ABSTRACT
Objectives: To evaluate efficacy and safety of the anti-IL-17 drug secukinumab in a real-life large cohort of patients with moderate-to-severe plaque psoriasis in Central Italy.
Methods: Multicenter, retrospective study with an observation period of up to 52 weeks. Efficacy was assessed by Psoriasis Area and Severity Index (PASI) score; clinical and laboratory examinations were performed at baseline and at weeks 4, 12, 24, 36, and 52.
Results: A 90% and a 100% PASI score reduction (PASI90 and PASI100) were reported in 67.5% and 55% of patients at week 12, respectively. A rapid improvement of skin lesions was observed particularly in young patients and in patients naïve to biologics: at week 4, the achievement of PASI90 and PASI100 was higher in younger patients (odds ratio [OR] 0.95, and 0.95; p = 0.003, and 0.005, respectively); PASI90 was achieved by 42.0% of patients naïve to biologics and by 17.0% of patients with prior exposure to biologics (PBT) (OR 0.24; p = 0.001); and PASI100 was reached by 25.5% of naïve patients and 9.8% of PBT (OR 0.28; p = 0.015).The drug was well tolerated.
Conclusion: Secukinumab was effective in this real-life analysis, with rapid clinical improvement and long-term maintenance of results.
1. Introduction
Over the last few years, several studies have demonstrated the key role of interleukin-17 (IL-17) in the pathogenesis of psoriasis, providing a new therapeutic target for treating the disease. IL-17, a pro-inflammatory cytokine produced mainly by T helper 17 (Th-17) lymphocytes, is involved in biological responses that lead to inflammation, neutrophilic chemotaxis, and angiogenesis. IL-17 increases the expression, by keratinocytes, of chemokines involved in recruiting myeloid dendritic cells, Th17 cells, and neutrophils in the site of the lesion [Citation1–Citation4]. Based on these findings, several new biologics agents for the treatment of plaque psoriasis (PsO), which target IL-17, have been developed [Citation5–Citation8]. These new drugs include secukinumab, a fully human immunoglobulin G1 kappa (IgG1k) antibody, and ixekizumab, a humanized IgG4 monoclonal antibody, both of which selectively bind the IL-17A cytokine, as well as brodalumab, a human monoclonal antibody that blocks the IL-17 receptor, inhibiting the activity of all types of IL-17 [Citation9]. Of these, secukinumab was the first anti-IL17 to be approved by the US Food and Drug Administration and European Medicines Agency for the treatment of moderate-to-severe psoriasis and psoriatic arthritis (PsA) in adult patients [Citation10]. The efficacy and tolerability of secukinumab have been investigated in several phase III trials, which compared two different doses of secukinumab (150 or 300 mg for 5 weeks and then every 4 weeks) with placebo and etanercept, and demonstrated that secukinumab was superior in terms of efficacy to etanercept, with similar safety. At week 12, 81.6% of patients treated with secukinumab 300 mg in the ERASURE study and 77.1% in the FIXTURE study had a 75% reduction in Psoriasis Area and Severity Index (PASI) scores (PASI75) compared with 44.0% of patients receiving etanercept [Citation11]. An additional ‘head-to-head’ study showed that secukinumab was superior to ustekinumab in clearing skin lesions of patients with moderate-to-severe psoriasis and improving quality of life (QoL), with comparable safety: at week 52, a 90% reduction in PASI (PASI90) was observed in 74.9% of patients in the secukinumab group compared with 60.6% of patients in the ustekinumab group [Citation12,Citation13]. Subsequent studies confirmed the effectiveness of secukinumab for the treatment of PsA [Citation14] and palmoplantar psoriasis [Citation15], with no differences in terms of efficacy and safety observed for the two different methods of administration (prefilled syringe or injector pen) [Citation16,Citation17]. When comparing ‘fixed dose’ or ‘as needed’ retreatment regimens, patients receiving a fixed-dose regimen of secukinumab every 4 weeks had better PASI75 outcomes than patients receiving as needed treatment [Citation18]. In particular, from week 12 to week 52, PASI75 response was maintained in a higher proportion of patients treated on fixed interval dosing (78.2%, 300 mg; 62.1%, 150 mg) compared with patients on retreatment as needed (41.0%; 40.0%).
