ABSTRACT
Introduction: Despite the relatively high efficacy of antifungal drugs, invasive fungal infections (IFIs) are still associated with tremendous morbidity and mortality, since late diagnosis makes an antifungal drug therapy inefficient. Therefore, antifungal immunotherapies to specifically strengthen the host´s own immune mechanisms constitute an additional promising strategy in taking action against fungal pathogens.
Areas covered: The authors summarize efforts in research and clinical trials to provide safe and efficient immunotherapeutic options against invasive fungal diseases. Treatment of IFIs is challenging as the number of available antifungals is limited and further complications include: toxicity, drug interactions and the emergence of drug resistance. Susceptibility is determined by the impaired immune status of the host. Hence, augmenting immunity by immunotherapeutic interventions may offer future directions to treat IFI.
Expert opinion: A much better understanding of fungus and host cell interactions is essential for the development of safe and successful immunotherapeutic strategies. Indeed, there is encouraging preliminary data available that such approaches are possible; however, current data is too limited to allow solid conclusions on the risks and benefits in the clinical setting. Clinical trials focusing on the role of adjuvant immunotherapeutics with or without a combination of antifungals are highly needed for further evaluation.
1. Introduction
Opportunistic fungal infections are the major cause of morbidity and mortality among immunocompromised individuals [Citation1]. Individuals at risk are patients with prolonged neutropenia, allogeneic hematopoietic stem cell transplant (HSCT), solid organ transplant (SOT), inherited or acquired immunodeficiencies i.e. AIDS, or corticosteroid use, among others [Citation1]. Severity and pattern of fungal diseases are usually directed by the interplay of the pathogen and host immunity. Hence, host susceptibility is determined by the activity of cell- and humoral-mediated immunity [Citation2]; in immunocompetent patients disease manifestation is linked to an excessed, in immunocompromised individuals to lack of adequate host response [Citation3]. For successful infection, fungi adapt to hosts stressors and evade hosts’ immunity; any failure to restore host immunity leads to worse outcome.
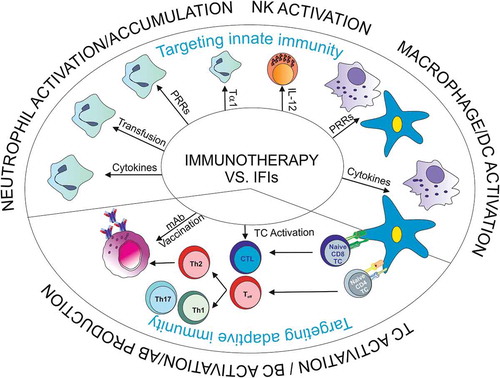
Approaches to modulate the host immune system in fighting against fungal pathogens include the application of effector and regulatory cells (e.g. granulocytes, antigen-specific T cells) as well as the administration of recombinant cytokines and growth factors (e.g. interferon-γ (IFN-γ), granulocyte- and granulocyte-macrophage colony stimulating factor (G-CSF, GM-CSF), TNF-α, IL-15) and vaccinations. Although promising results are reported on in vitro data and animal studies, current data are too limited to allow solid conclusions on the risks and the benefits of these strategies in the clinical setting.
Modulating host response may be associated with moderate success because of the onset of toxicity, inherent or acquired resistance, excessive immune response and inflammation, or pathogen replacement [Citation3]. Pathogen-modulating interventions may benefit from the fact that monoclonal antibodies (mAb) specifically target antigens; however, the induction of anti-mAB may raise unknown pharmacokinetic and pharmacodynamic principles thereby limiting their usage [Citation3]. The range of targets here is a broad one and runs from mAbs to synthetic antimicrobial peptides, signal transduction inhibitors, biological agents that may neutralize fungal pathogens by opsonizing directly and/or engaging the complement system, blocking their binding to cells, arresting their growth, or killing them altogether. Here, we outline efforts in research and clinical trials to provide safe and efficient immunotherapeutic options against invasive fungal infections (IFIs) and to strengthen innate and adaptive effector functions by various approaches.
2. An overview of potential immunotherapies for fungal diseases
2.1. Innate and adaptive host immune response to fungal infections
Upon entry into the host, fungi activate both – early immediate (innate) and sophisticated specific (adaptive) – immune response elements [Citation4]. Early antifungal defenses comprise pathogen destruction through phagocytic processes or the secretion of microbicides, immune cell recruitment through release of chemotactic factors or through activation of humoral components of the innate immune system. Neutrophils, monocytes, macrophages, natural killer (NK) cells and dendritic cells (DCs) comprise the most important cell types in sensing fungal pathogens once they have passed the physical barriers [Citation4–Citation6].
The innate immune components immediately recognize invading pathogens through pattern recognition receptors (PRRs), which are a limited number of germ line-encoded receptors expressed on endo- and epithelial cells and on immune cells underlying i.e. the skin or the mucous membranes of the respiratory, gastrointestinal, or urogenital tracts [Citation7]. PRRs recognize pathogen-associated molecular patterns (PAMPs) unique to fungi, bacteria, viruses, or parasites and the combined action of different PRR families leads to recognition and – at best – elimination of pathogens. Among PRRs expressed on myeloid cells i.e. DCs, neutrophils or macrophages are toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors, retinoic acid inducible gene I (RIG-I)-like receptors and C-type lectin receptors (CLRs) [Citation8]. PRRs induce signaling pathways to activate pathogen-specific immune responses thereby spontaneously controlling infection.
After several days of infection the adaptive immune response is then tailored via DCs, the sentinels of our immune system and the only cells capable of priming naïve T cells in the T cell zone of the lymph node [Citation5]. Dependent on the type of infection, DCs engage T cell receptors and create specific cytokine surroundings thereby polarizing CD4+ T cells into the appropriate T helper (TH) subset(s) and activating cytotoxic T lymphocytes (CTLs or CD8+ T cells). Stimulation and polarization of T cells is crucial for an optimal protective immunity against fungal infections, since both CD4+ and CD8+ T cells are required for clearance of fungal pathogens [Citation9].
Qualitative or quantitative defects in neutrophils – as given in immunocompromised individuals – display a high risk factor for IFIs. In addition, other immune defense mechanisms may play a major role since the number of IFIs increased in non-neutropenic patients [Citation10]. Factors involved may comprise defective recognition of the pathogen, cell recruitment, T cell priming or cell effector functions. Immunotherapies against IFIs, which are currently under investigation at the preclinical level, try to restore these failures in immune cell functions or in humoral immune responses (). Most of the immune therapeutic interventions are largely in development and are under extensive evaluation based on in vitro studies and animal models; hence no clear clinical recommendations can be drawn. Limited clinical data are available on the application of colony-stimulating factors, granulocyte transfusion, recombinant interferon (IFN)–γ in neutropenic patients with diagnosed or suspected invasive aspergillosis (IA) or T cell transfer.
