5:e329.
- Han G, Gu B, Cao L, et al. Hexose enhances oligonucleotide delivery and exon skipping in dystrophin-deficient mdx mice. Nat Commun. 2016;7:10981.
- Coley WD, Bogdanik L, Vila MC, et al. Effect of genetic background on the dystrophic phenotype in mdx mice. Hum Mol Genet. 2016;25:130–145.
- Young CS, Hicks MR, Ermolova NV, et al. A Single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell. 2016;18:533–540.
- Iyombe-Engembe J-P, Ouellet DL, Barbeau X, et al. Efficient restoration of the dystrophin gene reading frame and protein structure in DMD myoblasts using the cindel method. Mol Ther Nucleic Acids. 2016;5:e283.
- Maggio I, Stefanucci L, Janssen JM, et al. Selection-free gene repair after adenoviral vector transduction of designer nucleases: rescue of dystrophin synthesis in DMD muscle cell populations. Nucleic Acids Res. 2016;44:1449–1470.
- Tabebordbar M, Zhu K, Cheng JKW, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351:407–411.
- Nelson CE, Hakim CH, Ousterout DG, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351:403–407.
- Long C, Amoasii L, Mireault AA, et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351:400–403.
- Xu L, Park KH, Zhao L, et al. CRISPR-mediated genome editing restores dystrophin expression and function in mdx mice. Mol Ther. 2016;24:564–569.
- Ousterout DG, Kabadi AM, Thakore PI, et al. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat Communications. 2015;6:6244.
- Li HL, Fujimoto N, Sasakawa N, et al. Precise correction of the dystrophin gene in Duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Rep. 2015;4:143–154.
- Long C, McAnally JR, Shelton JM, et al. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA.. Science. 2014;345:1184–1188.
- Béroud C, Tuffery-Giraud S, Matsuo M, et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum Mutat. 2007;28:196–202.
- Butland RJ, Pang J, Gross ER, et al. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed). 1982;284:1607–1608.
- Bushby K, Finkel R, Wong B, et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve. 2014;50:477–487.
- Mayhew A, Mazzone ES, Eagle M, et al. Development of the performance of the upper limb module for Duchenne muscular dystrophy. Dev Med Child Neurol. 2013;55:1038–1045.
- Pane M, Mazzone ES, Fanelli L, et al. Reliability of the performance of upper limb assessment in Duchenne muscular dystrophy.. Neuromuscul Disord. 2014;24:201–206.
- Jirka S, Aartsma-Rus A. An update on RNA-targeting therapies for neuromuscular disorders. Curr Opin Neurol. 2015;28:515–521.