Although randomized controlled trials (RCTs) are necessary to assess drug efficacy and safety, clinical daily practice may often be different. For this reason, it is crucial to integrate data obtained from clinical trials with those of real life, which include different patients’ populations, patients with different disease severity and multiple concomitant diseases. To date, only three real-life studies have investigated the efficacy and safety of secukinumab, over a maximum follow-up period of 12 weeks [Citation19–Citation21]. The aim of this multicenter, retrospective study was to analyze the efficacy and safety of secukinumab in a patient population from Central Italy with moderate-to-severe psoriasis, over a 52-week treatment period.
2. Patients and methods
2.1. Patients
Data from a cohort of patients with chronic PsO with or without PsA who initiated treatment with secukinumab between September 2015 and May 2017 were analyzed. The study population consisted of patients attending the outpatient clinics of the three participating centers (Dermatology, University of Rome ‘Tor Vergata’; Dermatology, Catholic University of the Sacred Heart, Rome; Dermatology, University of L’Aquila). The study was conducted following the principles of the Declaration of Helsinki, and all patients provided their informed consent for participation.
2.2. Treatment and outcomes
For each patient, demographic and clinical data (age, sex, height, weight, body mass index [BMI], waist circumference, age of onset of psoriasis, duration of disease, comorbidities, involvement of difficult-to-treat areas as scalp, genital area, nails and palmoplantar psoriasis, PASI, and previous systemic therapies) were collected. In patients with PsA, the severity of arthritis was assessed using Disease Activity Score (DAs 44), and the patient-reported outcome Visual Analog Scale of pain (VASp) was also assessed.
Secukinumab was administered in a standard dosing regimen (300 mg subcutaneous once weekly for 5 weeks then once monthly thereafter) to patients with moderate-to-severe psoriasis who failed to respond or had contraindications to or did not tolerate at least one conventional treatment, including systemic therapy (methotrexate, cyclosporine, acitretin) or phototherapy (ultraviolet B, psoralen plus ultraviolet A), according to Italian regulations for prescription of biologic therapy. Patients with a baseline PASI <10.0, who presented involvement of sensitive areas such as the face, scalp, hands, or genital areas, thus affecting their QoL, were also considered eligible for secukinumab treatment.
Clinical evaluations (PASI, DAS 44, VASp) and laboratory examinations (complete blood count, alanine aminotransferase [ALT], aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), creatinine, high density lipoprotein and low-density lipoprotein cholesterol, triglycerides, urea nitrogen, glucose, uric acid, C-reactive protein, lactate dehydrogenase, and creatine phosphokinase) were performed at baseline and at weeks 4, 12, 24, 36, and 52. Quantiferon-TB gold tests and serology for hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) were performed at baseline and at week 52.
2.3. Statistical analysis
Efficacy data were analyzed by an intent-to-treat last observation carried forward imputation (ITT-LOCF), where if a patient dropped out of the study the last available value was ‘carried forward’ until the end of the treatment. Data were presented as mean ± standard deviation, median, and interquartile ranges. The significance of differences in mean values obtained at the different time points of treatment was assessed by unpaired Student’s t-test (statistical significance was set at p ≤ 0.05) and, whenever the values were not normally distributed, by Wilcoxon test. Simple logistic regression and multivariate logistic regression analysis considering all the variables collected were also performed. In all cases, a p < 0.05 was considered statistically significant; all p-values were two-sided. Statistical analysis was performed using STATA 11.2 software (StataCorp LP Inc., College Station, TX, USA).
Safety and tolerability were assessed by incidence of adverse events (AEs), including mild and serious AEs, clinically significant changes in laboratory values and physical examinations.
3. Results
This multicenter study included 107 patients (75% male), with a mean age of 47.5 years and a mean disease duration of 20.3 years (). Approximately, 51.3% of patients had comorbidities. The most frequent comorbidities were obesity (23.4%), hypertension (15%), hyperlipidemia (13.1%), type 2 diabetes (10.3%), and hyperuricemia (6.5%). A small proportion (3.7%) of patients had concomitant psychiatric disorders (bipolar syndrome, anxiety, and depressive syndrome). For each patient reporting comorbidities, concomitant medications were recorded (Supplemental Table 1). Sixteen (15%) patients had concomitant PsA (). The mean age at PsA onset was 36.1 years. Polyarthritis was present in 11 of 16 patients (68.7%): sacroiliitis and/or spondylitis were present in 31.3% (5/16) and enthesitis as a prominent manifestation was found in 31.3% (5/16) of these patients.
