ABSTRACT
Introduction
Immune-mediated inflammatory diseases (IMIDs) are increasingly managed effectively with biologic medicines. However, with relatively high unit costs, there remains a meaningful pressure to ensure streamlined, equitable, and inclusive prescription of biologics in the UK. Despite an increased awareness of the benefits of patient-centric shared decision making, patients remain on the periphery of biologic selection for the treatment of IMIDs.
Areas covered
We provide a patient perspective on core issues in the commissioning, prescription, and decision making around biologics for IMIDs in the UK, focusing on England. In particular, the crucial aspect of determining ‘value’ for different stakeholders, who necessarily have different priorities, is considered.
Expert opinion
There are disparities in commissioning, access to, and prescription of biologics for IMIDs in the UK. This creates an unequal treatment model and drives patient dissatisfaction with an ‘experience lottery’ for the management of disease. A more transparent approach to prescribing decisions, made in close consultation with patients, is essential for improving equity and experience with biologic treatment of IMIDs.
1. Introduction
In the past decade, biologic medicines have become a core part of treatment for many patients with immune-mediated inflammatory diseases (IMIDs), such as psoriasis, inflammatory arthritis (e.g. rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis), and inflammatory bowel disease (IBD; e.g. Crohn’s disease and ulcerative colitis) [Citation1–7]. Although undeniably effective, biologics can have a high unit cost, and their increasing use, combined with growing cost pressures, has contributed to recent reforms within the UK National Health Service (NHS), particularly in England [Citation8–10]. It is important to understand the patient perspective on the evolving healthcare service in England with regard to the prescription of biologics for IMIDs to improve their treatment experiences.
In the UK, evidence from randomized and real-world trials is assessed by the National Institute for Health and Care Excellence (NICE), which produces recommendations on reimbursement, as well as cost-effectiveness appraisals and guidance on medicines usage. Between 2012 and 2022, specific funding decisions and prescribing criteria for medicines (‘commissioning’) within NHS England were controlled by clinically led statutory bodies called clinical commissioning groups (CCGs) [Citation11]. They were instituted under the principle that local clinicians could most effectively judge how to allocate resources for their area and, collectively, they were responsible for approximately two-thirds of the NHS England budget [Citation11,Citation12]. However, during their existence, there were concerns about regional variation in medicines and provision of healthcare services, sometimes referred to in the UK as an ‘experience lottery’ or ‘postcode lottery.’ This means that having access or not can be highly dependent on where someone lives, rather than being equitable across the country [Citation9,Citation11,Citation13,Citation14]. Patients in the UK can also choose to supplement their NHS care with private healthcare provision, and under a system with regional variation, some patients may feel they have no choice but to take on the additional costs of such care [Citation15]. However, even this may not be a solution, as many UK private healthcare insurance companies do not cover continuous treatment for chronic conditions such as IMIDs.
Comparing CCG pathways across different regions revealed that some permitted a greater degree of clinical freedom than others. In particular, some regions allowed the use of biologics early in patient pathways, whereas others imposed strong restrictions regarding when biologics could be used or used language around cost-effectiveness that may have influenced prescribing decisions [Citation16–18]. Such disparities indicated that there were inconsistencies in how NICE guidelines were applied by CCGs. Clinicians treating patients with IMIDs with biologics reported that this led to an unacceptable degree of variation in outcomes for patients with similar clinical cases [Citation13,Citation19,Citation20].
In July 2022, 42 integrated care systems (ICSs) were established on a statutory basis. These are intended to be broader-based entities and include representation from the NHS integrated care board, authorities within the local area, place-based partnerships, and healthcare providers [Citation21]. ICSs are now responsible for commissioning in NHS England and have a specific remit to improve outcomes in population health and healthcare and to tackle inequalities in outcomes, experience, and access. In seeking to address inequities in the prescription of biologics, it is important that the lessons learned in the CCG era are now translated into opportunities for improvement under ICSs.
In light of recent evidence, the introduction and utilization of biologics for psoriatic arthritis (PsA) in the UK warrant further discussion, particularly in comparison to practices across continental Europe. Studies using data from a multinational cohort have highlighted that patients in the UK are more likely to be receiving conventional synthetic disease-modifying antirheumatic drugs (DMARDs) and less likely to be receiving biologics than their European counterparts. These have illuminated significant disparities in the timing of biologic therapy initiation for patients with PsA in the UK. Notably, the later use of these treatments in the UK may be contributing to higher disease activity rates among the UK PsA population compared with the population in Europe [Citation22,Citation23]. This delay underscores the critical need for evaluating and possibly revising current healthcare policies and practices to ensure timely access to effective treatments for patients with IMIDs.
In this article, we provide a patient perspective on key issues with healthcare delivery relevant to people living with IMIDs in England. We share opinions and insights from patient groups that have experienced inequities in biologic prescribing across England – the ‘postcode lottery’ – during the CCG era, moving from individual experiences to system-level considerations, and ultimately discussing how ‘value’ for different stakeholders, who necessarily have different priorities, can be determined. Finally, we suggest ways to improve the treatment experiences of people with IMIDs who are eligible for biologics, including how key actions can be implemented now that the ICS model is in place.
2. Methods
The authors of this article are representatives of two UK patient advocacy groups: Helen McAteer from the Psoriasis Association, Northampton, UK (referred to hereafter as ‘HM’) and Seb Tucknott from IBDrelief Ltd, Brighton, UK (referred to hereafter as ‘ST’). The authors discussed their experience of the issues affecting patients with IMIDs in England. For this article, their experiences have been contextualized by searching the literature for articles relevant to the use of biologic medicines to treat IMIDs in the UK since 2012 (no full systematic literature review was conducted). NICE guidelines have been used as the primary resource for the recommendations for treatment and care of patients in England, and other resources, such as patient surveys, have been utilized to provide a broader patient perspective on clinical practice.