2.2. Enhancement of innate immune effectors as immunotherapeutical strategies against IFIs
2.2.1. Neutrophil enrichment and activation
Since defects in neutrophils are a predisposing factor for opportunistic infections, one approach is to increase and activate neutrophils to augment the immune response to fungal pathogens ():
2.2.1.1. Neutrophil augmentation and activation by colony-stimulating factors (CSFs)
High cytokine concentrations are locally needed to activate non fungus-specific cells of the innate immune system, for example, neutrophils [Citation11]. Neutrophils are produced in the bone marrow in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF), which also inhibits neutrophil apoptosis [Citation12]. Furthermore, GM-CSF stimulates oxidative burst from neutrophils, phagocytosis as well as upregulation of phagocytizing surface receptors, for example complement receptors complement receptors [Citation13,Citation14]. In patients receiving cytotoxic regimens CSFs are commonly used to shorten the duration of neutropenia [Citation15]. Granulocyte CSF (G-CSF) influences survival, proliferation, and cell differentiation within the neutrophil lineage and boosts mature neutrophil functions. Also, G-CSF stimulates neutrophil recovery and effector functions and activates monocytes and macrophages. A meta-analysis of prophylactic G-CSF showed a reduction in the incidence of neutropenic fever and death in adult cancer patients [Citation16], and a survival benefit in randomized controlled clinical trials of cancer chemotherapy [Citation17]. In vivo and in vitro experiments showed that resolution of infection with filamentous fungi was closely associated with the activation of neutrophils, which jointly exert oxidative and nonoxidative mechanisms by causing hyphal damage of e.g. Aspergillus (A.) fumigatus and Rhizopus oryzae and efficiently keeping fungal invasion and dissemination under control [Citation18,Citation19]. Nevertheless, the risk of inducing a hyper-inflammatory response by extensive neutrophil activation has to be taken into account also for the applications described below and also questions the clinical value of CSFs for the treatment of fungal infections. Due to lack of clinical data the use of CSFs in patients suffering from IFIs cannot be recommended [Citation1].
2.2.1.2. Neutrophil augmentation and activation by recombinant IFN-γ (rIFN-γ)
The use of recombinant interferon gamma (rIFN-γ) as adjunctive therapy for patients with IA is limited to case reports and small studies. Beside TH1 polarization associated with antifungal control, IFN-γ was shown in vitro to initiate O2 production in polymorphonuclear leukocytes (PMNs) following Pneumocystis carinii exposure and was illustrated to enhance fungistatic and fungicidal activities of neutrophils against most fungal species tested [Citation20,Citation21]. A major concern related to the application of rIFN-γ in allogeneic HSCT recipients is the potential to worsen graft versus host disease (GVHD). However, a single-center retrospective analysis showed rIFN-γ to be safe in allogeneic HSCT recipients [Citation22]. Currently, data supporting the efficacy of adjunctive rIFN-γ for aspergillosis are weak [Citation1]. rIFN-γ has been used as salvage therapy for patients with cerebral cryptococcosis unresponsive to antifungal drugs [Citation23]. A short-course IFN-γ administration to standard treatment significantly increased the rate of clearance of cryptococcal infection. However, data supporting the routine usage of adjunctive rIFN-γ for cryptococcosis are weak [Citation24]. Respecting Scedosporium spp. infection and neutrophil activation combined treatment with GM-CSF/IFN-γ proved useful in in vitro experiments, but need to be further investigated in vivo to determine the efficacy of the cytokine combination [Citation21].
2.2.1.3. Effects of rTNF-α on neutrophils during fungal infection
In vitro, TNF-α – despite its high toxicity – was demonstrated to be a potent activator of fungicidal activity of PMNs against Candida and A. fumigatus [Citation25-Citation27]. In vivo experiments depicted that treatment with TNF-α resulted in a decreased mortality in corticosteroid-treated mice with invasive aspergillosis [Citation28]. Another study showed that intratracheal challenge of neutropenic and non-neutropenic mice with A. fumigatus conidia increased lung TNF-α levels associated with an increased infiltration of PMNs and a lower mortality. They further illustrated that neutralization of TNF-α resulted in an increase in mortality in normal and cyclophosphamide-treated animals associated with increased lung fungal burden and that pre-treatment of mice with a TNF-agonist before infection had a protective effect and enhanced resistance to A. fumigatus in neutropenic mice [Citation29].
2.2.1.4. PMN activation via IL-15
IL-15, another key immune-regulatory cytokine of innate immune cells i.e. PMNs or NK cells, was also studied with respect to its antifungal activity [Citation30]. In vitro experiments depicted that IL-15-treated PMNs produced increased levels of superoxides and released IL-8 in response to filamentous fungi in a time- and species-dependent fashion. Thus, IL-15 has a potential adjuvant role to conventional antifungal therapy in prevention and treatment of invasive aspergillosis, which needs to be further investigated.
2.2.1.5. Neutrophil augmentation via granulocyte transfusion
Granulocyte transfusion has been used for many years as adjunctive treatment for severe infections in neutropenic patients, with the use of unrelated donors being feasible [Citation31,Citation32]. Granulocyte transfusions augment the numbers of circulating neutrophils to support the patient´s immune system during neutropenia and suffering from IFIs. Neutrophil transfusions from healthy donors were shown to be safe in a phase I/II clinical trial for treatment of infections in HSCT patients [Citation33]. Patients who received >0.6 × 109 granulocytes/kg per transfusion tended to have a better outcome when compared to a lower dose [Citation33]. Further, granulocyte transfusion was demonstrated to increase survival rates in cancer patients with candidemia, to show good clinical efficacy in various case reports of patients with IA and mucormycosis and to prevent recurrence of fungal infections [Citation22,Citation32,Citation33]. On the other hand, a phase III randomized trial of 55 neutropenic patients suffering from IFIs did not show any clear effect of granulocyte transfusion [Citation34]. Acute lung injury and alloimmunization leading to graft failure after allogeneic HSCT are the major risks of granulocyte transfusions. Presently, there are insufficient data to recommend their use in patients to treat IFIs [Citation1].
2.2.1.6. Neutrophil augmentation via transfer of myeloid progenitors
Common myeloid and granulocyte-monocyte progenitors were illustrated to protect against lethal A. fumigatus infections following chemotherapy-induced neutropenia and HSCT in a mouse model [Citation35,Citation36], which may consider them as novel strategy to strengthen neutrophil numbers and functions during IFIs.
2.2.1.7. Neutrophil activation via thymosin-α1 (Tα1)
The thymus-derived immune-stimulatory agent thymosin-α1, which is approved in more than 30 countries to treat viral infections and as an immune adjuvant [Citation37], prolonged survival and reduced fungal burden in kidneys of immunosuppressed mice when used in combination with fluconazole [Citation38]. PMNs showed an increased intracellular killing of Candida (C.) albicans upon the combinatorial treatment with Tα1 and fluconazole in these mice [Citation38]. These data suggest that Tα1 boosts neutrophils in combination with an antifungal drug to efficiently kill fungi, but in vivo data are still lacking.
