1. Introduction
Bispecific antibodies (bsAbs) have emerged as a promising class of therapeutic molecules, designed to simultaneously target two distinct antigens or epitopes. Simultaneous targeting of antigens permits synergistic functionalities beyond what can be obtained even with combinations of conventional monospecific antibodies [Citation1]. A prime example is bispecific T-cell engagers that bridge tumor cells and T cells through binding of both a tumor associated antigen and CD3, thus targeting the T cells directly toward cells, expressing the tumor-associated antigen and mediating the CD8 T-cell killing of the tumor cells [Citation2–4]. This work presents considerations on the formation of bsAbs through the fusion of antigen binding fragments from heavy-chain only antibodies (VHH) onto IgG scaffold to form bispecific IgG-VHH antibodies.
2. Characteristics of IgG-VHH fusions
BsAbs can be assembled in numerous different ways from various molecular building blocks to form a myriad of structurally diverse bsAb molecules, and the molecular configuration can be optimized to fit the therapeutic purpose [Citation1]. Among these, IgG-VHH fusions, where small (~15 kDa) and monomeric VHH domains are fused onto IgG scaffolds, are a highly attractive way of forming stable and functional bsAbs [Citation5]. The VHH molecules are the smallest naturally occurring antibody binding domains derived from heavy-chain only antibodies that can be found naturally in camelids and sharks. The VHH domains are monomeric and thus do not rely on pairing with a cognate light chain. This largely prevents chain mispairing and undesired self-association, which are common issues for scFv-IgG bsAbs that formed through the fusion of single-chain variable fragments (scFvs) onto IgG scaffolds [Citation6]. Additionally, VHHs are known for their high stability and solubility, while generally being non-immunogenic, as they share high sequence identity with the human VH domains [Citation7], which can be further improved through humanization campaigns.
The IgG-VHH fusions can be formed as both symmetric bsAbs, that adhere to the paired heavy chain–light chain symmetry of conventional monospecific antibodies (HC2LC2), and as asymmetric bsAbs, e.g. by replacing one of the Fabs with a VHH domain [Citation5,Citation8]. Both types of bsAbs contain Fc domain that provides antibody effector functions through the binding of cognate Fcγ receptors as well as extended half-life through FcRn recycling and easy purification using Protein A. The construction of symmetric IgG-VHHs through simple the fusion of VHH domains is a reliable and conceptually easy way of forming bsAbs. The VHH can be fused to N- and/or C-termini of HC and/or LC, thus allowing flexibility with regard to valencies and molecular geometry. The HC2LC2 symmetry is beneficial, because only two polypeptide chains need to be co-expressed, and therefore, the workflows closely resemble those of conventional monospecific antibodies which are established and very efficient. The recent exploration of a comprehensive panel of symmetric IgG-VHH bsAbs showed that fusion of VHH domains on IgG scaffolds is a robust way of forming functional and structurally diverse bsAbs with high thermodynamic stability and low aggregation propensity. Binding studies further confirmed that the IgG-VHH bsAbs were able to bind each target individually as well as simultaneously by being functionally bispecific [Citation5]. The study also highlighted that the structural configuration influences the behavior of the molecules and that the molecular format is therefore a crucial factor to consider when developing IgG-VHH bsAbs. The molecular configuration of IgG-VHH bsAbs can be optimized to fit the therapeutic purpose, e.g. by tailoring the fusion site, the number of fused VHH domains (valency) as well as the spacing of antigen-binding domains by engineering the fusion site(s) and judicious linker design. It is also possible to form tandem VHH-Fc by replacing each Fab domain of the IgG with a tandem VHH to produce a tetravalent bsAb that does not contain any light chain. This construct requires expression of only a single polypeptide chain, thus greatly limiting the number of potential antibody-related impurities that can be formed from incomplete bsAb assembly. The simplicity of the tandem VHH-Fc construct makes it an attractive format that is currently being evaluated in a clinical setting (NCT03809624).