3. The benefits of supporting patients’ health literacy
Health literacy is defined as the extent to which patients have the skills and ability to understand and use information to make decisions regarding their health [Citation24]. Inadequate health literacy has been associated with a range of problems, including poor health, reduced life expectancy, increased healthcare costs, and health inequalities [Citation25]. Physicians can improve patients’ health literacy by communicating effectively to patients about their disease and treatment [Citation24].
A variety of tools are available to help physicians in this endeavor. Patient decision aids that provide information on the available treatment options and help patients to think about, clarify, and communicate their opinions on the value of each treatment can be valuable. To support healthcare professionals (HCPs) and people who use healthcare services, NICE provides a standard framework regarding patient decision aids, which includes the following aims: they should include evidence-based information on the treatment options available to the patient, as well as the risks, benefits, and likely outcome of a treatment; they should support patients with making informed decisions in collaboration with a HCP; and they should support the HCP in following a shared decision-making approach with their patient [Citation26] (see section 4 for further discussion on shared decision making). A study that examined what patients with IBD understand about biologic medicines concluded that the development of a tool to aid decision making regarding biologics would be useful due to the complexity of associated risks and benefits [Citation27]. Such a tool could also help to address inequalities in health literacy.
We believe that all patients who want to understand more about their condition should be given access to trustworthy, relevant, and up-to-date information (expert opinion, ST and HM). A recent survey commissioned by the National Axial Spondyloarthritis Society (NASS) asked 913 patients with axial spondyloarthritis to rate their approach to managing their condition. This was assessed using the Patient Activation Measure, which measures a person’s knowledge, skills, and confidence regarding managing their own health and well-being. The survey indicated that 62% of patients would like to work as a team with their HCPs to make decisions about their care; however, only 21% of patients reported that that they are confident making their own decisions about their care [Citation28]. Together, these findings underline the importance of increasing patient health literacy. Many organizations, including IBDrelief, are trying to address the issue of poor health literacy [Citation29], with patients and supporters expressing a desire to improve their understanding of the medications being used. If there is meaningful improvement in a patient’s understanding of their available treatment options, they will hopefully demonstrate improved medication adherence and feel empowered to make informed choices regarding their health (expert opinion, ST).
4. The advantages of shared decision making and the role of the patient in shaping care
In 2021, NICE published shared decision-making guidelines that encourage patient’s involvement in their own care, with the aim of empowering patients to make decisions that are right for them [Citation26]. Patients’ understanding of treatments can be improved by employing specific education strategies. Examples recommended by NICE include the ‘three-talk model,’ in which discussions are broken down into three stages addressing choices, options, and decisions, and the ‘teach-back method,’ which asks people to ‘teach back’ the information they have been given to confirm their understanding [Citation30].
There can be barriers to fully implementing a shared decision-making approach when situations cross multiple health disciplines. For example, a mixed-methods needs assessment of dermatologists and rheumatologists found a lack of confidence and suboptimal knowledge regarding pregnancy and contraception for women of reproductive age who had psoriasis, psoriatic arthritis, and/or rheumatoid arthritis, including a common perception that all biologics are strictly contraindicated during pregnancy, which is not the case [Citation31]. However, when fully implemented, shared decision-making approaches to biologic treatment have been shown to improve patient’s trust in their treatment [Citation32].
In the UK, despite significant work in this area [Citation14,Citation33,Citation34], some patients still do not feel heard when decisions are made regarding their care. In the 2022 IBDrelief medication survey, approximately one-quarter of patients had reservations about discussing their condition with their IBD specialist nurse or consultant [Citation35]. This can often relate to the impact of medication on a patient’s life, as illustrated by the following quotes:
I’ve felt over the years that doctors don’t listen to me, the patient, and they always feel they know best based on standard evidence; however, one size does not fit all.
I believe oftentimes doctors don’t want to listen to their patients and want to immediately label them as ‘non-compliant’ if they stop […] or don’t want to take a medication at all because of the side effects it causes. Doctors should be putting in more effort to get patients on medications they can tolerate.
Such findings are consistent with an international survey of patients with IBD and physicians, which found that only approximately two-thirds of patients felt comfortable discussing symptoms with their physician [Citation36]. This is also seen in other IMIDs: in a survey of 262 patients who were being treated with a biologic, conducted by The Patients Association, just over one-third of patients said that they were not consulted prior to a change in their medication, despite this being a requirement in the NHS England guidelines [Citation37]. Moreover, as may be expected, any reservations are often exacerbated among patients for whom English is a second language [Citation38]. Two studies in PsA also highlight this crucial gap in the patient–clinician dynamic, particularly in treatment intensification decisions for PsA. They show that treatment adjustments often depend more on the clinicians’ judgments than on patients’ self-reported outcomes, including their quality of life, as measured by the 12-item Psoriatic Arthritis Impact of Disease Questionnaire (PsAID-12). This highlights an ongoing challenge in achieving shared decision-making in clinical practice, underscoring the importance of incorporating patient-reported outcome measures (PROMs) into treatment planning [Citation22]. Further evidence suggests that, despite the known benefits of biologics in managing PsA, decision-making processes may not fully align with patient experiences and expectations, particularly in the UK [Citation23]. Our experience also suggests that patients may often receive a letter notifying them of changes regarding their medication, without any consultation between the patient and their physician (expert opinion, ST and HM)
Communication between patients and their practitioners is key to developing realistic treatment goals and optimizing outcomes through prompt treatment alterations when goals are not met (expert opinion, ST and HM). Specialist nurses are often the most accessible contact for patients, and whom patients are most likely to feel confident talking to about their care (expert opinion, HM). The desire of many patients to work closely with their HCPs, as reported in the NASS survey discussed in section 3, may provide a foundation for implementing shared decision-making processes.