2.2.1.8. Neutrophil activation via PRRs
Neutrophils recognize fungi via TLR2, TLR4, Dectin-1 and via complement receptor CR3 (CD11b/CD18) and CR1 interactions [Citation39,Citation40]. Ligation of these receptors on neutrophils in vitro results in mitogen-activated protein kinase (MAPK), especially ERK, activation and inhibition of the ERK pathway blocked Candida albicans-mediated neutrophil migration [Citation41]. TLR4 recognizes A. fumigatus hyphae leading to a release of proinflammatory cytokines in effector cells and further neutrophil recruitment [Citation40]. Different Aspergillus motifs interact with distinct TLR family members on neutrophils resulting in specialized antifungal effector functions such as activation and release of inflammatory cytokines [Citation42]. Also, liposomal amphotericin B was shown to stimulate antifungal activity of neutrophils by diverting TLR2 to TLR4 signaling in the absence of inflammatory toxicity [Citation43]. These data suggest that selective addressing of TLRs or other engaged PRRs, for example Dectin-1 or CRs, in vivo is eligible to induce improved fungicidal activity via neutrophils, which has to be further clinically evaluated.
Additionally, in response to primary inflammatory signals (e.g. TLR engagement, TNF-α, IL-1β), neutrophils release pentraxin 3 (PTX3), which is dealt as potential therapeutic agent with anti-inflammatory properties in aspergillosis [Citation44]. PTX3, an opsonin, binds with high affinity on the conidial surface of A. fumigatus [Citation45]. Thus, PTX3 amplifies the innate immune response by activating the classical complement pathway and facilitating efficient phagocytosis of opsonized fungi by macrophages and DCs [Citation46,Citation47]. Early PTX3 administration was shown to enhance the fungicidal (conidiocidal) activity of neutrophils by exerting anti-inflammatory properties in NADPH oxidase-deficient mice [Citation48].
2.2.2. Macrophage and dendritic cell activation
During suppressing treatment also macrophage, monocyte, and dendritic cell functions may be affected. Hence, another goal is to restore their functionality by various options ().
2.2.2.1. Macrophage and DC activation via cytokines
Notably, macrophage colony-stimulating factor (M-CSF) does not exert any effect on neutrophils but enhances monocyte and macrophage functions, such as superoxide production, phagocytosis, chemotaxis, and cytokine production [Citation49]. Mice infected with Candida albicans had a higher survival rate after prophylactic administration of M-CSF and in a related study [Citation50], also the survival of rats treated with a combination of M-CSF and fluconazole was increased [Citation51,Citation52]. A phase I clinical trial included patients with IFI and receiving recombinant human M-CSF displayed a 50% 2-year survival rate when compared to a historical cohort (15%) [Citation53].
Further, a retrospective study investigated the usage of granulocyte-macrophage colony-stimulating factor (GM-CSF) [Citation54] as GM-CSF alone or in combination with IFN-γ improved the fungicidal activity of innate phagocytic cells in animal studies [Citation55]. 66 patients with hematological malignancies or HSCT received rhGM-CSF as immune therapeutic adjuvant to treat IFIs [Citation54]. GM-CSF administration turned out to be safe and well tolerated, but contrary to the experiments in mice, the addition of GM-CSF during high-dose corticosteroid therapy resulted in treatment failure. A significantly better outcome occurred in more than 50% of the patients upon administration of GM-CSF during antineoplastic therapy [Citation54]. It needs further studies to evaluate GM-CSF efficiency and exact mode of action in IFI treatment of immunosuppressed individuals.
2.2.2.2. Macrophage and DC activation via PRRs
Murine macrophages are stimulated only by Aspergillus conidia, but not hyphae, via TLR4 to produce proinflammatory cytokines TNF-α and IL-1. Contrary, hyphae signal via TLR2-dependent mechanisms thereby initiating an anti-inflammatory cytokine milieu. This switch of the inflammatory response was suggested to contribute to fungal evasion from host defense [Citation56].
Similar to TLR4 stimulation of neutrophils, these data may support a selective targeting of specific TLRs to improve fungicidal activity of macrophages or other myeloid cells.
Murine and human DCs were found to upregulate the expression of TLR2 and TLR9 signaling upon Tα1-administration, to mature and to produce cytokines. Treatment of A. fumigatus-infected mice with Tα1 showed an increased survival after infection, a reduced fungal growth and a promotion of protective IFN-γ TH1 cells [Citation57]. Besides inducing immunogenic DCs, Tα1 activated DCs for IDO-mediated immune tolerance through TLR9 and type I inferferon signaling [Citation58]. By governing the balance between inflammatory and regulatory DCs, Tα1 or Tα1-exposed DCs administration resulted in protective immunity to A. fumigatus [Citation58]. These results are promising to use Tα1 or Tα1-exposed DCs as adjunct therapy to treat IFIs, but need to be evaluated in clinical trials.
Also CRs, expressed on macrophages, monocytes and DCs recognize opsonized pathogens and therefore influence the immune response induced by these antigen-presenting cells. After inhalation, fungal conidia are opsonized with complement fragments (C3) and it has been reported that opsonization of A. fumigatus influences the binding to and ingestion in phagocytes [Citation59]. Furthermore, opsonization of pathogens resulted in stronger production of pro-inflammatory cytokines as well as in a TH1/TH17 differentiation of naïve T cells in vitro when co-cultured with DCs [Citation60,Citation61]. Triggering CRs during fungal infection might represent a new strategy to restore myeloid cell functions and could provide another novel way to generate a protective T cell response.
2.2.2.3. Transducing or pulsing DCs to improve adaptive immune responses
Since appropriate stimulation of naïve T cells in the T cell zone of the lymph node by DCs is the key event in efficient adaptive host immune responses to pathogens, specifically targeting DCs may be promising for future vaccination strategies against fungal pathogens. Murine models showed that DCs transduced with an adenoviral vector encoding IL-12 acted as potent vaccine against invasive pulmonary aspergillosis [Citation62] and that DCs pulsed ex vivo with live Aspergillus fumigatus or A. fumigatus RNA induced an efficient and protective TH1 immune response [Citation63]. Ex vivo transduction or pulse of DCs is labor intensive and expensive and therefore not suited for broad use in clinical applications. Nevertheless, the results underline the translational potential of ex vivo transduced or pulsed DCs in therapeutic vaccination against IFIs [Citation64]. Direct targeting of DC subsets may result in strong immune responses as well as induction of fungus-specific CD8+ T cells due to cross-presentation of the delivered antigen [Citation65]. However, despite the progress in the field of DC subset characterization and targeting, still no vaccines are clinically available for any fungal pathogen.
