ABSTRACT
Introduction
Myasthenia gravis (MG) is a neurological B-cell mediated autoimmune disorder affecting the neuromuscular junction. MG therapeutics have always relied on nonselective immunosuppression with oral steroids and non-steroidal immunosuppressants, mainly with good clinical response. However, clinical stabilization is often reached at the cost of many troublesome side effects and up to 15% of MG patients are deemed as refractory to conventional immunosuppression. This highlights the need of a more targeted and efficacious therapeutic approach. Results from the randomized-controlled period of the CHAMPION study demonstrate a good safety, tolerability, and efficacy profile of ravulizumab compared to placebo. Like eculizumab, ravulizumab is an anti-C5 monoclonal antibody, but with an enhanced pharmacokinetic profile, that allows dosing every 8 weeks.
Areas covered
We provide an overview of ravulizumab biological features and results from the phase III CHAMPION MG (NCT03920293) study.
Expert opinion
Data of the CHAMPION MG trial demonstrate that ravulizumab is effective and safe in the treatment of generalized MG. Having a rapid clinical effect, with long-term clinical response, ravulizumab could represent a selective immunosuppressive drug of choice in the future therapeutic algorithm of MG, where conventional immunosuppressants slowly leave room for newer drugs with a more targeted mechanism of action.
1. Introduction
Myasthenia gravis (MG) is a rare neuroimmunological disorder affecting the post-synaptic membrane at the neuromuscular junction (NMJ) [Citation1]. Clinical hallmarks of MG are muscle fatigability and muscle weakness which can affect the ocular, bulbar, axial, limb, and respiratory muscles [Citation2]. Most frequently patients experience ocular involvement with diplopia and/or ptosis as symptoms of onset, and the majority of patients later develop a generalized muscle involvement within the first 3 years [Citation2]. Only about 10–15% of patients remain with a pure ocular form of MG [Citation3].
1.1. Pathophysiology
MG is caused by autoantibodies directed against functionally important molecules on the post-synaptic membrane at the NMJ, which ultimately leads to an impaired neuromuscular transmission. Most patients (about 85%) display antibodies directed against the nicotinic acetylcholine receptor (AChR), whereas only 9% present antibodies anti-muscle-specific kinase (MuSK) and 1% have antibodies anti-lipoprotein-receptor-related protein 4 (LRP4) [Citation4]. Anti-AChR antibodies are mainly if IgG1 and IgG3 subclass, and are therefore able to activate the complement cascade, which is the main pathogenic mechanism of AChR+ MG. Complement activation, as later discussed, ultimately leads to the disruption of the postsynaptic folds with loss and dispersion of AChRs [Citation5]. Another very important pathogenic mechanism is antigenic modulation, where bivalent antibodies crosslink AChRs with acceleration of AChR endocytosis and degradation. Finally, anti-AChR antibodies act also by directly blocking the acetylcholine binding site on AChRs [Citation6]. Anti-MuSK antibodies, on the other hand, are of IgG4 subclass, and are therefore unable to activate the complement cascade and to induce antigenic modulation as they are functionally monovalent [Citation7]. Their pathogenic effect is exerted by masking the binding sites in MuSK that interact with LRP4 and collagen Q, which is essential for AChR clustering. Indeed, the inactivation of MUSK leads to a decrease of AChR density and an impairment of their alignment on the postsynaptic membrane [Citation7,Citation8]. Anti-LRP4 are of IgG1 subclass and are thus able to activate the complement cascade. Moreover, these antibodies also inhibit AChR clustering, by blocking the agrin-LRP4 interaction [Citation9]. Finally, a small percentage of MG patients are defined as seronegative, as no specific pathogenic antibody has been identified [Citation4]. The mechanisms which lead to selective antibody production in MG are still unknown.