5. Understanding NICE guidelines, local commissioning, and clinical decision making in practice
In the UK healthcare system, NICE provides national guidance on the treatment of specific conditions, but the planning and purchasing of healthcare services (‘service commissioning’) – previously by CCGs, and moving forward, by ICSs – have a huge impact on clinical decisions and, therefore, the patient. One purpose of the reorganization from CCGs to ICSs is to drive a more patient-centric and integrated model of care [Citation21]. Historically, healthcare service commissioning has sometimes not given patients sufficient opportunity to provide their input because of a focus on only randomized controlled trial (RCT) data [Citation39,Citation40].
RCTs are considered the gold standard for examining the efficacy of treatments or interventions and are required for new approvals by regulatory authorities worldwide [Citation41]. However, the external validity of RCTs outside of the study population is often low, and relying on them for commissioning decisions can exclude important evidence emerging from outside of RCTs [Citation19,Citation40]. It is increasingly recognized in NICE guidance that it is important to complement RCT data with evidence from real-world studies, which collect clinical outcome data on the use, risks, and benefits of a medical therapy in a much broader range of patients and over a much longer period of time than typically covered in RCTs [Citation42]. In many cases, RCTs may have used a placebo comparator, which may not reflect routine practice or the experience the patient has with their treatment [Citation39,Citation42]. Commissioning decisions should also take such factors into account.
Moreover, decisions regarding medicine availability in local patient pathways are increasingly made at the payment level, long before the patient becomes involved [Citation14,Citation19,Citation20,Citation43]. This often involves the selection of biosimilars. Biosimilars are medicines that are very similar to an existing, already approved biologic medicine, defined as having no clinically meaningful differences in terms of safety, purity, and potency [Citation8,Citation37,Citation44,Citation45]. Biosimilars are often cheaper than the original biologic because any patents that maintain exclusivity of the original biologic have expired; such medication changes have the potential to save the NHS significant amounts of money, while increasing patient access to biologics [Citation8,Citation44,Citation46]. However, where treatment selection is driven by unit cost, it is feasible that there will be fewer opportunities for patient preference to be reflected in treatment choice [Citation44]. In the management of psoriasis, unless there is a very strong clinical reason against it, patients with psoriasis are likely to be first prescribed an antitumor necrosis factor biosimilar, regardless of patient preference or whether the administration regimen is considered to be convenient by the patient (expert opinion, HM) [Citation39,Citation47]. Physicians should also ensure that planned treatment switches are supported by available evidence; many biosimilar switch studies have been conducted, but some gaps still remain [Citation44,Citation48,Citation49].
An integrated model of care is becoming particularly important because, in an aging population, many patients have multiple complex conditions requiring individualized care utilizing different healthcare services for personalized medicine as well as long-term support. Going beyond the patient-centered treatment goal of a functional life, we can see how an integrated care model supports working toward the person-centered goal of a meaningful life. Under CCGs, there were notable limitations in the integration of care, leading to inefficient duplication of provision and poor experiences and outcomes for patients [Citation14,Citation50,Citation51]. The new integrated model of care creates an opportunity for physicians and their patients to discuss treatment options earlier in the treatment pathway. We strongly recommend that patient partners are involved in priority setting as this new model is developed (expert opinion, HM and ST); responses from a structured interview series confirm that formalized patient involvement must be actively nurtured to achieve success in this area [Citation52,Citation53].
6. Time to biologic treatment and the importance of ‘getting it right the first time’
There is evidence that some patients with IMIDs in the UK experience delays in biologic initiation; for instance, a retrospective review of patients with IBD in the UK found that the median time from the clinical decision to treat to the initiation of a biologic was 44.5 days, with some variation depending on the route of treatment administration [Citation54]. We have experienced similar delays (expert opinion, HM and ST). In IBD, delayed treatment increases the risk of complications (such as abscesses, fistulas, uncontrolled inflammation, and bowel perforation) that may require surgical intervention. In both IBD and psoriasis, delayed treatment is likely to negatively impact patient quality of life (expert opinion, HM and ST).
Delays in disease management may lead patients to seek private medical care at their own expense, regardless of their financial position (expert opinion, ST). Patients gave the following feedback to the Psoriasis Association regarding these issues via their helpline (telephone/e-mail) during 2021:
I’ve been waiting to see a dermatologist for 8 months with the hope of finally starting medication […]. There doesn’t seem to be any end in sight, and my psoriasis is the worst it has been, so I’ve been thinking of going private.
I went private because I still had months on the waiting list and my skin was so bad I could barely walk.
It took about 5 months […] to even get an appointment with a GP [general practitioner] for a referral and a whole year from that to see a dermatologist.
Patients also report treatment delays between the agreement to prescribe a biologic (first-line treatment or a later-line switch) and receipt of the new medication (expert opinion, HM and Dale Webb, chief executive officer of the NASS, London, UK), and treatment delays of this nature have been reported to the NASS. In order to obtain a prescription for a biologic, a patient with rheumatoid arthritis must attend an appointment with a rheumatology nurse, undergo testing, and have their results assessed, and a funding request must be submitted. Delays at any of these stages may result in the patient remaining untreated and in pain for weeks, affecting their work, social, and family life. Furthermore, the delay in receiving a biologic may necessitate additional visits to an HCP for treatment to manage symptoms, such as pain and fatigue. One NASS member wrote in the NASS online forum:
I am in quite severe pain a lot of the time due to the sacroiliitis. The pain wakes me in the night and trying to move in bed makes me howl. I am taking naproxen, which helps a little but it is not enough to stop this night-time waking, and I am losing a lot of sleep and suffering much discomfort throughout the day, affecting my work. I saw the rheumatology physician associate today to be assessed for secukinumab. The switch to secukinumab could take another month, then there is the wait for it to work. I have already been in pain some considerable time due to various delays (even this appointment was meant to happen 5 weeks ago). I asked what I could do about the pain in the meantime, and she said I should speak to my GP, and they could give me co-codamol. I found this response completely inadequate.