Table 1. Patient demographics and disease characteristics at baseline.
3.1. Effectiveness
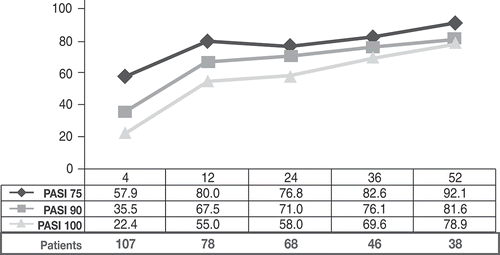
In the ITT-LOCF, 62 (57.9%) patients achieved a PASI75 at week 4, 38 (35.5%) achieved a PASI90, and 24 (22.4%) were clear of disease (defined as a 100% reduction in PASI [PASI100]). PASI scores continued to improve through to week 12, with 64 of 80 (80%) patients achieving PASI75, 54 (67.5%) patients achieving PASI90, and 44 (55%) patients achieving PASI100. At weeks 24, 53 of 69 (76.8%) patients reached PASI75, 49 (71.0%) patients reached PASI90, and 40 (58.0%) patients reached PASI100. At week 52, data were available for 38 of 107 (35.5%) patients, with 92.1% (35/38) of these patients achieving PASI75, 81.6% (31/38) achieving PASI90, and 78.9% (30/38) achieving PASI100 ().
Figure 1. Proportion of patients achieving a 75% reduction in Psoriasis Area Severity Index (PASI) scores (PASI75), a 90% reduction in PASI (PASI90) and a 100% reduction in PASI (PASI100) during the 52 weeks of secukinumab treatment.
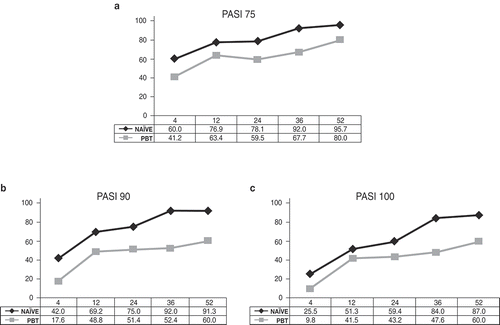
Fifty-five of 107 (51.4%) patients had prior exposure to biologics (PBT); secukinumab was a second-line, third-line, fourth-line, and fifth-line treatment for 20.0%, 33.3%, 27.7%, and 20.0% of patients, respectively. At week 4, PASI75 response was achieved by 41.2% of PBT patients, and by 60.0% of treatment-naïve patients, while at week 52, PASI75 was reached by 80.0% of PBT and 95.7% of treatment-naïve patients (), PASI90 by 60.0% and 91.3% (), and PASI100 by 60.0% and 87.0%, respectively ().
Figure 2. Proportion of patients naïve to biological therapies (naïve) and patients with prior exposure to biologics (PBT) achieving (a) 75% reduction in Psoriasis Area Severity Index (PASI) scores (PASI75), (b) 90% reduction in PASI (PASI90), and (c) 100% reduction in PASI (PASI100) during the 52 weeks of treatment with secukinumab.
In 15 of the 16 patients with PsA, both cutaneous and joint involvement showed improvement, and this clinical benefit was maintained until week 52 irrespective of the type of joint involvement (polyarthritis, sacroiliitis and/or spondylitis, and enthesitis). In addition, in patients with PsA, significant improvements in mean DAS 44 scores (2.1; p = 0.0272) and mean VASp scores (31.0; p = 0.0084) with secukinumab treatment were observed at week 12, with further improvements observed up to week 52. Although of interest, these observations should be considered preliminary, and the effect of secukinumab on PsA will be further investigated in a subsequent study.