1.2. Thymus involvement
So far it is well established the crucial role of the thymus in anti-AChR antibody production. In most patients with AChR+ MG the thymus is affected, either with thymoma (about 10%) or thymic hyperplasia, which affects more than 80% of patients with early-onset MG (onset of symptoms before 50 years of age) [Citation10,Citation11]. On the other hand, patients with late-onset MG (onset of symptoms after 50 years of age) generally present an involuted thymus [Citation11]. The thymus in AChR+ MG is rich in germinal centers, which are normally absent. These germinal centers are composed of anti-AChR-antibody-producing B cells, supporting the hypothesis that the thymus is the site responsible for the loss of immune tolerance to AChR [Citation11–13]. Up to now, there is no evidence of the role of the thymus in MUSK MG [Citation14]. In thymoma patients, thymectomy is nearly always indicated, however it does generally not lead to clinical remission. Indeed, in both thymoma and non-thymoma patients, the removal of the thymus does not reset AChR antibody levels, suggesting the existence of a stimulus outside the thymus that maintains autoantibody production [Citation15]. Efficacy of thymectomy in non-thymoma AChR+ generalized MG (gMG) patients was explored in the MGTX study and its extension trial [Citation16,Citation17]. Results showed that, compared to prednisone alone, the association of thymectomy with prednisone was able to reduce MG severity and steroid dosage. Efficacy of thymectomy was especially appreciable in patients younger than 40 years of age and with thymic hyperplasia. Current updated international consensus on the management of MG recommends early thymectomy in non-thymoma AChR+ gMG patients, aged 18 to 50 years, to gain early clinical stabilization and reduce the need of immunotherapy [Citation18].
1.3. Pharmacological treatment of MG
Aside from thymectomy, MG treatment is based on symptomatic treatment, immunosuppressive therapy, and immunomodulation. Symptomatic treatment is constituted by pyridostigmine bromide, which is the first-line treatment for all MG forms, except MUSK+ MG. However, its use is often limited by side effects such as diarrhea, nausea, muscle cramps, hypersalivation, and increase in respiratory secretions [Citation19]. It is however very difficult to reach clinical remission on symptomatic therapy alone, therefore the addition of an immunosuppressive drug is often required. First-line immunotherapy is represented by oral prednisone and prednisolone, which have a rapid effect, especially when given at high doses, since a higher dosage corresponds to a more rapid response, usually within 2–4 weeks [Citation19,Citation20]. Non-steroidal immunosuppressants (NSISTs) are employed as steroid sparing agents and to improve clinical stability, as there is a great variability in steroid-monotherapy response. NSISTs are constituted by antimetabolites (azathioprine, mycophenolate mofetil, methotrexate, or cyclophosphamide) and calcineurin inhibitors (tacrolimus and cyclosporine). NSISTs can also be used in monotherapy, however they have a delayed effect, which can require up to 6–9 months. Therefore, it is recommended, where possible, to perform a steroid bridging until peak of efficacy is obtained. Moreover, it is always advisable to start at low doses and then increase to induction regimen, due to their possible early toxicity [Citation19]. Finally, immunomodulation includes intravenous immunoglobulin (IVIg) and plasmapheresis (PLEX). Immunomodulation is usually employed as rescue therapy in case of MG crisis or MG worsening, however both IVIg and PLEX are often used in chronic therapy in patients that do not respond or have contraindications to conventional immunosuppression [Citation18]. The adoption of conventional immunosuppression is often effective, but clinical stable remission is hardly achieved. Moreover, up to 15% of patients are treatment refractory, meaning they do not respond or are intolerant to various immunosuppressive drugs [Citation21,Citation22]. Thus, refractory patients are often put on various immunosuppressants and experience the double burden of a non-stabilized chronic disease and side effects of immunosuppression. It is therefore fundamental to explore new molecules able to respond to such great unmet needs. Considering the central role of complement activation in the pathogenesis of MG, a very appealing target is the inhibition of the complement component 5 (C5). The cleavage of C5 results in the formation of C5a and C5b, which is necessary for the formation of membrane attack complex (MAC) [Citation5].