Moreover, some patients find the first prescribed biologic unsuitable for them; reasons for this include: adverse events, tolerability issues, and lack of efficacy. A real-world study reviewing current therapies may be particularly useful for providing insights into patient adherence on therapy and failure rate [Citation43]. This will help to ensure the right treatment is chosen for the right patient from the start [Citation55]. In turn, this will potentially lead to long-term savings, through reduction of disease flares, adverse events, medication changes, and other factors that contribute to healthcare utilization.
The coronavirus disease 2019 (COVID-19) pandemic exacerbated treatment delays for many patients, seriously affecting the prescription of new medications and making it difficult for patients to see or speak to an HCP; regular appointments were canceled as ‘non-essential’ care ceased [Citation56,Citation57]. Another NASS member wrote the following in the NASS online forum in June 2021:
I was supposed to have a phone appointment and twice they failed to call. I am being asked to have blood tests to go on biologic medication, and three times I did [have blood tests performed] before I was then told that it HAD to be at the hospital and not my GP surgery. Calls I was promised never happened.
The debate about whether there is sufficient evidence in the published literature to demonstrate how a delay in biologic initiation might affect patient outcomes is ongoing. Therefore, we feel that there is a need for evidence sharing to encourage further discussion and resolution of the issue. Specifically, in patients with IBD being treated with biologics, there can be additional delays, which vary across the country, depending on whether patients are treated as inpatients or outpatients (expert opinion, ST).
7. Accounting for the ‘experience lottery’
In February 2021, a group of specialists in dermatology, rheumatology, and gastroenterology from across England published a consensus statement on ‘Maintaining Clinical Freedom Whilst Achieving Value in Biologics Prescribing,’ which highlighted the need across all three disease areas to balance clinical freedom and cost restrictions to support equitable resource distribution within NHS England [Citation19]. Drawing on experience from the CCG era, the consensus statement highlighted local variations in care that contradicted NICE guidance. In our experience, these local variations continue to persist into the ICS era. This is supported by a recent analysis conducted by the Patient Experience Platform, which synthesizes publicly available data on patient experience in England under the new ICS structure [Citation58]. They identified variations both between English regions (e.g. Midlands vs. London) and within regions (e.g. North vs. South London). From personal experience, issues are not solely related to access to biologics but are compounded by variations in healthcare services, funding, and expertise, which may differ between district and academic health trusts or between tertiary and research hospitals (expert opinion, HM).
One consequence of such variations can be seen in the assessment of Psoriasis Area and Severity Index (PASI) scores. Some dermatologists will measure patients’ eligibility for biologic therapy based on baseline PASI scores taken prior to treatment initiation, whereas others will use current PASI scores. As patients are required to meet a threshold PASI score to be eligible for biologic therapy, there are accounts of patients having to let their psoriasis deteriorate to a point at which they qualify for biologic therapy, despite the increased burden of disease associated with this decline. Additionally, it should be noted that assessment of PASI scores is less reliable in patients with black skin versus white skin, which may contribute to unequal access to biologic therapies (expert opinion, HM). Patient feedback provided to the Psoriasis Association on this issue includes:
I had to have 6 months unmedicated to let my skin get to the point I was about 80% covered and kept getting infections […]. It’s not good but was worth the wait to get on Humira [adalimumab] and keep me clear for a few years.
Had my booking in appointment for my biologics today […] having been told I’d receive medication next week, to my horror, I was told I’m not severe enough […], this is while STILL being medicated by methotrexate; obviously my skin severity grade will be lower because I’m already medicated […]. I have been told that I should come off my medication and call them when my flare up becomes severe again. This can’t be right?! I have to go back to what I dread the most in order to get the treatment I need! Despite already having been passed to them by my consultant […].
Based on considerations like these, to work toward equity in the biologic treatment of IMIDs and a true shared decision-making model, we feel there is a need to understand wider points of view from any HCP who might be involved in the management of patients receiving biologics, not just specialists. Patients could potentially avoid deterioration of their condition if commissioners allow physicians to use their expertise to assess patient eligibility for biologic treatment.
8. Determining the value of biologic medicines for all stakeholders
The concept of ‘value’ in healthcare has different meanings for different stakeholders [Citation59]. Clinicians may define value as the best care based on research and clinical experience. Commissioners may consider value as the most cost-effective clinical benefit achieved. For example, RCTs often provide a ‘number needed to treat,’ which is the number of patients you need to treat to see a clinical benefit: the lower the number, the better. Patients are likely to see value differently; they are less likely to be concerned with cost and more likely to be concerned with how the treatment improves their symptoms, quality of life, and prognosis [Citation59]. Furthermore, different patients may value different aspects of a treatment option; for example, for some patients, convenient dosing might be taking a once-daily pill, whereas for others, a once-weekly injection may better meet their needs [Citation59].
Determining the ‘best value’ in the context of biologic treatment can, therefore, be challenging. The cheapest per- unit cost may not provide the best value for all stakeholders. For example, patients who are clinically stable receiving an originator biologic may not wish to switch to a biosimilar [Citation60,Citation61]. The consensus of dermatologists, rheumatologists, and gastroenterologists mentioned earlier recommended the use of expanded value frameworks to assess value in biologic treatment by including real-world evidence in addition to clinical trial data, and considering factors such as productivity, reduction in uncertainty of response to treatment, and the importance of hope [Citation62]. Current treatment guidelines rarely use such frameworks, and there are challenges in reflecting all factors that patients value. For instance, although it is common to assess health-related quality of life when evaluating psoriasis treatment, a commonly used measure such as the Dermatology Life Quality Index (DLQI) does not always provide a reliable assessment of an individual’s situation; research has found that there is a persistently high rate of ‘not relevant’ responses [Citation63,Citation64]. Despite this, NICE guidelines recommend the DLQI as a validated tool to assess a patient’s quality of life and determine their treatment path [Citation6]. A consensus of dermatologists, rheumatologists, and gastroenterologists from the UK recommends continued cooperation between clinicians and commissioners to balance clinical freedom for clinicians with the short-term cost restrictions that support the equity of patient access to biologics [Citation19].