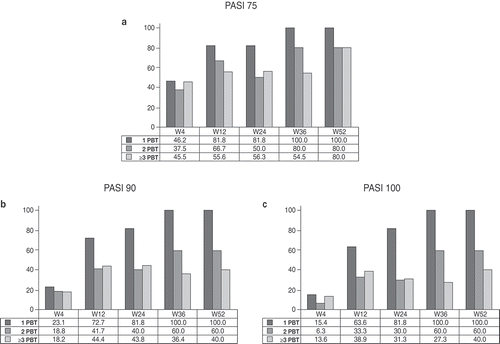
Univariate logistic regression analysis showed that, at week 4, PASI75, PASI90, and PASI100 were achieved more frequently by treatment-naïve patients (odds ratio [OR] 0.30, 0.24, and 0.28; p = 0.004, 0.001, and 0.015, respectively). Similarly, PASI75, PASI90, and PASI100 were achieved more frequently by treatment-naïve patients also at week 12 (OR 0.10, 0.19, and 0.39; p = 0.005, 0.002, and 0.043) and at week 24 (OR 0.11, 0.12, and 0.33; p = 0.006, 0.002, and 0.032). At week 36, a significant association between treatment-naïve patients and achievement of PASI90 and PASI100 (OR ratio 0.12 and 0.21; p = 0.012 and 0.025, respectively), but not PASI75 was observed. At week 52, a similar trend was observed; however, this was not statistically significant due to the small number of patients followed up to 52 weeks (). Furthermore, in the PBT population, a significant difference associated with the number of previous biologics was observed. The proportion of patients achieving PASI75, PASI90, and PASI100 was inversely proportional to the number of biological drugs received by patients before secukinumab, at each end point considered ().
Table 2. Univariate logistic regression analysis on PASI75, PASI90, and PASI100 response.
Figure 3. Proportion of patients achieving (a) 75% reduction in Psoriasis Area Severity Index (PASI) scores (PASI75), (b) 90% reduction in PASI (PASI90), and (c) 100% reduction in PASI (PASI100) during the 52 weeks of secukinumab treatment, according to the number of previous biological therapies (PBT).
Univariate logistic regression analysis also showed that younger patients responded faster to treatment: the achievement of PASI75, PASI90, and PASI100 was higher in younger patients at week 4 (OR 0.97, 0.95, and 0.95; p = 0.050, 0.003, and 0.005, respectively; ). In our analysis, we considered the independent variable age (x) as a continuous variable, without cutoffs for this variable. The results show that the achievement of PASI75, PASI90, and PASI100 is inversely proportional to the patient’s age. As the age of patients increases, the achievement of PASI75, PASI90, and PASI100 is reduced. This association between clinical efficacy and age, although statistically significant, is a weak association, as demonstrated by ORs.
Furthermore, comorbidities appeared to have a negative influence on complete skin clearance: there was a negative association between number of comorbidities and achievement of PASI100 at various time points (OR 0.42, 0.45, 0.34, and 0.34; p = 0.031, 0.011, 0.003, and 0.027 at weeks 4, 12, 24, and 36, respectively; ).
No significant association between age; sex; height; weight; BMI; waist circumference; PASI at baseline; age of onset of psoriasis; duration of disease; comorbidities; involvement of difficult-to-treat areas (like scalp, genital area, nails, and palmoplantar psoriasis); number of biological drugs used before secukinumab variables; and PASI75, PASI90, and PASI100 responses at weeks 4, 12, 24, 36, and 52 of secukinumab treatment in multivariate models was observed. In particular, our findings showed that obesity (BMI ≥ 30 kg/m2), the most frequent comorbidity affecting 25 of 107 patients (23.3%), did not affect PASI response to secukinumab despite the fact that the median PASI baseline score in obese patients was generally higher than in normal-weight patients. Additionally, in the small percentage (3.7%) of patients in our population suffering from psychiatric disorders, the treatment with secukinumab did not influence the course of this type of disease and, conversely, the psychiatric comorbidity did not affect the achievement of PASI75, PASI90, or PASI100.
3.2. Safety and tolerability
Two of the 107 patients (two men aged 65 and 63 years, respectively) had a previous HBV infection and were anti-HBc Ag positive, HBs Ag, and HBV DNA negative. In these patients, liver enzymes (ALT, AST, and GGT) were checked once monthly during treatment and did not show any alteration. Another 45-year-old male patient, with chronic HBV infection (HBs Ag positive, anti-HBc Ag positive, HBV DNA <13 UI/mL), was on treatment with entecavir when he started secukinumab. After 4 months, he achieved complete clinical remission of cutaneous lesions, but he showed a slight increase of HBV DNA copies (100 UI/mL). Therefore, secukinumab was discontinued and the patient switched to tenofovir since a mutation in the viral HBV genotype was detected. After 2 months of tenofovir treatment, viral load (HBV DNA <13 UI/mL) was not detectable, and secukinumab was administered again at the dosage of 300 mg for 4 weeks.