2. Overview of the market
MG is a chronic disease where clinical stabilization is often reached at the cost of many burdensome side effects given by conventional immunosuppressants, especially chronic steroid therapy. Also considering the proportion of refractory patients, it is evident the urgent need of new drugs with a higher profile in terms of efficacy and tolerability. Notably, except pyridostigmine which has been FDA approved for the treatment of MG back in 1955, all other drugs used in the chronic and acute phase (e.g. steroids, NSISTs, PLEX, IVIg, Rituximab) are not licensed for the treatment of MG by the FDA. In many European countries, and recently also in Australia, IVIg are licensed for the treatment of severe acute MG exacerbations, while in Japan Tacrolimus is approved for MG. However, this trend has undergone a significant change of course in the very recent years, where three new selective immunosuppressants have been FDA-approved for the treatment gMG: eculizumab, an anti-C5 monoclonal antibody (mAb) FDA-approved on 23 October 2017 for AChR+ gMG patients (in Europe it has been approved only for refractory patients); efgartigimod, an inhibitor of the human neonatal Fc receptor (FcRn), has been FDA-approved on 21 December 2021 for the treatment of AChR+ gMG patients as well, while an expanded access is available for both AChR+ and AChR- gMG patients in countries different from US before its regulatory approval (NCT04777734); ravulizumab, another anti-C5 mAb FDA-approved on 28 April 2022 for the treatment of AChR+ gMG. The FDA-approval of ravulizumab followed the encouraging results of the CHAMPION MG study, a multicenter, double-blind, phase III trial to evaluate safety and efficacy of ravulizumab for the treatment of AChR+ gMG (NCT03920293), which is still active in its open-label (OLE) phase ().
: Drug summary box
Lately, thanks to the increasing knowledge of MG pathogenesis, there has been flourishing research on newer therapeutic molecules with a more targeted effect, compared to conventional immunosuppressants (). Apart from eculizumab and ravulizumab, other complement inhibitors are currently under study: zilucoplan, an anti-C5 small molecule delivered subcutaneously (NCT04115293); the combination of pozelimab and cemdisiran, respectively a mAb and a si-RNA both targeting C5 also administered subcutaneously (NCT05070858); ALXN2050, an orally-administered inhibitor of the complement factor D, which is a key component of the alternative pathway of the complement cascade (NCT05218096). As for FcRn inhibitors, at the moment efgartigimod is under study to evaluate its efficacy with different intravenous (iv) dosing regimens (NCT04980495) and its efficacy with subcutaneous (sc) administration (NCT04735432; NCT04818671). Other FcRn inhibitors currently under investigation in clinical trials are rozanolixizumab (NCT04650854) and nipocalimab (NCT04951622). Another appealing target for MG is represented by B-cells, due to their crucial role in antibody production. Rituximab is an anti-CD20 mAb, which is currently recommended as early treatment in MUSK+ MG patients with no initial response to immunosuppressive treatment. In AChR+ MG it is recommended only in refractory patients who do not respond or do not tolerate other immunosuppressants, as its efficacy in AChR+ MG is still very debated [Citation18]. Inebilizumab could prove to be more effective in this subgroup of patients, as it targets CD-19, which unlike CD20 is expressed on long-lived plasma cells responsible for anti-AChR antibody production. Another direct B-cell inhibitor with an ongoing phase II trial is TAK-079, which targets CD38, expressed on plasmablasts, plasma cells and natural killer cells, and is also induced on activated T and B cells (NCT04159805). B-cells can also be targeted indirectly, as in the case of belimumab, which neutralizes the biologically active soluble B-lymphocyte stimulator (BAFF) [Citation23], and iscalimab (NCT02565576), an anti-CD40. Both drugs have completed a phase II clinical trial on AChR+ and MUSK+ gMG patients, but failed to meet their primary endpoint. Currently a phase III study investigating satralizumab, an inhibitor of the soluble IL6 receptor (IL6-R) is ongoing for AChR+, MUSK+ and LRP4+ gMG patients (NCT04963270).
Table 1. New myasthenia gravis therapies approved and under study.