2.2.3. Natural killer (NK) cell activation via IL-12
Very recently, also NK cells were implicated for therapeutic approaches to combat fungal infections. In particular HIV-1-infected individuals suffering from Cryptococcus were illustrated to express reduced levels of (i) NKp30 on NK cells, (ii) perforin content and release from NK cells, and (iii) cryptococcal binding and killing by NK cells compared to healthy adults [Citation66]. Treatment with IL-12 reversed this defective NK cell anti-cryptococcal activity in HIV-1 infected individuals [Citation66]. Therefore, modulation of NK cells via specific cytokines might also provide an immunotherapeutic target in treating fungal infections.
2.3. Targeting adaptive cellular immunity during IFIs
2.3.1. Adoptive T cell transfer
CD4+ T cells in particular are critical for successful elimination and development of protective immunity against fungi. A clinically highly relevant study investigated the transfer of specific T cells against A. fumigatus after HSCT [Citation67]. Haploidentical recipients with aspergillosis had marked immune defects with delayed TH1 cell reconstitution. Adoptive T cell transfer of functionally active Aspergillus-specific TH1 cells significantly reduced Aspergillus galactomannan antigenemia and resulted in a clinical benefit [Citation68]. Stühler et al. illustrated that IFN-γ-secreting TH1 cells increased innate immune responses against Aspergillus by activating macrophages and neutrophils [Citation67]. These observations might also be transferred to other IFIs, for example mucormycosis, and suggest that reconstitution of cellular immunity by adoptive T cell transfer is able to improve the prognosis of allogeneic HSCT recipients suffering from mucormycosis [Citation69], but this remains to be elucidated in vivo. Patients with IA and allo-SCT showed a low rate of T cell proliferation when exposed to Aspergillus antigens and a predominant Aspergillus-specific TH2 response, which was associated with a worse outcome [Citation70]. Promising results were achieved in a preliminary study, when in vitro stimulated and selected Aspergillus-specific T cell clones were transferred to allo-SCT patients with uncontrolled IA [Citation68].
Not only the transfer of functionally active fungus-specific TH1 cells for adoptive transfer, but also Treg-based immunotherapies have been studied [Citation71]. In this experimental approach, co-infusion of Treg cells and conventional T cells favored immune reconstitution, improved immunity to opportunistic pathogens and prevented lethal graft-versus-host disease (GVHD) in the absence of any post-transplantation immunosuppression. Nevertheless, the benefit of adoptive transfer of Tregs in combination with functionally active TH1 cells to treat IFIs has to be further investigated.
Though CD4+ T cells play the major role regarding control of pathogenic fungal infections, it was demonstrated in a murine model infected with yeasts that protective antifungal memory CD8+ T cells persisted for more than 5 months without CD4+ T cell help and remained fully functional [Citation72]. Earlier studies in mice and human peripheral blood mononuclear cells also illustrated that CD8+ T cells mount protective antifungal immune responses [Citation73]. In human PBMCs, these actions required CD4+ T cell help as well as exposure to IL-15 [Citation73]. These findings suggest that not only CD4+ T cells, but also CD8+ T cell memory might be targeted for designing novel antifungal vaccines.
Additionally, it was shown, that isolated T cells expressing the alternative gamma/delta (γδ) T cell receptor and recognizing fundamentally different ligands from the short peptides that are seen by alpha/beta (α/β) T cells in the context of MHC class I or class II molecules, mediated anti-Aspergillus activity [Citation70]. γδ T cells rapidly produce a variety of cytokines and usually exert potent cytotoxic activity and as DCs or complement they functionally link innate and adaptive immunity. They were easily activated and expanded in vivo by stimulation with aminobisphosphonates [Citation74]. The γδ T cell population does not mediate alloreactivity, hence may be a promising candidate for adoptive immunotherapy to treat IFIs.
2.3.2. Increase of T cell immunity via cytokines (e.g. recombinant IFN-γ)
Several studies have focused on a combinatorial therapy of antifungal drugs and cytokines, that is, IFN-γ and GM-CSF. A pilot study showed that IFN-γ, which is normally produced by T cells and NK cells, increased the cytotoxicity of antigen presenting cells in patients with Candida and Aspergillus infections [Citation75]. Moreover, rIFN-γ treatment was responsible for enhanced secretion of pro-inflammatory cytokines (IL-1β and TNFα) and up-regulation of MHC class II receptors on leukocytes, which in turn results in higher induction of T cell responses [Citation75]. Additionally, it has been reported that rIFN-γ treatment enhanced secretion of IL-17 and IL-22 by antigen presenting cells – both cytokines responsible for stimulating TH17 cells. Differentiation of naïve T cells into TH17 cells is associated with protective immune response in patients with candidemia and IA [Citation75,Citation76]. rIFN-γ is a valuable cytokine already used as prophylactic agent but also in combination with antifungal drugs to stimulate cellular immunity and thereby limiting fungal burden [Citation77].
2.4. Improvement of humoral adaptive immunity
Humoral immunity against IFIs can be strengthened by application of antibodies or vaccination (). In contrast to this, there are also reports questioning the effectiveness of antibody-mediated protection [Citation78,Citation79].
2.4.1. Increase in humoral immunity via antibody administration
Individuals suffering from primary antibody deficiency, for example, X-linked agammaglobulinemia (XLA) or common variable immunodeficiency (CVID) have a higher incidence of fungal infection [Citation80,Citation81]. Immunotherapeutic studies characterizing protective monoclonal antibodies (mAbs) have been described for e.g. A. fumigatus, C. albicans, Histoplasma (H.) capsulatum, or Paracoccidiodes (P.) brasiliensis [Citation82]. These studies elucidated the complex nature of antibody-mediated protection during fungal infections. Variations in efficiency depended on isotype and titer of the fungus-specific Ab, presence of protective and non-protective Abs, host MHC background or other host factors [Citation83,Citation84].