9. Conclusion: addressing the issues raised
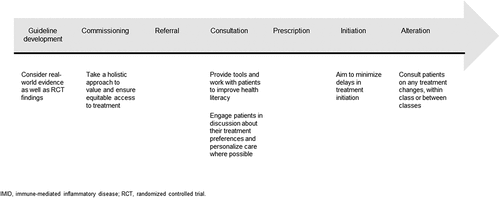
Addressing the inequities identified in this article will require action by clinicians, commissioners, and patients. Clinicians should review existing NICE guidance and quality standards on shared decision making and patient support tools [Citation26,Citation30], and evaluate their own practice against the recommended standards. Commissioners should ensure that the frameworks they use set out the importance of shared decision making in the treatment process. Patients should be encouraged to elevate their health literacy, not only to ensure they are involved in treatment decisions, but also to help reduce health inequalities more broadly [Citation65]. With many competing pressures facing the NHS, including a challenging funding environment, staff shortages, and an aging population, the existing challenges will be complex to solve. Additionally, for patients to help lead some aspects of change by asking for the care they are entitled to and deserve, there will be a requirement for support in the forms of education and voice amplification. A collaborative approach by all stakeholders is likely to be the most effective route to ensuring the patient voice is heard on the issues that matter the most to them, ultimately leading to person-centered care () [Citation19]. Where patients do not wish to or are unable to have extensive involvement in their care, patient advocacy groups can be a powerful representative voice.
10. Expert opinion
Biologics are now a vital part of the treatment arsenal for patients with IMIDs. Current prescribing practices, typically driven by budget pressures, take a narrow view of treatment value. However, a holistic approach to value must include the patient perspective, obtained through and respected during shared decision making – an approach already advocated in the NICE guidelines. For prescribers, this means ensuring consideration of the impact biologic medications have on the patient’s lifestyle and quality of life.
Physicians caring for people with IMIDs should take advantage of continuing professional development opportunities to ensure they are fully informed about relevant biologic treatment options. They should also work to ensure that the patient voice is heard and considered at all stages. This should include consultation with patients before decisions regarding switching or stopping medications are made, either within or between treatment classes. In addition, to maximize the patient’s role in shared decision making, it is important that healthcare systems provide tools to improve patient health literacy and that patients use these tools to address gaps in their understanding of their disease and treatment.
Across England, disparities in healthcare delivery means that access to biologics varies geographically. Although the full effects of the transition from a CCG to an ICS model remain to be seen, we can immediately identify some steps for optimizing treatment. A central goal recommended by the NHS when initiating biologic treatment in people with IMIDs to ensure the best treatment outcomes and highest patient satisfaction should be ‘getting it right the first time,’ which requires involvement of the patient in decisions regarding their care, alongside stakeholders from primary and secondary care, and treatment awareness from commissioners [Citation66]. We reiterate that patients need to be given treatment options in line with national guidelines as well as the information necessary for them to make informed decisions in collaboration with their HCP. Successful inclusion of the patient in this process is likely to lead to better patient health, quality of life, and adherence. The challenge with the development of new biologics is the time taken to collect data that support clinical decision making.
Delays in access to both secondary care and biologics must also be examined and addressed. We believe patients may currently be undertreated for extended periods of time, affecting them both physically and psychologically. Changes must be made to the structure of healthcare services and guidelines on biologic prescribing to mitigate these effects and eliminate the ‘experience lottery’ to ensure patients are treated consistently, adequately, and in a timely fashion. This will help to achieve better treatment adherence and, consequently, improved disease control and quality of life.
We challenge those governing healthcare pathways in the UK to redefine the focus of patient care so that patients are recognized as key stakeholders and to make patient choice not just rhetoric, but a reality.
We are confident that meaningful steps are possible, based on our experience-led insights and suggestions, that can increase transparency, reduce inequality, and improve disease outcomes for patients in the UK with IMIDs who require biologic treatment. This would ultimately increase patient confidence in and satisfaction with their healthcare service, to the benefit of all.
Article highlights
In the UK, commissioning of local healthcare services has a key influence on medicine prescribing. The recent move to an integrated care system model provides an opportunity to end the segregation of primary and secondary care pathways, and feature patient-centric decision making.
Integration of care pathways can help to address biologic prescribing decisions being made in isolation from routine disease management.
Understanding a patient’s determination of the value of treatment is essential in making informed treatment decisions for immune-mediated inflammatory diseases (IMIDs).
By bringing together integrated pathways, transparency in prescribing decisions, understanding of stakeholder perceptions of treatment value, and encouraging patient health literacy, meaningful steps can be taken toward minimizing the ‘experience lottery’ and improving treatment access and outcomes in the management of IMIDs.
Abbreviations
CCG, clinical commissioning group; COVID-19, coronavirus disease 2019; DLQI, Dermatology Life Quality Index; DMARD, disease-modifying anti-rheumatic drug; GP, general practitioner; HCP, healthcare professional; HM, Helen McAteer; IBD, inflammatory bowel disease; ICS, integrated care system; IMID, immune-mediated inflammatory disease; NASS, National Axial Spondyloarthritis Society; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; PASI, Psoriasis Area and Severity Index; PROM, patient-reported outcome measure; PsA, psoriatic arthritis; PsAID-12, 12-item Psoriatic Arthritis Impact of Disease Questionnaire; RCT, randomized controlled trial; ST, Seb Tucknott.