By replacing only a single Fab of the IgG with VHH or fusing the VHH on one of the HCs while also including mutations for HC heterodimerization, such as knobs-into-holes [Citation9], asymmetric IgG-VHH bsAbs can be constructed. These asymmetric bsAbs have the distinct advantage of containing only a single light chain which eliminate the risk of incorrect HC-LC pairing. The asymmetric nature of these IgG-VHH molecules allows full control of the valencies, and the bsAbs can also therefore be used for targets that require monovalent targeting of antigens where antigen crosslinking is highly undesired, such as CD3 [Citation10]. The modular nature of VHH domains and IgG molecules allow very high flexibility with regard to fusion sites and the number of fused binding partners. This is not only applicable for tailoring the valencies of IgG-VHH but can also be used in the formation of tri- and tetraspecific antibodies [Citation11].
While the fusion of VHH domains onto IgG scaffolds represents an attractive and robust means of forming bsAbs, it should be noted that the added complexity of molecules compared to the parent IgG risk introducing unexpected liabilities. This has been shown in a recent study where certain IgG-VHH configurations were found to be more aggregation prone than the parent IgG scaffold. This was particularly evident for two hexavalent bsAbs with VHH fused C-terminally on both HC and LC, suggesting that an added structural complexity of bsAbs can compromise colloidal stability [Citation5]. Furthermore, the subclass of IgG scaffold should carefully be considered to match the intended IgG-VHH functionality [Citation12].
3. Expert opinion
VHH domains are being increasingly recognized as attractive modular building blocks for constructing complex bsAbs with advanced functionalities. Despite their smaller size and relatively fewer complementarity-determining regions (CDRs), their binding affinities and versatilities are fully comparable to Fab and scFv. A recent comparison of a large number of paratope–epitope interfaces showed that VHH domains compensate for their smaller size by applying a larger proportion of both CDR and framework residues in the binding interface. This allows them to establish a similar number of total atom–atom contacts between the paratope and the epitope as conventional Fv antibodies, containing both VH and VL [Citation13]. The efficient construction of IgG-VHH bsAbs can be broken down into the processes of (1) identifying suitable antigen-binding partners with the desired binding functionality and (2) judiciously combining the building blocks in an optimal molecular configuration that will allow the IgG-VHH to exert the desired biological function.
3.1. Identifying molecular building blocks
VHHs for therapeutic approaches can be obtained through the immunization of llamas in combination with the construction of large antibody libraries, such as phage or yeast display libraries, or via synthetic VHH antibody libraries with human or humanized frameworks [Citation14]. As shown recently, VHH domains exhibit comparatively longer CDR3 than their Fv counterparts [Citation13,Citation15], thus making human VHH libraries with longer CDR3 desirable for therapeutic approaches which have been reported recently (Goletz, PEGS Europe, 2023). Since these processes to identify lead candidate VHH can be laborious and time consuming [Citation12], computational tools are increasingly used to improve antibody discovery campaigns. Examples include deep mining of antibody repertories through next-generation sequencing coupled with advanced analytics [Citation16] as well as modeling efforts to avoid selecting hits with liabilities that will negatively affect the developability profile and make them unsuitable as drug candidates [Citation17]. The small and monomeric nature of VHH antibodies is also advantageous in these in silico workflows, because the lack of a VL partner makes uncertainties associated with proper VH-VL pairing obsolete. These uncertainties include reconstitution of native VH-VL pairs from individually sequenced genes or VH-VL packing during structural modeling.