3. Mechanism of action
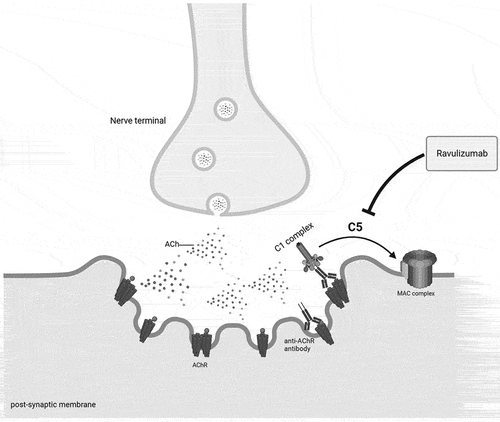
The main pathogenic mechanism of AChR-antibody-mediated damage is complement activation. AChR antibodies are of IgG1 and IgG3 subclass, and thus activate the complement cascade through the classical pathway which leads to the formation of the C5 convertase and ultimately to the formation of the membrane attack complex (MAC). The MAC complex is constituted by C5b, C6, C7, C8 and polymeric C9, which together form a lytic pore on the post-synaptic membrane, which leads to the disruption of the postsynaptic folds and subsequent impairment of the neuromuscular transmission [Citation24,Citation25]. Ravulizumab is a humanized recombinant mAb IgG2/4 K that specifically binds C5 with high affinity, thus blocking the terminal pathway and preventing MAC formation (). Ravulizumab spares the early components of the complement cascade that are fundamental for the opsonization of microorganisms and clearance of immunocomplexes [Citation26].
Figure 1. Pathophysiology of complement activation in MG: anti-acetylcholine (Ach) receptor (AChR) antibodies activate the complement cascade through the classical pathway by binding C1q con the Fc domain. The subsequent formation of C5 convertase initiates the terminal pathway which ultimately leads to the formation of the membrane attack complex (MAC), a lytic pore on the postsynaptic membrane. The final effect is a focal lysis of the neuromuscular junction (NMJ), with disruption of the postsynaptic folds and loss of AChRs. Ravulizumab binds with high affinity to C5, inhibiting its enzymatic cleavage and thereby preventing the MAC formation. Created with Biorender.com.
4. Chemistry
Ravulizumab is a long-acting humanized monoclonal IgG2/4 K antibody produced in Chinese hamster ovary (CHO) cell culture by recombinant DNA technology. It consists of 2 identical 448 amino acid heavy chains and 2 identical 214 amino acid light chains, with a molecular weight of about 148kDa [Citation26].
5. Pharmacodynamics
Results of the randomized placebo-controlled phase (RCP) of the CHAMPION MG study on the pharmacodynamic effect of ravulizumab on AChR+ gMG patients are currently available [Citation27]. Data from 86 ravulizumab-treated patients were analyzed, demonstrating that ravulizumab was able to induce immediate and complete inhibition of the terminal pathway (serum free C5 < 0.5 μg/mL), which was sustained throughout treatment in all patients. Moreover, in the treated population, no anti-drug antibodies (ADA) were detected.
6. Pharmacokinetics and metabolism
Ravulizumab was engineered from eculizumab (h5G1.1-mAb), by substituting 4 aminoacids in the complementary binding and neonatal Fc regions of the eculizumab backbone. This resulted in an increased endosomal dissociation of C5 with an effective recycling of ravulizumab in the vascular compartment via the FcRn pathway [Citation28]. This ultimately leads to an increased half-life of ravulizumab, which requires iv infusions once every 8 weeks (q8w), compared to eculizumab which requires iv infusions every 2 weeks. Ravulizumab dose regimen is weight-based, with an initial loading dose of 2400–3000 mg, followed after 2 weeks by a maintenance dose of 3000–3600 mg every 8 weeks [Citation26]. Results of the RCP phase of the CHAMPION MG study indicate that therapeutic serum concentrations of ravulizumab (>175 μg/mL) were reached by the end of the first infusion and maintained throughout the whole 26-week treatment period. Mean elimination half-life of ravulizumab was 56.4 days (SD: 7.9 days). After the last maintenance dose, mean Cmax was 1558 μg/mL, and mean Ctrough was 663 μg/mL. No differences were observed among weight groups [Citation27].
As an immunoglobulin gamma (IgG) mAb, ravulizumab is expected to be metabolized just like any other endogenous IgG, via catabolic pathways that lead to degradation into small peptides and amino acids. Ravulizumab is made of only naturally occurring amino acids and does not have any known active metabolites [Citation26].