Declaration of interests
S Tucknott is an employee (Chief Executive) of IBDrelief Ltd. This organization has received grants and funding from AbbVie, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Galapagos, Janssen, Norgine, PredictImmune, Roche, and Takeda. H McAteer is an employee (Chief Executive) of the Psoriasis Association (charity registered in England and Wales [charity number: 1180666] and in Scotland [charity number: SC049563]). Over the past 36 months, this organization has received funding from AbbVie, Almirall, Amgen, Eli Lilly, Janssen/Janssen-Cilag, LEO Pharma, Novartis, and UCB. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to acknowledge the input of Dale Webb (NASS, London, UK) into the discussions that led to this manuscript. Helen McAteer acknowledges the support and input of Laura Stevenson (Deputy Chief Executive, Psoriasis Association, Northampton, UK). Medical writing support was provided by Natalie Griffiths, PhD, of OPEN Health Communications, London, UK; Colin Griffin, PhD (Griffin Scientific Ltd., Hereford, UK), on behalf of OPEN Health Communications, London, UK; Charles Geary, MSci, of OPEN Health Communications, London, UK; and Ellie Hughes, PhD, of OPEN Health Communications, London, UK, and funded by Janssen-Cilag Ltd., in accordance with Good Publication Practice guidelines (https://ismpp.org/gpp-2022).
Additional information
Funding
References
- Smolen JS, Landewe RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):3–18. doi: 10.1136/ard-2022-223356
- Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis: updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022 Aug;18(8):465–479. doi: 10.1038/s41584-022-00798-0
- Gomollon F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017 Jan;11(1):3–25. doi: 10.1093/ecco-jcc/jjw168
- Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017 Jul 1;11(7):769–784. doi: 10.1093/ecco-jcc/jjx009
- National Institute for Health and Care Excellence [Internet]. NG219: Crohn’s disease: management. Manchester (UK): NICE; 2019 [cited 2023 Sep]. Available from: https://www.nice.org.uk/guidance/ng129/chapter/Recommendations
- National Institute for Health and Care Excellence [Internet]. CG153: psoriasis: assessment and management. Manchester (UK): NICE; 2017 [cited 2023 Sep]. Available from: https://www.nice.org.uk/guidance/cg153/chapter/1-Recommendations
- National Institute for Health and Care Excellence [Internet]. NG130: ulcerative colitis: management. Manchester (UK): NICE; 2019 [cited 2023 Sep]. Available from: https://www.nice.org.uk/guidance/ng130/chapter/Recommendations
- NHS England [Internet]. Commissioning framework for biological medicines (including biosimilar medicines). Leeds (UK): NHS; 2017 [cited 2023 Oct]. Available from: https://www.england.nhs.uk/wp-content/uploads/2017/09/biosimilar-medicines-commissioning-framework.pdf
- The King’s Fund [Internet]. The rising cost of medicines to the NHS – what’s the story? London (UK): The King’s Fund; 2018 [cited 2023 Aug]. Available from: https://assets.kingsfund.org.uk/f/256914/x/063e13bd4d/rising_cost_of_medicines_april_2018.pdf
- Alnahar S, Elliott RA, Smith MD. Biosimilar uptake by British local formularies: a cross sectional study. Int J Clin Pharm. 2017 Oct;39(5):1055–1060. doi: 10.1007/s11096-017-0523-6
- NHS Confederation [Internet]. What were clinical commissioning groups? London (UK): NHS Confederation; 2021 [cited 2023 Oct]. Available from: https://www.nhsconfed.org/articles/what-are-clinical-commissioning-groups
- Checkland K, McDermott I, Coleman A, et al. Complexity in the new NHS: longitudinal case studies of CCGs in England. BMJ Open. 2016 Jan 7;6(1):e010199. doi: 10.1136/bmjopen-2015-010199
- Mistry J, Hill D, Kaul A. P87 CCG biologics pathways: re-introduction of the postcode lottery by stealth. Rheumatology (Oxford). 2020 Apr;59(Supplement_2):87. doi: 10.1093/rheumatology/keaa111.085
- Wenzl M, McCuskee S, Mossialos E. Commissioning for equity in the NHS: rhetoric and practice. Br Med Bull. 2015 Sep;115(1):5–17. doi: 10.1093/bmb/ldv031
- NHS [Internet]. ‘If I pay for private treatment, how will my NHS care be affected?’ Newcastle Upon Tyne (UK): NHS; 2022 [cited 2023 Sep]. Available from: https://www.nhs.uk/common-health-questions/nhs-services-and-treatments/if-i-pay-for-private-treatment-how-will-my-nhs-care-be-affected/
- NHS Bath and North East Somerset Swindon and Wiltshire Clinical Commissioning Group [Internet]. Moderate to severe plaque psoriasis biologic treatment pathway in adults. Chippenham (UK): Bath and North East Somerset, Swindon and Wiltshire Partnership; 2019 [cited 2023 Oct]. Available from: https://bswtogether.org.uk/medicines/wp-content/uploads/sites/3/2023/04/BSW-Plaque-Psoriasis-Pathway-April-2023.pdf
- Pan Mersey Area Prescribing Committee [Internet]. Psoriasis in adults, sequential use of biological agents. Leeds (UK): Pan Mersey Area Prescribing Committee; 2020 [cited 2023 Oct]. Available from: https://www.panmerseyapc.nhs.uk/media/2132/psoriasis_biologics.pdf
- Surrey and North West Sussex Area Prescribing Committee [internet]. Plaque psoriasis drug treatment pathway. Surrey, (UK): Surrey and North West Sussex Area Prescribing Committee; 2019 [cited 2023 Sep]. Available from: https://surreyccg.res-systems.net/PAD//Content/Documents/2/FINAL%20Psoriasis%20treatment%20pathway%20september%202019.pdf
- Raine T, Gkini MA, Irving PM, et al. Maintaining clinical freedom whilst achieving value in biologics prescribing: an integrated cross-specialty consensus of UK dermatologists, rheumatologists and gastroenterologists. BioDrugs. 2021 Mar;35(2):187–199. doi: 10.1007/s40259-020-00464-5
- Kaul A, Mistry J, Iagnocco A, et al. Inequality of access to advanced therapies for patients with inflammatory arthritis: a postcode lottery? Rheumatol Adv Pract. 2021 Nov;5(3):rkab081. doi: 10.1093/rap/rkab081
- NHS England [Internet]. What are integrated care systems? Leeds (UK): NHS; 2022 [cited 2023 Oct]. Available from: https://www.england.nhs.uk/integratedcare/what-is-integrated-care/
- Coyle C, Watson L, Whately-Smith C, et al. How do patient reported outcome measures affect treatment intensification and patient satisfaction in the management of psoriatic arthritis? A cross sectional study of 503 patients. Rheumatology (Oxford). 2024 [cited 2024 Jan 8]. doi: 10.1093/rheumatology/kead679
- Watson L, Coyle C, Whately-Smith C, et al. An international multi-centre analysis of current prescribing practices and shared decision-making in psoriatic arthritis. Rheumatology (Oxford). 2023 [cited 2023 Nov 27]. doi: 10.1093/rheumatology/kead621
- Nutbeam D, Lloyd JE. Understanding and responding to health literacy as a social determinant of health. Annu Rev Public Health. 2021 Apr 1;42(1):159–173. doi: 10.1146/annurev-publhealth-090419-102529
- Liu C, Wang D, Liu C, et al. What is the meaning of health literacy? A systematic review and qualitative synthesis. Fam Med Community Health. 2020 May;8(2):e000351.