3.2. Assembling IgG-VHH bsAbs
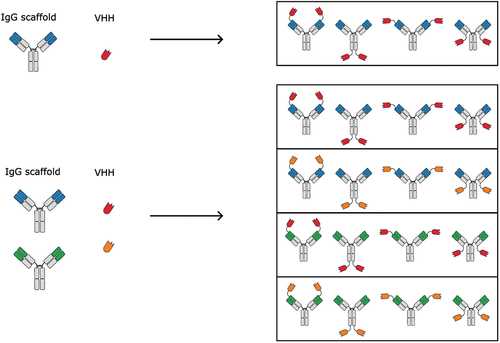
After identifying an IgG scaffold and a suitable VHH domain to use as the exogenous fusion partner, the components must be combined to form the final IgG-VHH. The structural search space for IgG-VHHs grows exponentially with linearly increasing number of parental antibodies, because the components can be combined in numerous different ways () [Citation18]. Several parameters are important, such as fusion site, spatial orientation, valency, and linker lengths and design, which need to be carefully selected to achieve the desired biological activity and developability profile. While the optimal molecular configuration is likely to depend on target combinations and target availability, some trends were observed from a systematic study of a comprehensive panel of structurally diverse IgG-VHH bsAbs with mirrored specificities [Citation5,Citation8]. In these studies, the fusion of VHH to the HC in a symmetric format is favorable over fusion to LC. While all IgG-VHH bsAbs in the panel were functionally bispecific, i.e. able to bind both antigens individually and simultaneously, the molecular geometry showed influences on the binding affinity. The fusion of VHH to LC reduced the affinity of the scaffold Fv when linked N-terminally (3–10 fold affinity reduction), whereas fusion C-terminally on LC impaired the binding of VHH itself (4–5 fold affinity reduction). In contrast, VHH fusion to the HC showed much less pronounced effect on the binding affinity for both C- and N-terminally fused constructs. Since bsAbs often exhibit higher complexity than conventional IgGs, there is a need for assays that are capable of selectively characterizing the intricate binding functionality of bsAbs. Recently, an in-solution assay for dissecting bsAb binding functionality in complex dual binding environments without relying on potentially obstructive surface immobilization was introduced. The assay permits the assessment of simultaneous antigen binding through incremental increases in antibody–antigen complex sizes as well as determination of individual binding affinities even in higher order bsAb complexes, where more than one antigen is bound [Citation8]. This in-solution assay based on flow-induced dispersion analysis also allows the determination of binding cooperativity and interference and can be applied even with complex sample matrices.
Figure 1. Combinatorial diversity of symmetric bsAb binder formats increases exponentially with increasing number of parental antibody building blocks. Only a selected set of symmetric tetravalent bsAbs are shown, but the structural search space will become even bigger while also including asymmetric bsAbs or higher valency symmetric bsAbs.
Another important aspect of bsAbs besides the direct targeting of the antigen and exerting the biological functionality is the developability profile, broadly referring to physicochemical properties that describes how likely it is a candidate can become an efficacious, safe, and manufacturable drug. Such important features include production yield, biophysical stability, solubility, polyreactivity and immunogenicity. The bispecific IgG-VHH format can be seen as favorable in respect to biophysical stability and yield. The thermal stabilities of the symmetric IgG-VHH bsAb panel were generally high, and aggregation propensities were low, both comparable to IgG1 molecules [Citation5]. Asymmetric formats using VHH as fusion partner are also seen to be robust in respect to the VHH part, while the thermal stability is somewhat reduced compared to IgG1 and symmetric formats [Citation7], since most platforms for HC heterodimerization of asymmetric bsAbs are based on CH3 engineering known to negatively affect thermal stability. In respect to manufacturability, all the IgG-VHH bsAbs with VHH fused on HC fusion of VHH to HC showed an increase in productivity compared to the parent IgG1, while fusion of VHH to LC appeared less favorable [Citation5]. Downstream workflows can make efficient use of Protein A purification and closely resemble those of conventional IgG1 for symmetric IgG-VHH. Most research on antibody developability has been conducted with conventional monospecific IgG molecules, and there is thus a need for a more systematic evaluation of developability liabilities for bsAbs such as IgG-VHH.