7. Clinical efficacy
In the CHAMPION MG study, adult AChR+ patients with a Myasthenia Gravis Foundation of America (MGFA) class between II and IV, and with a Myasthenia Gravis-Activities of Daily Living (MG-ADL) score ≥6 were randomized (1:1) to receive iv ravulizumab infusion or placebo. Stable standard-of-care (SoC) therapy was permitted throughout the study. Patients had to be vaccinated against meningococcal infection within three years before initiating the trial agent. Exclusion criteria included active or untreated thymoma, a history of thymic carcinoma or thymic malignancy (unless deemed cured with no recurrence in the last 5 years prior to screening), history of thymectomy in the last 12 months prior to screening, IVIg treatment in the 4 weeks before randomization, rituximab therapy in the past 6 months before screening or previous treatment with complement inhibitors. Dose regimen was bodyweight-based: 2400–3000 mg loading dose (or placebo) on Day 1, then 3000–3600 mg every 8 weeks beginning on Day 15, or placebo for 26 weeks. After the 26-week period of the RCP phase, patients entered the OLE period, where they received iv ravulizumab, with a blind induction or bridging dose for those previously receiving respectively placebo or ravulizumab, followed by 3000–3600 mg q8w depending on body weight. Primary efficacy endpoint was change in the MG-ADL score from baseline to Week 26, while secondary endpoints included change from baseline in Quantitative Myasthenia Gravis (QMG) total score, in the Revised 15 Component Myasthenia Gravis Quality of Life (MG-QOL15r) score, and in the Neurological Quality of Life (Neuro-QoL) Fatigue Score. The percentage of patients with a QMG total score reduction of at least 5 points at week 26, as well as the percentage of patients with a MG-ADL total score reduction of at least 3 points at week 26 were also considered as secondary endpoints. Primary data on the RCP phase are currently available as well as interim data of the OLE phase. In the RCP a total of 175 patients were enrolled from 85 different centers worldwide. A statistically significant improvement in MG-ADL total score at Week 26 (−3.1 vs −1.4 for placebo; p < 0.001) was observed in the ravulizumab-treated group. Moreover, ravulizumab displayed statistically significant improvements from baseline through Week 26 also in the QMG total score (−2.8 vs −0.8 for placebo; p < 0.001). Improvements in both MG-ADL and QMG scores were witnessed within 1 week, and the benefit was maintained through Week 26. Furthermore, a significantly greater proportion of ravulizumab-treated patients experienced an improvement of 5 points or more in QMG total scores compared to the placebo group (30.0% vs. 11.3%; P = 0.005) [Citation29]. For the OLE phase, an ad interim analysis included 79 patients (38 received ravulizumab, 41 received placebo in the RCP) who rolled-over into the OLE phase at least 26 weeks before data cutoff. The analysis demonstrated that ravulizumab administered q8w was able to provide sustained improvements in symptoms, with a good tolerability profile, for up to 1 year. Indeed, improvements reached in the MG-ADL score among the ravulizumab-treated group in the RCP were preserved, while patients who switched from placebo to ravulizumab experienced a rapid improvement in the MG-ADL score, which was also maintained for 26 weeks. Similarly, improvements in the QMG score were maintained in the previously ravulizumab-treated group, while patients that were previously on placebo displayed a significant improvement from OLE baseline [Citation30].
8. Safety and tolerability
Regarding the safety of complement inhibitors, it is important to underline that inhibition of C5 increases the risk of Neisseria Meningitidis infection. Therefore, it is mandatory to vaccinate the patients with both quadrivalent and B-serotype vaccines, at least 2 weeks before starting therapy. Otherwise, a prophylactic antibiotic therapy should be started and continued for at least two weeks after anti-meningococcal vaccination. Patients must be informed of the risks and symptoms of meningococcal infection and should always carry an informational safety card to show whenever needed. No cases of meningococcal infection were reported in the CHAMPION MG RCP or OLE study, while eculizumab registered only one non-fatal case during the REGAIN OLE [Citation31].
In the previous trials investigating safety and tolerability of eculizumab in PNH, a HUS and gMG, most common adverse events were headache, nasopharyngitis and gastro-intestinal disorders [Citation31–34].