Fungal cell wall polysaccharides, proteins and non-cell wall surface molecules, such as heat-shock proteins (HSPs), provide attractive targets for antifungal mAb-based immunotherapies. The mAb 2G8 directed against laminarin, which is composed of β-glucans, showed effective protection against C. albicans by directly inhibiting the fungal growth in vitro and was also active against A. fumigatus and Cryptococcus neoformans [Citation85,Citation86]. Another mAb, scFv anti-HSP90 (Efungumab, Mycograb®), reduced Candida-associated mortality in a clinical trial and improved fungal clearance, but due to production difficulties and other issues this successfully starting mAb therapy was discontinued [Citation87]. Therefore, putting efforts in designing efficient and safe mAb immunotherapies for treatment of IFIs is promising and problems arising in vaccine development, e.g. immune status of the host, are bypassed, since mAbs act directly on the fungus or indirectly by mediating specific destruction via other host immune mechanisms. Of course, adverse effects of mAb immunotherapy have to be tightly investigated. Other mechanisms of antibody-mediated protection (AMP) against fungal infection are often distinguished by their mode of action and therefore they are arranged into an indirect and a direct group. The indirect group contains the most studied and most described mechanisms of antibody-mediated protection. Among these are phagocytosis, complement activation, antibody-directed cellular cytotoxicity (ADCC), phagosome activation and modulation of the inflammatory response [Citation82,Citation88]. Compared to this, the direct group uses AMP such as inhibition of biofilm formation, inhibition of polysaccharide release, inhibition of replication and cytotoxicity, interference with dimorphic changes, alterations in gene expression, iron starvation as well as mimicking killer toxin using anti-idiotypic antibodies [Citation82,Citation88]. All these mechanisms in antibody-mediated protection were mostly studied in infections with Cryptococcus neoformans and Candida albicans and very promising results were observed. For example, it has been shown that administration of mAbs to Candida albicans mannoprotein, aspartyl proteinase and 1,2 b mannotriose inhibited adherence and germination of the fungus and direct activity due to iron starvation. Studies with mABs targeting Cryptococcus neoformans capsule structures also reported on direct and indirect mechansims on fungal pathogenesis. Although, in these studies antibodies directed against the capsule were able to interfere with the polysaccharide release and biofilm formation which directly inhibited fungal infection also indirect mechanisms of antibody-mediated immunity were associated with the demonstrated protection. Especially modification on inflammatory responses and enhanced interaction and activation of immune cells (i.e. macrophages) were reported to have a beneficial outcome for the host because of improved fungal clearance and reduced damage to the tissue. Other approaches for antifungal therapy include radioimmunotherapy, which is mainly used in cancer therapy, and the use of anti-idiotypic antibodies. For radioimmunotherapy antibodies are linked to radioactive isotopes and these conjugated antibodies then deliver cytotoxic radiation to the target cell. Anti-idiotypic antibodies mimic the structure of the antigen and an anti-idiotypic antibody can be effective against various fungal species. These techniques have potential and represent an opportunity for the development of new antifungal treatments.
2.4.2. Increase in humoral immunity via vaccination
Fungal vaccine trials are rare and the goal of a fungal vaccine is to induce efficient adaptive immune responses leading to immunological memory to avoid infections [Citation89]. Recently, vaccine candidates were tested against various opportunistic fungi, that is, C. albicans, Aspergillus spp., Cryptococcus spp [Citation90].
Fungal proteins, e.g. Als3p, cell wall polysaccharides, and/or live attenuated Candida strains as vaccines and the use of different adjuvants or delivery systems to enhance the antifungal efficacy have been studied [Citation69,Citation91]. Recombinant Aspergillus proteins as Asp f3, Gel1, Asp f9, Asp f16, and Pep1 demonstrated protection in murine aspergillosis models [Citation92,Citation93]. Additionally, vaccination of 40 healthy adults using the Als3p protein coupled to aluminium hydroxide as an adjuvant resulted in rapid production of anti-Als3p antibodies after the first dose, while enhancing the anti-IgA1 as well as a robust Th1 and Th17 cell response after the second dose [Citation94]. Though not tested in patients yet, these results provide promising new strategies against opportunistic infections with Candida. A panfungal vaccine using β-glucans of Saccharomyces cerevisiae was illustrated to induce a protective immune response against various pathogenic fungi, among these A. fumigatus, without the addition of an adjuvant [Citation95], whereas no protection was detectable when vaccinated with galactomannan [Citation92]. This study was performed in immunocompetent mice and not under immune-suppressive regimen, therefore not giving a clue whether the protective immunity is also generated in immunodeficient conditions. Another study shows the protection of mice from five different genera of fungi by using heat-inactivated Saccharomyces cerevisiae yeast, assuming that memory innate immune defenses are stimulated within these mice. Wuthrich et al. identified a T cell epitope within calnexin, which is a protein highly conserved in Ascomycota, and vaccination of mice with calnexin increased the expansion of antigen-specific CD4+ T cells [Citation96]. Nonetheless, the group did not examine calnexin-dependent protection against Aspergillus. Other approaches focus on whole-organism vaccines consisting of live or killed A. fumigatus or fractions of it. Cenci et al. could show, that the protection of mice was only prolonged, when live Aspergillus fumigatus or filtrates thereof were used, in comparison to mice receiving heat-killed fungi. Higher CD4+ T cell numbers, IFN-γ and IL-2 were shown to be responsible for the protection, whereas heat-killed conidia up-regulated CD4+ T cells producing IL-4 and IL-13 [Citation96]. However, vaccination strategies using live conidia are of concern due to the risk of developing autoimmune diseases [Citation96]. Nevertheless, developing a panfungal vaccine protecting against an array of pathogenic fungi would be a very promising novel immunotherapeutic strategy. The fact that IFIs affect immune-suppressed hosts restricts the design of fungal vaccines without risking worsening the underlying disease state [Citation97]. In addition, autoimmunity is an issue upon generating a vaccine against a commensal fungus, that is, Candida albicans as well as high costs creating antifungal vaccines.
3. Conclusion
Since IFIs still have a high morbidity and mortality the development of new therapeutic strategies is of major concern. Immunotherapy with or without antifungals may be a promising candidate.
Among the first cells that encounter fungal pathogens are the cells of the innate immune system. Neutrophils were demonstrated to play a major role in combating fungal infections since in neutropenic patients a predisposition for opportunistic infections has been reported. Activating and stimulating factors such as CSFs, IFN-γ or thymosin-α1 are used to restore neutrophil functions and to boost survival, proliferation and cell differentiation. Alternatively to stimulation of neutrophils via cytokines or chemokines granulocyte transfusion can be used to improve the numbers of circulating neutrophils in patients with neutropenia. Macrophage-, monocyte- and dendritic cell-functions are also impaired in immunocompromised patients thereby favoring the development of IFIs. To activate and recover myeloid cell responses, M-CSF was demonstrated to enhance superoxide production, phagocytosis, chemotaxis, and cytokine production. Macrophages, monocytes, and DCs express PRRS – selective triggering of these PRRs results in establishment of a proinflammatory milieu thus improving the fungicidal activity of these immune cells.
Adaptive immune responses are essential in elimination of pathogens and in development of protective immunity. Especially CD4+ T cells polarized into the IFN-γ secreting TH1 cell subset were reported to establish a protective immune response and thereby to limit fungal burden in patients with candidemia and IA. Next to stimulating T cell responses also antibody-mediated protection against fungal infections has been investigated. Attractive targets for antifungal mAb-based immunotherapies are cell wall components (e.g. polysaccharides) and non-cell wall surface molecules (e.g. HSPs). The few data available proved that mAb immunotherapies reduced Candida-associated mortality and improved fungal clearance. Since mAb immunotherapy is very promising and acts directly against fungal components more research should be conducted in the identification of alternative fungal motifs as well as in the development of new adjuvants to create an efficient and safe vaccine.