- National Institute for Health and Care Excellence [Internet]. ECD8: standards framework for shared-decision-making support tools, including patient decision aids. Manchester (UK): NICE; 2021 [cited 2023 Sep]. Available from: https://www.nice.org.uk/corporate/ecd8
- Martinez B, Dailey F, Almario CV, et al. Patient understanding of the risks and benefits of biologic therapies in inflammatory bowel disease: insights from a large-scale analysis of social media platforms. Inflamm Bowel Dis. 2017 Jul;23(7):1057–1064. doi: 10.1097/MIB.0000000000001110
- National Axial Spondyloarthritis Society [Internet]. What do patients value and need in the diagnosis, treatment and care of axial spondyloarthritis? London (UK): NASS; 2022 [cited 2023 Sep]. Available from: https://nass.co.uk/wp-content/uploads/2022/11/NAS-Patient-Values-Report-DIGITAL.pdf
- NHS: Health Education England [Internet]. Improving health literacy - better health through improved health literacy. Leeds (UK): NHS; [cited 2023 Oct]. Available from: https://www.hee.nhs.uk/our-work/knowledge-library-services/improving-health-literacy
- National Institute for Health and Care Excellence [Internet]. NG197: shared decision making. Manchester (UK): NICE; 2021 [cited 2023 Sep]. Available from: https://www.nice.org.uk/guidance/ng197
- Murray S, Augustyniak M, Murase JE, et al. Barriers to shared decision-making with women of reproductive age affected by a chronic inflammatory disease: a mixed-methods needs assessment of dermatologists and rheumatologists. BMJ Open. 2021 Jun 16;11(6):e043960. doi: 10.1136/bmjopen-2020-043960
- Zisman-Ilani Y, Thompson KD, Siegel LS, et al. Crohn’s disease shared decision making intervention leads to more patients choosing combination therapy: a cluster randomised controlled trial. Aliment Pharmacol Ther. 2023 Jan;57(2):205–214. doi: 10.1111/apt.17286
- Tierney S, Wong G, Mahtani KR. Current understanding and implementation of ‘care navigation’ across England: a cross-sectional study of NHS clinical commissioning groups. Br J Gen Pract. 2019 Oct;69(687):e675–e681. doi: 10.3399/bjgp19X705569
- Taylor PC, Betteridge N, Brown TM, et al. Treatment mode preferences in rheumatoid arthritis: moving toward shared decision-making. Patient Prefer Adherence. 2020 Jan 20;14:119–131. doi: 10.2147/PPA.S220714
- IBDrelief [Internet]. Impact of IBD on physical and emotional health: findings from an IBDrelief survey. Shoreham-by-Sea. (UK): IBD Relief Ltd; 2022 [cited 2023 Oct]. Available from: https://www.ibdrelief.com/ibd-medication-adherence-survey-report
- Rubin DT, Sninsky C, Siegmund B, et al. International perspectives on management of inflammatory bowel disease: opinion differences and similarities between patients and physicians from the IBD GAPPS survey. Inflamm Bowel Dis. 2021 Nov 15;27(12):1942–1953. doi: 10.1093/ibd/izab006
- The Patients’ Association [Internet]. Understanding patient needs in switching from biologic to biosimilar medicines: final report of survey and focus group findings. San Francisco (CA): WordPress; 2018 [cited 2023 Oct]. Available from: https://allcatsrgrey.org.uk/wp/download/pharmacy/Understanding_patient_needs_in_switching_from_biologic_to_biosimilar_medicines.pdf
- Kumar K, Raizada SR, Mallen CD, et al. UK-South Asian patients’ experiences of and satisfaction toward receiving information about biologics in rheumatoid arthritis. Patient Prefer Adherence. 2018 Apr 4;12:489–497. doi: 10.2147/PPA.S153741
- Faraoni D, Schaefer ST. Randomized controlled trials vs. observational studies: why not just live together? BMC Anesthesiol. 2016 Oct 21;16(1):102. doi: 10.1186/s12871-016-0265-3
- Pearce W, Raman S, Turner A. Randomised trials in context: practical problems and social aspects of evidence-based medicine and policy. Trials. 2015 Sep 1;16(1):394. doi: 10.1186/s13063-015-0917-5
- Hariton E, Locascio JJ. Randomised controlled trials - the gold standard for effectiveness research: study design: randomised controlled trials. BJOG. 2018 Dec;125(13):1716. doi: 10.1111/1471-0528.15199
- Chodankar D. Introduction to real-world evidence studies. Perspect Clin Res. 2021 Jul;12(3):171–174. doi: 10.4103/picr.picr_62_21
- Patel A. Getting It Right First Time (GIRFT) – dermatology. A clear path to clear skin: a pharmacist’s perspective. J Med Optim. 2021;7(2):54–59.