Data from the CHAMPION MG study did not detect any notable difference in adverse events between treatment groups and ravulizumab displayed a good tolerability profile [Citation29,Citation30]. In the RCP phase the most frequent adverse event was headache (19% of patients in the ravulizumab group and 16% of patients in the placebo group), while the most frequent serious adverse events were linked to MG worsening (3 patients receiving placebo and 1 patient receiving ravulizumab) and Covid-19 infection (1 patient receiving placebo and 2 patients receiving ravulizumab) [Citation29].
9. Regulatory affairs
Following the positive results of the CHAMPION-MG study, ravulizumab gained FDA approval on 28 April 2022, marking the first and only approval of a long-acting complement inhibitor for the treatment of gMG. On August 2022 ravulizumab was approved in Japan for the treatment of adult patients affected by AChR+ gMG whose symptoms are difficult to control with high-dose IVIg or PEX. On September 2022 ravulizumab was also approved in Europe as an add-on to standard therapy for the treatment of adult AChR+ gMG patients.
10. Conclusion
Despite substantial improvement in care over time, a non-negligible proportion of gMG patients continues to experience significant burden of disease, due to non-responsiveness to conventional therapy and/or to the frequent and burdensome side effects of conventional immunosuppression. Therefore, the need of a more specific and targeted approach has continuously emerged in the last years. Since complement activation is considered the main pathogenic mechanism of AChR+ MG, its inhibition could represent a successful treatment strategy. Results of the CHAMPION MG study and interim data of its OLE phase show that ravulizumab, administered q8w can guarantee a rapid and sustained clinical improvement and is well tolerated for up to 1 year in adults with AChR+ gMG. Therefore, ravulizumab holds great promise in finally responding to the unmet need of a more efficacious and well tolerated immunosuppressive therapy for gMG. Moreover, given that MG is a chronic disease, the convenience of ravulizumab dosing regimen may also increase patient satisfaction and treatment adherence.
11. Expert opinion
The fact that inhibiting the complement cascade could represent a turning point in MG management was demonstrated by the phase III REGAIN study, where results showed that treatment with eculizumab brought clinical benefit, which is particularly important also since patients had to be defined as treatment-refractory in order to enter the study [Citation31]. The recognition of its efficacy led to the engineering of another molecule with an improved pharmacokinetic and pharmacodynamic profile since dosing-regimen of eculizumab every 2 weeks could reduce patient adherence. Ravulizumab, with its enhanced half-life with dosing intervals q8w represents a very attractive alternative, not only compared to eculizumab, but to other drugs as well. Data of the CHAMPION MG study also demonstrated a similar profile compared to eculizumab, in terms of safety and tolerability, with also a long-term efficacy [Citation27,Citation29,Citation30].
Considering the great limitations of the current therapeutic algorithm of MG, in terms of unpredictable response and troublesome side effects, it is critical to shift toward a more targeted approach, where drugs are engineered based on the specific pathogenic mechanisms that underlie the disease. In this sense, ravulizumab, as well as other novel drugs currently under study as previously discussed, was conceived to act on a very specific and pivotal pathogenic system, the complement cascade. It is likely that the current therapeutic approach, characterized mainly by nonselective immunosuppression, will be replaced by newer drugs with a more precise mechanism of action, that should decrease side effects while guaranteeing a satisfactory clinical benefit. Hence, a possible new therapeutic approach could be to start ravulizumab either before or in association to conventional immunosuppressants. Ravulizumab also demonstrated to have a rapid clinical effect, as improvements in the MG-ADL and QMG scores were observed already within 1 week of treatment.
However, in clinical practice, the likelihood of using ravulizumab as an immunosuppressant of choice strongly depends on two factors: i) the confirmation in the completed CHAMPION MG OLE study as well in the real-world of its efficacy and good safety profile; ii) its costs, especially considering the need of life-long treatment. While the use of ravulizumab can be justified in refractory patients or in patients that have failed at least 1 conventional immunosuppressant, its possible high costs may limit its prescription particularly in naïve patients, thus losing precious information on ravulizumab effectiveness in this subset of patients.