4. Expert opinion
Epidemiological data show that IFIs are increasing worldwide as cause of a growing number of immunosuppressive infections, immunosuppressive treatment and invasive medical interventions. Invasive infections are of great concern because they are associated with unacceptably high mortality rates. A healthy immune system is essential to prevent invasive diseases; risk factors are diverse and include fungal multi-colonization, prior or concomitant exposure to antibiotics, neutropenia, ICU stay, surgical procedures, and use of steroids. Outcome largely depends on the early initiation of adequate therapy. It was shown that mortality was lowest in patients who received antifungal therapy on day 0 (15% mortality) – related to the first positive blood sample for yeasts, followed by patients who were treated on day 1 (24%), day 2 (37%), or day 3 (41%) (p = 0.0009 for trend) [Citation98]. However, mortality and morbidity of IFIs improved over the years due to a raised awareness of fungal diseases, the development of better and faster diagnostics, the availability of appropriate antifungal therapeutics, and the growing knowledge of antifungal immunity for novel immunotherapeutic strategies [Citation99,Citation100]. The introduction of the echinocandins and third-generation triazoles improved the therapeutic options, yet these drugs differ from their spectrum of activity, route of administration, and bioavailability in target tissues. Further complications include toxicity, drug interactions and the emergence of drug resistance. These factors and the high costs of antifungals steam down the success, and for infections that origin from failures in the immune function, cure may be improved by modulating immune deficits; thus, immunotherapy with or without antifungals may be a promising treatment strategy. Recently published studies display that approaches, combining immunotherapy with antifungals, target cells of the innate as well as of the adaptive immune system by cytokine or chemokine administration. Stimulation of immune cells with cytokines and chemokines showed an increased activation, proliferation and survival of immune cells which in turn resulted in a proinflammatory response able to clear fungal infections. Others induced protection from mycosis via granulocyte transfusion, adoptive T cell transfer, antibody administration or vaccination. Preliminary promising results were given with antibody administration (e.g. anti-HSP90) and vaccination (e.g. Als3p), but more research in designing efficient and safe mAb or in designing vaccines and adjuvants is highly needed. Vaccine target populations normally are immunocompromised hence immune responses may be diminished in these patients, and the use of live vaccines is generally contraindicated. Few fungal vaccine candidates have already gone through clinical phase I trials and show good progress of an efficient immunization [Citation3]. However, most data are available on animals and healthy humans, or are not yet available for the public. Vaccines derived from live attenuated strains may face safety issues for use in humans, and the fact that fungal pathogens affect mostly immunocompromised individuals limit the generation of fungal vaccines in these settings. A vaccine against commensal organisms (e.g. Candida spp) could be a challenge as autoimmunity against the commensal fungal organism may become an issue. Overall, the development of effective vaccines is lagging behind when compared to bacterial and/or viral infections. New adjuvants support to promote a stronger T cell response in immunocompromised patients. Hence, more clinical trials have to be conducted to explore the potential of these immunotherapeutic strategies in vivo since so far only a few fungal vaccine candidates have already gone through clinical trials [Citation3]. However, all these new strategies are of great interest and a combined therapy consisting of an antifungal drug with an immunotherapeutic agent could act as novel treatment option in fighting fungal infections in high-risk patients. The development of pan-fungal vaccines represents a very ambitious goal and to achieve this vigorous basic science researches as well as clinical studies have to be performed.
Article highlights
The high numbers of patients undergoing hematopoetic stem cell transplantation, organ transplantation or suffering from various immunodeficiency lead to an increase of invasive fungal infections worldwide.
Treatment with antifungal drugs such as polyenes, echinocandins and azoles represent the main standard of care.
The number of available antifungal drugs is limited, and drugs differ from their spectrum of activity, route of administration, and bioavailability in target tissues. Further complications include toxicity, drug interactions and the emergence of drug resistance.
Immunotherapy against fungal infections aims to activate cells of the innate as well as of the adaptive immune system by cytokine or chemokine administration.
Other techniques support protection via granulocyte transfusion, adoptive T cell transfer, antibody administration or vaccination.
Antibody administration and vaccination have shown very promising results, but more research in designing efficient and safe mAb for immunotherapies or in designing adjuvants is needed.
Only a few clinical trials have been conducted so far; hence there is a need for additional studies to explore the potential of these immunotherapeutic strategies in the clinical setting.
This box summarizes key points contained in the article.
Declaration of interest
The authors have relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63:433–442.
- Lass-Florl C, Roilides E, Loffler J, et al. Minireview: host defence in invasive aspergillosis. Mycoses. 2013;56:403–413.
- Medici NP, Del Poeta M. New insights on the development of fungal vaccines: from immunity to recent challenges. Mem Inst Oswaldo Cruz. 2015;110:966–973.
- Romani L. Immunity to fungal infections. Nat Reviews Immunol. 2004;4:1–23.
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22.
- Ogbomo H, Mody CH. Granule-dependent natural killer cell cytotoxicity to fungal pathogens. Front Immunol. 2016;7:692.
- Janeway CA Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216.
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
- Romani L. Immunity to fungal infections. Nat Reviews Immunol. 2011;11:275–288.
- Ruping MJ, Vehreschild JJ, Cornely OA. Patients at high risk of invasive fungal infections: when and how to treat. Drugs. 2008;68:1941–1962.
- Puccetti P, Romani L, Bistoni F. A TH1-TH2-like switch in candidiasis: new perspectives for therapy. Trends Microbiol. 1995;3:237–240.
- Casadevall A, Pirofski LA. Adjunctive immune therapy for fungal infections. Clin Infect Dis. 2001;33:1048–1056.
- Kapp A, Zeck-Kapp G. Activation of the oxidative metabolism in human polymorphonuclear neutrophilic granulocytes: the role of immuno-modulating cytokines. J Invest Dermatol. 1990;95:94S–9S.
- Al-Shami A, Mahanna W, Naccache PH. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Selective activation of Jak2, Stat3, and Stat5b. J Biol Chem. 1998;273:1058–1063.
- Bensinger WI, Price TH, Dale DC, et al. The effects of daily recombinant human granulocyte colony-stimulating factor administration on normal granulocyte donors undergoing leukapheresis. Blood. 1993;81:1883–1888.
- Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–3167.
- Lyman GH, Dale DC, Wolff DA, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J Clin Oncol. 2010;28:2914–2924.
- Diamond RD, Clark RA. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect Immun. 1982;38:487–495.
- Ellett F, Jorgensen J, Frydman GH, et al. Neutrophil interactions stimulate evasive hyphal branching by Aspergillus fumigatus. Plos Pathog. 2017;13:e1006154.
- Gaviria JM, Van Burik JA, Dale DC, et al. Comparison of interferon-gamma, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor for priming leukocyte-mediated hyphal damage of opportunistic fungal pathogens. J Infect Dis. 1999;179:1038–1041.
- Gil-Lamaignere C, Winn RM, Simitsopoulou M, et al. Inteferon gamma and granulocyte-macrophage colony-stimulating factor augment the antifungal activity of human polymorphonuclear leukocytes against Scedosporium spp: comparison with Aspergillus spp. Med Mycol. 2005;43:253–260.