Only 9.3% (10/107) of the study population experienced AEs, the most common of which were mucocutaneous Candida infections (6.5%) (). Upper respiratory tract infections were reported in only one patient (0.9%), although they were the most common AEs reported in RCTs.
Table 3. Adverse events during secukinumab exposure period.
A total of 14 of 107 (13%) patients discontinued secukinumab during treatment. In 1 patient, secukinumab was discontinued for primary inefficacy (lack of initial clinical response, i.e. patients failed to reach PASI50 at week 12), in 11 of 107 patients for secondary inefficacy (development of an inadequate response over time after an initial clinical response, i.e. loss of PASI50 after week 12) and in 2 of 107 for worsening of PsA (the treatment was suspended in 1 patient at week 24 and in another 1 at week 52). Discontinuation of secukinumab due to AEs occurred in 5 of 107 (4.7%) patients (). Seven of 107 (6.5%) patients experienced mucocutaneous Candida infection, which was successfully treated with antimycotic therapy; only in 2 of 7 patients, secukinumab was discontinued for the inefficacy of antimycotic therapy. A 64-year-old male patient developed severe esophageal candidiasis associated with dysphagia and anorexia, after 4 weeks of secukinumab treatment. He also had diabetes, obesity, and bipolar disorder. Secukinumab was discontinued, and the patient was treated with fluconazole 200 mg/day for 3 weeks with regression of AEs.
4. Discussion
This multicenter, retrospective study analyzed a large cohort of patients from Central Italy with moderate-to-severe psoriasis, treated with secukinumab with a maximum follow-up period of 52 weeks. The results of this study confirm the efficacy and safety of secukinumab in psoriatic patients, providing a rapid improvement of skin lesions particularly in young patients and in patients naïve to biologics.
Three reports concerning the efficacy and safety of secukinumab in real life for a 12-week observation period have been published so far. Georgakopoulos and colleagues [Citation19] described a cohort of 47 patients treated in two Canadian centers: at week 12, 34 of 47 (72.3%) patients had achieved PASI75 response with secukinumab (300 mg) treatment. A low percentage of AEs were reported, and discontinuation was lower than that observed in RCTs. In another multicenter Danish study [Citation21], 36 patients were randomized to secukinumab 300 mg and 33 patients were randomized to secukinumab 150 mg (an off-label dose). The 300-mg schedule dosing was found to be more effective than 150 mg: at week 12, PASI75 response was observed in 66.7% of patients on secukinumab 300 mg versus 52.9% on 150 mg. Finally, an Italian case series by Magnano and colleagues [Citation20] has reported the efficacy of secukinumab in 16 psoriatic patients who had been previously treated with at least three biologic agents. At week 12, PASI90 responses were observed in 87.5% of patients without any serious AEs.
Our study describes real-life data on secukinumab treatment in a higher number of patients and with a longer follow-up compared with the three previous real-life reports [Citation19–Citation21]. The demographic data in our cohort are similar to those reported in previous RCTs [Citation11–Citation18], except for the average age of patients, which was 47.5 years in our study versus 44.9 and 44.5 years in the phase III RCTs. In addition, the baseline mean PASI score (17.9) in our cohort was lower than the PASI of patients treated in the RCTs (22.5 for ERASURE and 23.9 for FIXTURE [Citation11]) but higher than that in the Canadian study and the Danish study (14.8 and 7.1, respectively [Citation19,Citation21]).
PASI75 was achieved by 80% of our patients and PASI90 was obtained in 67.5% at week 12. Finally, PASI100 at week 12 was reached in 55% of our patients. Thirty-one of 38 (81.6%) patients who reached week 52 maintained PASI90 response.
A direct correlation was found between the achievement of PASI reduction and the patients’ age, as we observed that younger patients achieved more rapid cutaneous clearance (PASI100) and maintained it longer than did elderly patients. This finding is in line with the Canadian study and could be possibly linked to alterations of drug pharmacokinetics and pharmacodynamics in elderly subjects [Citation19].