Both eculizumab and ravulizumab act by inhibiting the C5 component of the complement cascade, with a similar safety, tolerability, and efficacy profile. However, it is more likely to expect a higher usage of ravulizmab than eculizumab in the future mainly for its enhanced pharmacodynamic profile, but also because in Europe the prescription of ravulizumab is not restricted to refractory cases, thus increasing its potential application. However, more real-world data are available for eculizumab, especially in some categories like pregnant women, for which the prescription of eculizumab could be thus preferable.
So far, complete data on the RCP phase of the CHAMPION MG study have been recently published, while only interim data are available for the OLE phase (which completion is estimated for the end of 2023). Safety, tolerability, and effectiveness of ravulizumab will have to be demonstrated also in the real-world context. If these expectations will be met, it is very likely that in the next immediate years ravulizumab will be one of the main selective immunosuppressants of choice for AChR+ gMG.
Article highlights
Non-selective immunosuppression with oral steroids and non-steroidal immunosuppressants is still the mainstay of MG therapy. However, clinical response is often reached at the cost of important troublesome side effects and about 15% of patients are refractory to conventional immunosuppression. This underlines the presence of a remarkable unmet need for newer drugs with a higher profile in terms of efficacy and tolerability.
Ravulizumab could finally respond to the unmet need for a more effective and well tolerated drug, as it inhibits complement activation, which is the main pathogenic mechanism of AChR+ MG.
Ravulizumab is a long-acting humanized monoclonal IgG2/4K antibody that specifically binds with high-affinity C5, preventing MAC formation. It was engineered from eculizumab, with an increased half-life which allows dosing every 8 weeks (vs 2 weeks in case of eculizumab), therefore increasing patient adherence.
The randomized, phase III, placebo-controlled study CHAMPION MG provided strong evidence of clinical efficacy, tolerability and safety of ravulizumab in the treatment of AChR+ generalized MG, while the open-label phase is still active.
Following the encouraging results of the CHAMPION MG study, on 28 April 2022 the FDA approved ravulizumab for the treatment of AChR+ gMG, further widening the therapeutic opportunities for MG.
Declaration of interest
R Mantegazza has received personal compensation for consulting, serving on a scientific advisory board, speaking, travel or other activities with: Alexion Pharmaceuticals, argenx, Biomarin, Catalyst Pharmaceuticals, Sanofi-Aventis and UCB. R Mantegazza is also recipient of a grant RF-2016-02364384 of the Italian Ministry of Health entitled ‘Identification of B cell-related biomarkers to predict response to immunosuppressive and B cell targeting therapies in myasthenia gravis.’ F Vanoli received compensation for consulting by Alexion Pharmaceuticals and Argx. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they received small fees from Alexion to present data on myasthenia gravis in seminars. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Company review
Alexion provided a scientific accuracy review at the request of the journal editor.
Additional information
Funding
References
- Gilhus NE, Longo DL. Myasthenia gravis. N Engl J Med. 2016;375:2570–2581.
- Gilhus NE, Tzartos S, Evoli A, et al. Myasthenia gravis. Nat Rev Dis Primers. 2019 May 2;5(1):30.
- Kerty E, Elsais A, Argov Z, et al. EFNS/ENS Guidelines for the treatment of ocular myasthenia. Eur J Neurol. 2014;21:687–693.
- Vincent A, Huda S, Cao M, et al. Serological and experimental studies in different forms of myasthenia gravis. Ann N Y Acad Sci. 2018;1413:143–153.
- Tuzun E, Christadoss P. Complement associated pathogenic mechanisms in myasthenia gravis. Autoimmun Rev. 2013;12:904–911.
- Kordas G, Lagoumintzis G, Sideris S, et al. Direct proof of the in vivo pathogenic role of the AChR autoantibodies from myasthenia gravis patients. PLOS ONE. 2015;10:e0117673.
- Koneczny I, Stevens JAA, De Rosa A, et al. IgG4 autoantibodies against muscle-specific kinase undergo Fab-arm exchange in myasthenia gravis patients. J Autoimmun. 2017;77:104–115.
- Huijbers MG, Zhang W, Klooster R, et al. MuSK IgG4 autoantibodies cause myasthenia gravis by inhibiting binding between MuSK and Lrp4. Proc Natl Acad Sci USA. 2013;110:20783–20788.