4. Conclusion
Conclusively, IgG-VHH bsAbs present as attractive therapeutic agents that can be robustly assembled with high versatility to fit the intended mechanism of action. The small and monomeric nature of VHH domains make them highly suitable as exogenous fusion partners that bypass many of the issues often encountered for scFvs, containing both VH and VL. While the ability to assemble IgG-VHH bsAbs with diverse molecular geometries allows for tailoring the bsAb to fit the therapeutic need, it also creates a large structural search space. The molecular configuration has a profound impact on the activity and behavior of the bsAbs. Recently described patterns for preferable fusion sites and geometries can help effectively exploring the search space to identify optimal bsAb binder formats and to generate optimized bispecific therapeutic candidates.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Labrijn AF, Janmaat ML, Reichert JM, et al. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608. doi: 10.1038/s41573-019-0028-1
- Hoffmann P, Hofmeister R, Brischwein K, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115:98–104. doi: 10.1002/ijc.20908
- Löffler A, Kufer P, Lutterbüse R, et al. A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. doi: 10.1182/blood.V95.6.2098
- Seckinger A, Majocchi S, Moine V, et al. Development and characterization of NILK-2301, a novel CEACAM5xCD3 κλ bispecific antibody for immunotherapy of CEACAM5-expressing cancers. J Hematol Oncol. 2023;16(1):117. doi: 10.1186/s13045-023-01516-3
- Madsen AV, Kristensen P, Buell AK, et al. Generation of robust bispecific antibodies through fusion of single-domain antibodies on IgG scaffolds: a comprehensive comparison of formats. MAbs. 2023;15(1):2189432. doi: 10.1080/19420862.2023.2189432
- Cao M, Wang C, Chung WK, et al. Characterization and analysis of scFv-IgG bispecific antibody size variants. MAbs. 2018;10(8):1236–1247. doi: 10.1080/19420862.2018.1505398
- Muyldermans S. Nanobodies: natural single-domain antibodies. Kornberg R, editor. Annu Rev Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449
- Madsen AV, Mejias-Gomez O, Pedersen LE, et al. Immobilization-free binding and affinity characterization of higher order bispecific antibody complexes using size-based microfluidics. Anal Chem. 2022;94(40):13652–13658. doi: 10.1021/acs.analchem.2c02705
- Atwell S, Ridgway JBB, Wells JA, et al. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J Mol Biol. 1997;270(1):26–35. doi: 10.1006/jmbi.1997.1116
- Lee HY, Contreras E, Register AC, et al. Development of a bioassay to detect T-cell-activating impurities for T-cell-dependent bispecific antibodies. Sci Rep. 2019;9(1):3900. doi: 10.1038/s41598-019-40689-1
- Yanakieva D, Pekar L, Evers A, et al. Beyond bispecificity: controlled fab arm exchange for the generation of antibodies with multiple specificities. MAbs. 2022;14(1):2018960. doi: 10.1080/19420862.2021.2018960
- Yu J, Song Y, Tian W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J Hematol Oncol. 2020;13(1):45. doi: 10.1186/s13045-020-00876-4
- Madsen AV, Mejias-Gomez O, Pedersen LE, et al. Structural trends in antibody-antigen binding interfaces: a computational analysis of 1833 experimentally determined 3D structures. Computat Struct Biotechnol J. 2024;23:199–211. doi: 10.1016/j.csbj.2023.11.056
- Mandrup OA, Friis NA, Lykkemark S, et al. A novel heavy domain antibody library with functionally optimized complementarity determining regions. PLoS One. 2013;8(10):e76834. doi: 10.1371/journal.pone.0076834
- Mejias-Gomez O, Madsen AV, Skovgaard K, et al. A window into the human immune system: comprehensive characterization of the complexity of antibody complementary-determining regions in functional antibodies. MAbs. 2023;15(1):2268255. doi: 10.1080/19420862.2023.2268255
- Greiff V, Miho E, Menzel U, et al. Bioinformatic and statistical analysis of adaptive immune repertoires. Trends Immunol. 2015;36(11):738–749. doi: 10.1016/j.it.2015.09.006
- Raybould MIJ, Marks C, Krawczyk K, et al. Five computational developability guidelines for therapeutic antibody profiling. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:p. 4025–4030.
- Dengl S, Mayer K, Bormann F, et al. Format chain exchange (FORCE) for high-throughput generation of bispecific antibodies in combinatorial binder-format matrices. Nat Commun. 2020;11(1):4974. doi: 10.1038/s41467-020-18477-7