- Safdar A, Rodriguez G, Ohmagari N, et al. The safety of interferon-gamma-1b therapy for invasive fungal infections after hematopoietic stem cell transplantation. Cancer. 2005;103:731–739.
- Jarvis JN, Meintjes G, Rebe K, et al. Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. Aids. 2012;26:1105–1113.
- Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50:291–322.
- Roilides E, Lamaignere CG, Farmaki E. Cytokines in immunodeficient patients with invasive fungal infections: an emerging therapy. Int J Infect Dis. 2002;6:154–163.
- Ferrante A. Tumor necrosis factor alpha potentiates neutrophil antimicrobial activity: increased fungicidal activity against Torulopsis glabrata and Candida albicans and associated increases in oxygen radical production and lysosomal enzyme release. Infect Immun. 1989;57:2115–2122.
- Djeu JY, Blanchard DK, Halkias D, et al. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunology. 1986;137:2980–2984.
- Nagai H, Guo J, Choi H, et al. Interferon-gamma and tumor necrosis factor-alpha protect mice from invasive aspergillosis. J Infect Dis. 1995;172:1554–1560.
- Mehrad B, Strieter RM, Standiford TJ. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J Immunology. 1999;162:1633–1640.
- Winn RM, Gil-Lamaignere C, Roilides E, et al. Selective effects of interleukin (IL)-15 on antifungal activity and IL-8 release by polymorphonuclear leukocytes in response to hyphae of Aspergillus species. J Infect Dis. 2003;188:585–590.
- Hubel K, Carter RA, Liles WC, et al. Granulocyte transfusion therapy for infections in candidates and recipients of HPC transplantation: a comparative analysis of feasibility and outcome for community donors versus related donors. Transfusion. 2002;42:1414–1421.
- Price TH, Bowden RA, Boeckh M, et al. Phase I/II trial of neutrophil transfusions from donors stimulated with G-CSF and dexamethasone for treatment of patients with infections in hematopoietic stem cell transplantation. Blood. 2000;95:3302–3309.
- Price TH, Boeckh M, Harrison RW, et al. Efficacy of transfusion with granulocytes from G-CSF/dexamethasone-treated donors in neutropenic patients with infection. Blood. 2015;126:2153–2161.
- Seidel MG, Peters C, Wacker A, et al. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplant. 2008;42:679–684.
- BitMansour A, Burns SM, Traver D, et al. Myeloid progenitors protect against invasive aspergillosis and Pseudomonas aeruginosa infection following hematopoietic stem cell transplantation. Blood. 2002;100:4660–4667.
- BitMansour A, Cao TM, Chao S, et al. Single infusion of myeloid progenitors reduces death from Aspergillus fumigatus following chemotherapy-induced neutropenia. Blood. 2005;105:3535–3537.
- Goldstein AL, Goldstein AL. From lab to bedside: emerging clinical applications of thymosin alpha 1. Expert Opin Biol Ther. 2009;9:593–608.
- Di Francesco P, Gaziano R, Casalinuovo IA, et al. Combined effect of fluconazole and thymosin alpha 1 on systemic candidiasis in mice immunosuppressed by morphine treatments. Clin Exp Immunol. 1994;97:347–352.
- Braem SG, Rooijakkers SH, Van Kessel KP, et al. Effective neutrophil phagocytosis of Aspergillus fumigatus Is mediated by classical pathway complement activation. J Innate Immun. 2015;7:364–374.
- Mambula SS, Sau K, Henneke P, et al. Toll-like receptor (TLR) signaling in response to Aspergillus fumigatus. J Biol Chem. 2002;277:39320–39326.
- Wozniok I, Hornbach A, Schmitt C, et al. Induction of ERK-kinase signalling triggers morphotype-specific killing of Candida albicans filaments by human neutrophils. Cell Microbiol. 2008;10:807–820.
- Bellocchio S, Montagnoli C, Bozza S, et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunology. 2004;172:3059–3069.
- Bellocchio S, Gaziano R, Bozza S, et al. Liposomal amphotericin B activates antifungal resistance with reduced toxicity by diverting Toll-like receptor signalling from TLR-2 to TLR-4. J Antimicrob Chemother. 2005;55:214–222.
- Carvalho A, Cunha C, Bistoni F, et al. Immunotherapy of aspergillosis. Clin Microbiol Infect. 2012;18:120–125.
- Garlanda C, Hirsch E, Bozza S, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186.
- Mantovani A, Cassatella MA, Costantini C, et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Reviews Immunol. 2011;11:519–531.
- Cunha C, Aversa F, Lacerda JF, et al. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N Engl J Med. 2014;370:421–432.
- D’Angelo C, De Luca A, Zelante T, et al. Exogenous pentraxin 3 restores antifungal resistance and restrains inflammation in murine chronic granulomatous disease. J Immunology. 2009;183:4609–4618.
- Nemunaitis J. Macrophage function activating cytokines: potential clinical application. Crit Rev Oncol Hematol. 1993;14:153–171.
- Cenci E, Bartocci A, Puccetti P, et al. Macrophage colony-stimulating factor in murine candidiasis: serum and tissue levels during infection and protective effect of exogenous administration. Infect Immun. 1991;59:868–872.
- Nemunaitis J. Use of macrophage colony-stimulating factor in the treatment of fungal infections. Clin Infect Dis. 1998;26:1279–1281.
- Aukerman SL, Middleton S, Sampson-Johannes A, et al. Biological preclinical activity of macrophage colony-stimulating factor, M-CSF. In: Symann M, Morstyn PQ,G, editor. Growth Factors and Interleukins: biology and Clinical Applications 1992. Macclesfield: Caldwell Communications; 1992. p. 79–93.
- Nemunaitis J, Shannon-Dorcy K, Appelbaum FR, et al. Long-term follow-up of patients with invasive fungal disease who received adjunctive therapy with recombinant human macrophage colony-stimulating factor. Blood. 1993;82:1422–1427.
- Safdar A, Rodriguez G, Zuniga J, et al. Granulocyte macrophage colony-stimulating factor in 66 patients with myeloid or lymphoid neoplasms and recipients of hematopoietic stem cell transplantation with invasive fungal disease. Acta Haematol. 2013;129:26–34.
- Roilides E, Blake C, Holmes A, et al. Granulocyte-macrophage colony-stimulating factor and interferon-gamma prevent dexamethasone-induced immunosuppression of antifungal monocyte activity against Aspergillus fumigatus hyphae. J Med Vet Mycol. 1996;34:63–69.
- Netea MG, Kullberg BJ, Jacobs LE, et al. Van der Meer JW. Chlamydia pneumoniae stimulates IFN-gamma synthesis through MyD88-dependent, TLR2- and TLR4-independent induction of IL-18 release. J Immunology. 2004;173:1477–1482.
- Romani L, Puccetti P. Controlling pathogenic inflammation to fungi. Expert Rev Anti Infect Ther. 2007;5:1007–1017.
- Romani L, Puccetti P. Protective tolerance to fungi: the role of IL-10 and tryptophan catabolism. Trends Microbiol. 2006;14:183–189.