A recent post hoc analysis of three phase III trials was performed to evaluate efficacy and safety of the label dose of secukinumab 300 mg in subjects with moderate-to-severe PsO stratified by age into an elderly and a younger group [Citation22]. The two groups of patients included in this analysis showed several distinct differences at baseline. The mean age of both patient groups differed significantly (69.9 years vs. 42.8 years, p < 0.0001); elderly subjects had a significantly higher frequency of cardiovascular and metabolic comorbidities and significantly longer disease duration than younger subjects. Despite these differences, in this pooled analysis, secukinumab 300 mg provided rapid and sustained PASI75, PASI90, PASI100 and Investigator’s Global Assessment responses in all subjects regardless of age. There were no differences in the speed of onset or duration of response between elderly and younger subjects treated with secukinumab.
In our study population, 55 of 107 (51.4%) patients were previously treated with other biologic therapies, and 52 of 107 (48.6%) were naïve to biologics. We observed that patients who had never been exposed to previous biologic drugs tended to achieve PASI75 faster than previously treated patients (60% vs. 41.2%, respectively, at week 4). Interestingly, the number of previous biologic drugs influenced the achievement of PASI75, PASI90, and PASI100 at each end point considered. The multidrug-treated patients who received more than two biologic drugs reported a loss of efficacy, especially after 24 weeks. Compared with our population, a higher percentage of naïve patients were included in the RCTs (65% in the ERASURE study and 63% in the FIXTURE study).
Our finding that in most patients with PsA an improvement of both cutaneous and joint involvement was observed, and was maintained until week 52 independently of the type of joint involvement, supports the efficacy described in previous studies focusing on PsA [Citation14,Citation20].
In our experience, secukinumab has been demonstrated to be safe also in patients with previous HBV infection and active HBV infection. Three of 107 patients (male, aged 45–65 years) had a previous HBV infection, and they did not show any HBV reactivation or elevation in liver enzymes during secukinumab treatment. In one of these patients, a slight increase of HBV DNA copies (100 UI/mL) was detected because of a mutation in the viral HBV genotype. Indeed, the recombination among HBV virus genotypes is well known and not infrequent [Citation23], and is not linked to treatment with biologic drugs. To our knowledge, only five patients with HBV infection have been treated so far with secukinumab and, similarly to our case, HBV reactivation or elevation in liver enzymes was not found [Citation24,Citation25]. In this regard, several studies have shown that Th17 cells play a critical role in the pathogenesis of viral hepatitis, with serum levels of IL-17 being able to predict the severity of liver damage and fibrosis [Citation26]. For this reason, inhibitors of IL-17 as secukinumab could have a protective role in the progression of liver injury and may be preferred in psoriatic patients with HBV or HCV infection. Further long-term studies are needed to confirm the safety of secukinumab in these patients.
In our population, secukinumab showed a safety profile similar to that reported in previous trials, with a low percentage (9.3%) of patients experiencing AEs, the most common of which were mucocutaneous Candida infections. The incidence of such infections in our cohort of patients (6.5%) was higher compared with RCTs (0.9–4.7%), even though most (6/7) were mild or moderate in severity and responded to standard oral or topical therapy without discontinuation of secukinumab. Only one case of esophageal Candida infection required secukinumab withdrawal and resolved with fluconazole treatment. Considering that IL-17 has an important role in innate and adaptive responses against infections at mucosal and cutaneous interfaces and it is essential for the protection of skin and mucous membrane against Candida albicans, a higher incidence of Candida infection during secukinumab treatment was expected [Citation27]. On the other hand, it has been demonstrated that psoriatic patients have an increased rate of colonization by Candida in the oral cavity as well as in other anatomic areas [Citation28–Citation30]. A recent systematic review of studies with anti-IL17 drugs revealed that the frequency of Candida infection is dose-related and is increased (from 0% to 5%) compared with the frequency observed in patients treated with placebo, etanercept, or ustekinumab [Citation31]. Our results confirm that patients receiving secukinumab should be advised to report any sign and symptom that might be related to mucocutaneous candidiasis in order to establish an early diagnosis and to start an appropriate treatment.