- Barker JB. Biosimilars. J Eur Acad Dermatol Venereol. 2018 Jul;32(7):1054. doi: 10.1111/jdv.15066
- US Food and Drug Administration [Internet]. Biosimilars: review and approval. White Oak, MD (US); 2022. [cited 2024 Mar]. Available from: https://www.fda.gov/drugs/biosimilars/review-and-approval
- Gecse KB, Cumming F, D’Haens G. Biosimilars for inflammatory bowel disease: how can healthcare professionals help address patients’ concerns? Expert Rev Gastroenterol Hepatol. 2019 Feb;13(2):143–155. doi: 10.1080/17474124.2019.1553617
- Warren RB, Marsden A, Tomenson B, et al. Identifying demographic, social and clinical predictors of biologic therapy effectiveness in psoriasis: a multicentre longitudinal cohort study. Br J Dermatol. 2019 May;180(5):1069–1076. doi: 10.1111/bjd.16776
- Moots R, Azevedo V, Coindreau JL, et al. Switching between reference biologics and biosimilars for the treatment of rheumatology, gastroenterology, and dermatology inflammatory conditions: considerations for the clinician. Curr Rheumatol Rep. 2017 Jun;19(6):37. doi: 10.1007/s11926-017-0658-4
- Allocati E, Godman B, Gobbi M, et al. Switching among biosimilars: a review of clinical evidence. Front Pharmacol. 2022 Aug;13:917814. doi: 10.3389/fphar.2022.917814
- The King’s Fund [Internet]. Integrated care systems explained: making sense of systems, places, and neighbourhoods. London (UK): The King’s Fund; 2022 [cited 2023 Oct]. Available from: https://www.kingsfund.org.uk/publications/integrated-care-systems-explained
- Lewis RQ, Checkland K, Durand MA, et al. Integrated care in England - what can we learn from a decade of national pilot programmes? Int J Integr Care. 2021 Oct;21(4):5.doi: 10.5334/ijic.5631
- Coultas C, Kieslich K, Littlejohns P. Patient and public involvement in priority-setting decisions in England’s transforming NHS: an interview study with clinical commissioning groups in South London sustainability transformation partnerships. Health Expect. 2019 Dec;22(6):1223–1230. doi: 10.1111/hex.12948
- Smiddy J, Reay J, Peckham S, et al. Developing patient reference groups within general practice: a mixed-methods study. Br J Gen Pract. 2015 Mar;65(632):e177–83. doi: 10.3399/bjgp15X683989
- Liu E, Jatale R, DeSilva D, et al. PMO-39 Factors influencing delays in biologic initiation in inflammatory bowel disease. Gut. 2021;70(Suppl 4):A96–A97.
- Romao VC, Vital EM, Fonseca JE, et al. Right drug, right patient, right time: aspiration or future promise for biologics in rheumatoid arthritis? Arthritis Res Ther. 2017 Oct 24;19(1):239. doi: 10.1186/s13075-017-1445-3
- Goyal DK, Mansab F, Naasan AP, et al. Restricted access to the NHS during the COVID-19 pandemic: is it time to move away from the rationed clinical response? Lancet Reg Health Eur. 2021 Sep;8:100201. doi: 10.1016/j.lanepe.2021.100201
- Majeed A, Maile EJ, Bindman AB. The primary care response to COVID-19 in England’s National Health Service. J R Soc Med. 2020 Jun;113(6):208–210. doi: 10.1177/0141076820931452
- Patient Experience Platform Health [internet]. What Patients Think 2: trends and variation in patient experience across hospital trusts in England 2020-21. London (UK): Statica Research Ltd; 2021 [cited 2023 Sep]. Available from: https://www.pephealth.ai/what-patients-think-2021.
- Marzorati C, Pravettoni G. Value as the key concept in the health care system: how it has influenced medical practice and clinical decision-making processes. J Multidiscip Healthc. 2017 Mar;10:101–106. doi: 10.2147/JMDH.S122383
- Moots RJ, Curiale C, Petersel D, et al. Efficacy and safety outcomes for originator TNF inhibitors and biosimilars in rheumatoid arthritis and psoriasis trials: a systematic literature review. BioDrugs. 2018 Jun;32(3):193–199. doi: 10.1007/s40259-018-0283-4
- Dylst P, Vulto A, Simoens S. Barriers to the uptake of biosimilars and possible solutions: a Belgian case study. PharmacoEconomics. 2014 Jul;32(7):681–691. doi: 10.1007/s40273-014-0163-9
- Garrison L Jr., Kamal-Bahl S, Towse A. Toward a broader concept of value: identifying and defining elements for an expanded cost-effectiveness analysis. Value Health. 2017 Feb;20(2):213–216. doi: 10.1016/j.jval.2016.12.005
- Rencz F, Poor AK, Pentek M, et al. A detailed analysis of ‘not relevant’ responses on the DLQI in psoriasis: potential biases in treatment decisions. J Eur Acad Dermatol Venereol. 2018 May;32(5):783–790. doi: 10.1111/jdv.14676
- Poor AK, Brodszky V, Pentek M, et al. Is the DLQI appropriate for medical decision-making in psoriasis patients? Arch Dermatol Res. 2018 Jan;310(1):47–55. doi: 10.1007/s00403-017-1794-4
- Public Health England [Internet]. Improving health literacy to reduce health inequalities: practice resource summary. London (UK): Public Health England; 2015 [cited 2023 Oct]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/460710/4b_Health_Literacy-Briefing.pdf
- NHS England [Internet]. Dermatology GIRFT programme national specialty report. Leeds (UK): NHS; 2021 [cited 2023 Oct]. Available from: https://gettingitrightfirsttime.co.uk/wp-content/uploads/2021/09/DermatologyReport-Sept21o.pdf