- Steger MB,M, Latgé JP, Wagener J, et al. An efficient first line of immune defense is initiated by Aspergillus fumigatus devoid of cell wall β-1,3-glucan. Under review.
- Posch W, Steger M, Knackmuss U, et al. Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells. Plos Pathog. 2015;11:e1005005.
- Wilflingseder D, Schroll A, Hackl H, et al. Immediate T-Helper17 Polarization Upon Triggering CD11b/c on HIV-Exposed Dendritic Cells. J Infect Dis. 2015;212:44–56.
- Shao C, Qu J, He L, et al. Dendritic cells transduced with an adenovirus vector encoding interleukin-12 are a potent vaccine for invasive pulmonary aspergillosis. Genes Immun. 2005;6:103–114.
- Bozza S, Perruccio K, Montagnoli C, et al. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood. 2003;102:3807–3814.
- Roy RM, Klein BS. Dendritic cells in antifungal immunity and vaccine design. Cell Host Microbe. 2012;11:436–446.
- Abdel-Motal UM, Dahmen J, Liu T, et al. External glycopeptide binding to MHC class-I in relation to expression of TAP transporters, beta 2-microglobulin and to pH. Immunol Lett. 1996;54:31–35.
- Li SS, Kyei SK, Timm-McCann M, et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe. 2013;14:387–397.
- Stuehler C, Khanna N, Bozza S, et al. Cross-protective TH1 immunity against Aspergillus fumigatus and Candida albicans. Blood. 2011;117:5881–5891.
- Perruccio K, Tosti A, Burchielli E, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106:4397–4406.
- Schmidt S, Tramsen L, Perkhofer S, et al. Characterization of the cellular immune responses to Rhizopus oryzae with potential impact on immunotherapeutic strategies in hematopoietic stem cell transplantation. J Infect Dis. 2012;206:135–139.
- Hebart H, Bollinger C, Fisch P, et al. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood. 2002;100:4521–4528.
- Di Ianni M, Falzetti F, Carotti A, et al. Immunoselection and clinical use of T regulatory cells in HLA-haploidentical stem cell transplantation. Best Pract Res Clin Haematol. 2011;24:459–466.
- Nanjappa SG, Heninger E, Wuthrich M, et al. Protective antifungal memory CD8(+) T cells are maintained in the absence of CD4(+) T cell help and cognate antigen in mice. J Clin Invest. 2012;122:987–999.
- Ma LL, Spurrell JC, Wang JF, et al. CD8 T cell-mediated killing of Cryptococcus neoformans requires granulysin and is dependent on CD4 T cells and IL-15. J Immunology. 2002;169:5787–5795.
- Kunzmann V, Kimmel B, Herrmann T, et al. Inhibition of phosphoantigen-mediated gammadelta T-cell proliferation by CD4+ CD25+ FoxP3+ regulatory T cells. Immunology. 2009;126:256–267.
- Delsing CE, Gresnigt MS, Leentjens J, et al. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infect Dis. 2014;14:166.
- Kullberg BJ, Van De Veerdonk F, Netea MG. Immunotherapy: a potential adjunctive treatment for fungal infection. Curr Opin Infect Dis. 2014;27:511–516.
- Segal BH, Kwon-Chung J, Walsh TJ, et al. Immunotherapy for fungal infections. Clin Infect Dis. 2006;42:507–515.
- Clemons KV, Martinez M, Chen V, et al. Protection against experimental aspergillosis by heat-killed yeast is not antibody dependent. Med Mycol. 2014;52:422–426.
- Diaz-Arevalo D, Bagramyan K, Hong TB, et al. CD4+ T cells mediate the protective effect of the recombinant Asp f3-based anti-aspergillosis vaccine. Infect Immun. 2011;79:2257–2266.
- Marr KA, Datta K, Pirofski LA, et al. Cryptococcus gattii infection in healthy hosts: a sentinel for subclinical immunodeficiency? Clin Infect Dis. 2012;54:153–154.
- Malphettes M, Gerard L, Galicier L, et al. Good syndrome: an adult-onset immunodeficiency remarkable for its high incidence of invasive infections and autoimmune complications. Clin Infect Dis. 2015;61:e13–9.
- Casadevall A, Pirofski LA. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe. 2012;11:447–456.
- Rivera J, Casadevall A. Mouse genetic background is a major determinant of isotype-related differences for antibody-mediated protective efficacy against Cryptococcus neoformans. J Immunology. 2005;174:8017–8026.
- Maitta RW, Datta K, Pirofski LA. Efficacy of immune sera from human immunoglobulin transgenic mice immunized with a peptide mimotope of Cryptococcus neoformans glucuronoxylomannan. Vaccine. 2004;22:4062–4068.
- Torosantucci A, Chiani P, Bromuro C, et al. Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence. Plos One. 2009;4:e5392.
- Rachini A, Pietrella D, Lupo P, et al. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect Immun. 2007;75:5085–5094.
- Pachl J, Svoboda P, Jacobs F, et al. Mycograb Invasive Candidiasis Study G. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis. 2006;42:1404–1413.
- Casadevall A, Pirofski LA. A new synthesis for antibody-mediated immunity. Nat Immunol. 2011;13:21–28.
- Iannitti RG, Carvalho A, Romani L. From memory to antifungal vaccine design. Trends Immunol. 2012;33:467–474.
- Nanjappa SG, Klein BS. Vaccine immunity against fungal infections. Curr Opin Immunol. 2014;28:27–33.
- Edwards JE Jr. Fungal cell wall vaccines: an update. J Med Microbiol. 2012;61:895–903.
- Bozza S, Clavaud C, Giovannini G, et al. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J Immunology. 2009;183:2407–2414.
- Ito JI, Lyons JM, Hong TB, et al. Vaccinations with recombinant variants of Aspergillus fumigatus allergen Asp f 3 protect mice against invasive aspergillosis. Infect Immun. 2006;74:5075–5084.
- Schmidt CS, White CJ, Ibrahim AS, et al. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine. 2012;30:7594–7600.
- Liu M, Clemons KV, Bigos M, et al. Immune responses induced by heat killed Saccharomyces cerevisiae: a vaccine against fungal infection. Vaccine. 2011;29:1745–1753.
- Levitz SM. Aspergillus vaccines: hardly worth studying or worthy of hard study? Med Mycol. 2017;55:103–108.
- Cassone A. Fungal vaccines: real progress from real challenges. Lancet Infect Dis. 2008;8:114–124.
- Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–3645.
- Aigner M, Lass-Florl C. Treatment of drug-resistant Aspergillus infection. Expert Opin Pharmacother. 2015;16:2267–2270.
- Springer J, Lackner M, Nachbaur D, et al. Prospective multicentre PCR-based Aspergillus DNA screening in high-risk patients with and without primary antifungal mould prophylaxis. Clin Microbiol Infect. 2016;22:80–86.