5. Conclusion
In conclusion, our real-life study showed a good efficacy and safety profile of secukinumab over a period of 52 weeks in a large cohort of patients with moderate-to-severe psoriasis. PASI90 and PASI100 were achieved very rapidly especially in young patients and in patients naïve to biologics. On the other hand, our study showed that secukinumab can also be considered an effective treatment for obese and multidrug-resistant patients.
Author contributions
All authors conceived the study, collected the data, analyzed the data, and prepared the manuscript.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Supplemental_Table_1.docx
Download MS Word (30.6 KB)Acknowledgments
The authors would like to thank Simone Boniface of Springer Healthcare Communications who styled the manuscript prior to submission.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Chiricozzi A. Pathogenic role of IL-17 in psoriasis and psoriatic arthritis. Actas Dermosifiliogr. 2014;105(Suppl 1):9–20.
- Ghoreschi K, Laurence A, Yang XP, et al. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011;32:395–401.
- Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102.
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79–88.
- AbuHilal M, Walsh S, Shear N. The role of IL-17 in the pathogenesis of psoriasis and update on IL-17 inhibitors for the treatment of plaque psoriasis. J Cutan Med Surg. 2016;20:509–516.
- Chen Y, Qian T, Zhang D, et al. Clinical efficacy and safety of anti-IL-17 agents for the treatment of patients with psoriasis. Immunotherapy. 2015;7:1023–1037.
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776.
- Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014;18:156–169.
- Farahnik B, Beroukhim K, Nakamura M, et al. Anti-IL-17 agents for psoriasis: a review of phase III data. J Drugs Dermatol. 2016;15:311–316.
- Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015;75:329–338.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326–338.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400–409.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60–9 e9.
- Mease P, McInnes IB. Secukinumab: a new treatment option for psoriatic arthritis. Rheumatol Ther. 2016;3:5–29.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70–80.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484–493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082–1090.
- Mrowietz U, Leonardi CL, Girolomoni G, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015;73:27–36 e1.
- Georgakopoulos JR, Ighani A, Zhou LL, et al. Efficacy and safety of secukinumab in treating moderate to severe plaque psoriasis in two real-world Canadian dermatology clinics: a multicenter retrospective study. J Eur Acad Dermatol Venereol. 2018;32:e32–e4.
- Magnano M, Loi C, Patrizi A, et al. Secukinumab in multi-failure psoriatic patients: the last hope?. J Dermatolog Treat. 2018;1–3.
- Schwensen JF, Clemmensen A, Sand C, et al. Effectiveness and safety of secukinumab in 69 patients with moderate to severe plaque psoriasis: a retrospective multicenter study. Dermatol Ther. 2017;30:e12550.
- Körber A, Papavassilis C, Bhosekar V, et al. Efficacy and safety of secukinumab in elderly subjects with moderate to severe plaque psoriasis: a pooled analysis of phase III studies. Drugs Aging. 2018;35:135–144.
- Araujo NM. Hepatitis B virus intergenotypic recombinants worldwide: an overview. Infect Genet Evol. 2015;36:500–510.
- Bevans SL, Mayo TT, Elewski BE. Safety of secukinumab in hepatitis B virus. J Eur Acad Dermatol Venereol. 2018;32:e120–e1.
- Yanagihara S, Sugita K, Yoshida Y, et al. Psoriasis vulgaris in a hepatitis B virus carrier successfully treated with secukinumab and entecavir combination therapy. Eur J Dermatol. 2017;27:185–186.
- Yang C, Cui F, Chen LM, et al. Correlation between Th17 and nTreg cell frequencies and the stages of progression in chronic hepatitis B. Mol Med Rep. 2016;13:853–859.
- Ling Y, Puel A. IL-17 and infections. Actas Dermosifiliogr. 2014;105(Suppl 1):34–40.
- Bedair AA, Darwazeh AM, Al-Aboosi MM. Oral Candida colonization and candidiasis in patients with psoriasis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:610–615.
- Picciani BL, Michalski-Santos B, Carneiro S, et al. Oral candidiasis in patients with psoriasis: correlation of oral examination and cytopathological evaluation with psoriasis disease severity and treatment. J Am Acad Dermatol. 2013;68:986–991.
- Taheri Sarvtin M, Shokohi T, Hajheydari Z, et al. Evaluation of candidal colonization and specific humoral responses against Candida albicans in patients with psoriasis. Int J Dermatol. 2014;53:e555–e560.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47–62.