- Higuchi O, Hamuro J, Motomura M, et al. Autoantibodies to low‐density lipoprotein receptor–related protein 4 in myasthenia gravis. Ann Neurol. 2011 Feb;69(2):418–422.
- Strobel P, Moritz R, Leite MI, et al. The ageing and myasthenic thymus: a morphometric study validating a standard procedure in the histological workup of thymic specimens. J Neuroimmunol. 2008;201:64–73.
- Marx A, Pfister F, Schalke B, et al. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev. 2013;12:875–884.
- Cron MA, Maillard S, Villegas J, et al. Thymus involvement in early-onset myasthenia gravis. Ann NY Acad Sci. 2018;1412:137–145.
- Cavalcante P, Le Panse R, Berrih‐aknin S, et al. The thymus in myasthenia gravis: site of “innate autoimmunity”? Muscle Nerve. 2011 Oct;44(4):467–484.
- Leite MI, Ströbel P, Jones M, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol. 2005;57:444–448.
- Okumura M, Ohta M, Takeuchi Y, et al. The immunologic role of thymectomy in the treatment of myasthenia gravis: implication of thymus-associated B-lymphocyte subset in reduction of the anti-acetylcholine receptor antibody titer. J Thorac Cardiovasc Surg. 2003 Dec 1;126(6):1922–1928.
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016Aug11;375(6):511–522.
- Wolfe GI, Kaminski HJ, Aban IB, et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol. 2019Mar1;18(3):259–268.
- Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021Jan19;96(3):114–122.
- Vanoli F, Mantegazza R. Antibody therapies in autoimmune neuromuscular junction disorders: approach to myasthenic crisis and chronic management. Neurotherapeutics. 2022;19(3):897–910. DOI:10.1007/s13311-022-01181-3.
- Mantegazza R, Bonanno S, Camera G, et al. Current and emerging therapies for the treatment of myasthenia gravis. Neuropsychiatr Dis Treat. 2011;7:151.
- Mantegazza R, Antozzi C. When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther Adv Neurol Disord. 2018;11:1756285617749134.
- Silvestri NJ, Wolfe GI. Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis. 2014;15:167–178.
- Hewett K, Sanders DB, Grove RA, et al. BEL115123 study group. Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology. 2018 Apr 17;90(16):e1425–34.
- Sahashi K, Engel AG, Lambert EH, et al. Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J Neuropathol Exp Neurol. 1980 Mar 1;39(2):160–172.
- Mantegazza R, Vanoli F, Frangiamore R, et al. Complement inhibition for the treatment of myasthenia gravis. Immunotargets Ther. 2020;9:317.
- Alexion Europe SAS. Ultomiris (ravulizumab): summary of product characteristics. [cited 2023 March 03]. Available from: https://www.ema.europa.eu/en/documents/product-information/ultomiris-epar-product-information_en.pdf
- Vu Thuan, Ortiz Stephan, Katsuno Masahisa, et al. Pharmacokinetics and pharmacodynamics of ravulizumab in adults with generalized myasthenia gravis: results from the phase 3 CHAMPION MG study. Neurology. 2022;98:18 Supplement 850.
- Sheridan D, Yu Z-X, Zhang Y, et al. Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PloS one. 2018;13.4:e0195909.
- Vu Tuan, Meisel Andreas, Mantegazza Renato, et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evid. 2022;1.5:EVIDoa2100066.
- Howard JF, Vu Thuan, Mantegazza Renato, et al. Long-term efficacy and safety of ravulizumab, a long-acting terminal complement inhibitor, in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension. Neurology. 2022;98:18 Supplement 853.
- Howard JF Jr, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in antiacetylcholine receptor antibody-positive refractory generalized myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16:976–986.
- Muppidi S, Utsugisawa K, Benatar M, et al. Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve. 2019;60:14–24.
- Dmytrijuk A, Robie‐Suh K, Cohen MH, et al. FDA report: eculizumab (Soliris®) for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Oncologist. 2008 Sep;13(9):993–1000.
- Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N Engl J Med. 2013 Jun 6;368(23):2169–2181.
